Abstract
Sustainable poultry practices are needed to maintain an adequate supply of poultry products to the increasing human population without compromising human wellbeing. In order to achieve the understanding of the core microbiome that assumes an imperative role in digestion, absorption, and assimilation of feed as well as restrict the growth of pathogenic strains, a proper meta-data survey is required. The dysbiosis of the core microbiome or any external infection in chickens leads to huge losses in the poultry production worldwide. Along with this, the consumption of infected meat also impacts on human health as chicken meat is a regular staple in many diets as a vital source of protein. To tackle these losses, sub-therapeutic doses of antibiotics are being used as a feed additive along with other conventional approaches including selective breeding and modulation in feed composition. Altogether, these conventional approaches have improved the yield and quality of poultry products, however, the use of antibiotics encompasses the risk of developing multi-drug resistant pathogenic strains that can be harmful to human beings. Thus, there is an urgent need to understand the chicken microbiome in order to modulate chicken gut microbiome and provide alternatives to the conventional methods. Although there is now emerging literature available on some of these important microbiome aspects, in this article, we have analysed the relevant recent developments in understanding the chicken gut microbiome including the establishment of integrated gene catalogue for chicken microbiome. We have also focussed on novel strategies for the development of a chicken microbial library that can be used to develop novel microbial consortia as novel probiotics to improve the poultry meat production without compromising human health. Thus, it can be an alternative and advanced step compared to other conventional approaches to improve the gut milieu and pathogen-mediated loss in the poultry industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major requirement of human consumption for protein is directly met by the poultry industry in the form of chicken meat. As of 2016, the overall global production of poultry eggs and meat were approximately 74 and 119 million tons respectively [1, 2]. Poultry meat production was expected to increase to 122 million tons by November 2018 [2]. However, it has been estimated that by 2050, the global human populace is predicted to reach approximately 9.8 billion [3]. Hence, to maintain an adequate supply without compromising meat quality and human health, the livestock production needs to be intensified by using rational approaches.
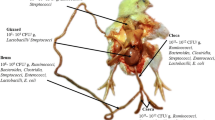
On the basis of their productivity, there are three main breeds of chicken livestock viz., broilers (meat productive poultry breed), layers (egg productive poultry breed) and backyard (dual-purpose poultry breed). The commercial production of meat is majorly (92%) from the broiler breed [4]. Due to continuous conventional techniques majorly by extensive selective breeding programs, enhancement in feed quality and by implementing antibiotics as feed additives during the past several decades, the yield and quality of meat have been improved substantially. In all these approaches feed has been the most important input for poultry production. So far, the diet ingredients mainly consist of fish, fish oil, soybeans, corn, and animal products. To retain the high protein value and overcome the high cost of protein diets, currently, insects are being used as a sustainable alternative, as their amino acids content is of much superior quality than conventional plant fed poultry ingredients [5, 6]. The overall utilization of these nutrient sources depends on the improved microbial diversity present in the gut of chickens. It is pertinent to mention, that the feed conversion ratio (FCR) is very crucial in the poultry sector as it measures the quantity of feed that can produce per kilogram of meat along with the reduction in greenhouse gas (GHG) emission. Due to the high rate of feed conversion (FCR = 1.4–2.8) in the commercial broiler, it is the preferred breed for meat production [7, 8]. There is nearly a saturation stage that has been attained by conventional improvement methods and further improvements without affecting the quality of meat and human health are difficult. To feed the ever-increasing world population and hence meet the increasing demand, we have to devise new approaches to improve and substantially increase the quality and quantity of poultry products. The study of chicken microbiome using metagenomics and culturomics techniques can provide possible remedies not only to tackle the problems of infection and antibiotic resistance in chicken but also to devise cost-effective production methods (Fig. 1).
Due to the recent advances in culturomics and sequencing technologies, the current emphasis is shifting to understand host-microbe interactions. A quick literature survey revealed that these approaches have been emphatically used in the past to study microbes inhabiting a wide range of ecological niches including hot water springs [9, 10], deep oceans [11], space [12], highly contaminated niches [13, 14], human and animal gut [15]. While free-living microbes play many important roles including the production of antibiotics [16], degradation of xenobiotic compounds [17] and production of several molecules important for mankind [18, 19], the intestinal bacteria have an important role in maintaining homeostasis and health in various organisms and their dysbiosis have been linked with various diseases [20,21,22].
There has been a rapid upsurge in the number of reports that suggest the role of the microbiome in sustaining the normal physiological functions and demonstrated the impact of microbial imbalance (dysbiosis) [23]. Similarly, studies have shown the impact of diet in shaping the gut microbial community in humans as well as animals [24,25,26]. As mentioned before in this context the chicken gut microbiome is very important and it provided the spur for further research in recent years and has engrossed on the role of the microbiome present in the chicken gut [27, 28]. More recently the establishment of an integrated gene catalogue (IGC) for chicken microbiome [29] and compilation of the taxonomical and microbial functional hierarchy at different developmental stages and regions of gastrointestinal tract (GIT) microbiota has provided impetus to devise microbiome based tools for the improvement of poultry production by using culturomics based microbial consortia [30]. In addition, wild chicken breeds that are less susceptible to diseases can also be used to raise specific microbial consortia to develop probiotics in order to improve global poultry health [31]. The selective data analysis, encompassing the core microbiota, IGC, the role of microbes in gut immunity and the new era culturomics approach as presented below can now provide an insight into the development of alternative methods to improve the pathogen-mediated loss in the poultry industry. These approaches aim to develop methods to improve the quality and quantity of poultry products without affecting human health (Fig. 1).
Core Microbiome and Co-evolution of Pathogens
The sum total of all the microbes (archaea, bacteria, viruses, protozoa, and fungi) occupying a particular niche is termed microbiome. In chicken, the microbiome is predominantly dominated by bacteria [32]. Initial studies on the ecological diversity of the microbiome primarily based on amplicon sequencing focussed on 16S rRNA gene sequence have revealed that the chicken microbiome is represented by at least 13 major bacterial phyla and out of these Firmicutes, Bacteroidetes, and Proteobacteria are the majority (> 90%) with relative proportion varying with breed [33]. More than 900 species-equivalent operational taxonomic units (OTUs) were identified in chicken, that represented 117 established bacterial genera [34]. In addition to these, the gut of chicken has also been occupied by pathogens like Salmonella, Escherichia coli, and Campylobacter that cause gastroenteritis in human [35] (Table 1). While most of these studies are based on culture-independent approaches, the majority of the culturable approaches have also identified that these bacteria inhabit the gut of almost all birds up to 107 CFU g−1 (colony forming unit per gram) and also reflected in the poultry environment including litter [36, 37]. These bacteria are either present as minor taxon or generally accepted to be non-pathogenic in healthy avian host. Being minor taxon of chicken intestinal microflora, Salmonella has been marked with sporadic and transient colonization of its avian host [37]. Whereas Campylobacter was thought to be a non-pathogenic commensal of chicken, it has now been shown to affect bird health [38] and is also a major foodborne pathogen [39]. The infections caused by these pathogenic bacteria lead to reduced meat production and present critical dangers to human wellbeing making poultry a noteworthy vector of infection [40]. Several stains of Campylobacter (C. concisus, C. ureolyticus C. lari, and C. upsaliensis) [41], Listeria monocytogenes [42] Shiga toxin-producing E. coli (STEC) [43], and non-typhoidal Salmonella [44] have been listed under the category of emerging pathogens, primarily because of their new emerging genotypes as well as the acquisition of antimicrobial resistance genes present in animal, environment and human intestinal tract [45,46,47,48].
Recent studies have revealed the role of microbiome interaction in empowering the host health to achieve the best possible fitness in a given environment [49]. The major role of diet in shaping the microbiome structure through the long-term symbiosis of host and the microbiome has been proved [50, 51]. The mapping of microbial communities in the chicken GIT based on shotgun sequencing revealed the spatial pattern, suggesting the different functional aspects of microbiome at different locations [52]. The major niche in ileum is occupied by more of Lactobacillus, Streptococcus, Enterococcus than Clostridiaceae (11%), while cecum is mostly dominated by members of the Clostridiaceae (65%) family [53]. Although the cecum is mostly dominated by Clostridiaceae, still the diversity of cecum is significantly more intricate than that of the small intestine and crop. Due to the spatial difference in microbial composition, hindgut harbouring healthy microbiota has been involved in converting dietary fibres into short chain fatty acids (SCFAs), that deliver more energy for the host and increases the FCR [54]. In contrast to this, the dysbiosis of the healthy microbiome leads to the passing of the excess nutrients to the lower intestine. This in-turn provides substrates for the growth of pathogenic bacteria which otherwise don’t compete in that habitat [55]. All this reflects that chicken microbiome plays a vital role in maintaining chicken health (see details below).
Chicken Gut Microbiome and Its Role in Maintaining Chicken Health
In addition to providing nutrition, the gut microbiota also attaches to the epithelial walls of the intestinal cells (enterocytes) forming a protective blockade and keep pathogenic bacteria at bay by restricting their growth [56]. Presence of pathogenic bacteria like Salmonella, E. coli and Campylobacter pose a stern risk to human health and the poultry industry. They act as a reservoir for antibiotic resistance and can easily disseminate to humans through infected meat (Table 1). To suppress the growth of these pathogenic strains, the feed is augmented with sub-therapeutic doses of antibiotics. The mechanism of growth promotion by sub-therapeutic antibiotics is partially known. But the microbiome has been made known to play a key part in chicken’s growth by maintaining an optimum immune system, physiology, and protection against pathogens. On the other hand, germ-free chickens do not gain weight even if subjected to feed additives thus highlighting the importance of the local microbiome [57]. There are however major losses that still occur as a result of bacterial and viral infections. To prevent the negative impact of bacterial pathogens on poultry, higher doses of antibiotics like tetracycline, bacitracin, salinomycin, tylosin etc. are very often used [58]. Tetracycline alone accounts for more than 2/3rd of antimicrobials productivity in poultry farms [59]. The studies have also shown a significant reduction in probiotic Lactobacillus population and post-antibiotic treatment [60, 61]. This can reduce the intestinal activity of bile hydrolase salt as Lactobacillus is known to produce the bile hydrolase salt [62]. The bile salt hydrolase converts conjugated bile salts into unconjugated bile salts i.e., more efficient to less efficient lipid emulsification and utilization form [62]. Thus, the reduction in bile salt hydrolase promotes lipid metabolism and increases the energy harvest leading to more weight gain. Further long-term exposure to antibiotics leads to the development of antibiotic-resistant pathogenic strains. It is also becoming increasingly clear that the gut provides a perfect environment for the transfer of multidrug-resistant genes via extra-chromosomal elements including plasmids and transposons among the bacteria [63]. In addition to antibiotics, the use of antibiotic growth promoters (AGPs) to tackle the problem of antibiotic resistance and to increase the muscle mass is also becoming popular in the poultry industry [64, 65]. In fact, AGPs have a dual role to play as while they increase muscle mass, these also act against pathogens by altering the microbial community in GIT of chicken [66]. However, AGPs are also not free from side effects and have been reported to even affect the beneficial microbes in the microbiome. However, AGPs have also been banned in many countries mainly due to the appearance of bacteria becoming resistant to antibiotics. Further, the acquisition of antimicrobial resistance genes leads to the emergence of novel strains, causing an augmented microbial hazard and these can be further passed on to humans.
Research on the ecology of chicken gut microbiome from healthy and diseased individuals is being unraveled with the increasing availability of literature in this field [57, 67]. Most of the studies have revealed that gut microbiome assumes a vital role in nutrition, physiology, and growth of chickens [56]. Lactobacillus and Bacillus have been identified as the major probiotic bacteria in chicken microbiome [68]. These probiotic bacteria have been linked with increasing the biosynthesis of vitamins majorly vitamin K and vitamin B groups, bacteriocins, SCFAs particularly butyric acid, acetic acid, and propionic acid, and organic acids viz. lactic acid. They also decrease triglycerides and induce non-pathogenic immune responses [67]. The contribution of gut microbiota in supplementing the amino acids such as glutamine and lysin is also observed [69]. The assimilation of uric acid in the cloaca to yield amino acids is one of the major roles played by the microbial community in birds. Chicken gut inhabitants like Bifidobacterium [70], Bacteroides [71] and Akkermansia muciniphila [72] are the important providers of carbon and nitrogen sources by degrading the available cell wall mucin. Thus, a two-way host-microbe exchange of nutrients has evolved.
Attempts to minimize the use of antibiotics and AGPs have been made by using probiotics, enzymes, organic acids, prebiotics, immune-stimulants, essential oils, bacteriocins, bacteriophages, phytogenic feed additives, phytoncides, and nanoparticles [73], but these are not very effective for maintaining the good health of chicken. These can be regarded as the recent therapeutic approaches that are being attempted for suppressing the use of antibiotics but there is a need for generating healthy pathogen-free inoculum to maintain the gut homeostasis and lessen the use of antibiotics.
Major Pathogens in Poultry and Use of Antibiotics
Due to the increasing consumption of poultry products, it is important to ensure proper food safety and shelf-life. In hatcheries, the newly hatched chickens have no contact with the adult birds, so their initial microbial inoculum source is the litter that shapes the development of gastrointestinal microbiome [37]. The presence of several putative pathogens has been reported from these litter samples [37] (Table 1). In the entire GIT, the cecum is the ideal habitat for diverse and complex microbiota, harbouring as many as 1010–1011/g of microbes [57]. The presence of pathogens in the gut and in the slaughtering environment increases the risk of contamination during and after slaughter. The pathogens like Salmonella spp., Campylobacter spp., Clostridium perfringens, L. monocytogenes, Staphylococcus aureus, and Aeromonas are the main contaminants in the poultry industry. A recent survey in Europe revealed S. aureus (38.5%) as the main pathogen, followed by Campylobacter (33.3%), L. monocytogenes (19.3%) and Salmonella (7.1%) [74]. In the USA, during 1998–2011, the highest number of outbreaks were caused by Salmonella (43%), followed by C. perfringens (26%), Campylobacter spp. (7%), S. aureus (5%) and L. monocytogenes (3%) [75]. In Australia, there were approx. 4.1 million annual cases of foodborne gastroenteritis and pathogenic E. coli, Campylobacter spp. and non-typhoidal Salmonella spp. were the most commonly known causative agents [76]. After China, USA, and Brazil, India is the fourth largest producer of chicken, while the third largest producers of eggs, just behind China and USA [1]. In a developing country like India, the microbial risk assessment system is not so robust, therefore the data is limited. However, the presence of pathogens like S. aureus, Salmonella spp. and Bacillus cereus in poultry products has been reported [77] and therefore, it is important that the management practices should be improved to overcome the microbial hazard.
Chicken Gut Microbiome: A Panorama of Immunity Interactions
In fact, most changes in the bacterial community in the broiler chickens have been reported to impact the immunity of the birds. As a result, Campylobacter (principally C. jejuni and C. coli), Salmonella enterica, E. coli, and C. perfringens pathogens frequently appear or overpopulate [37]. Recently, the mechanism of microbiota in impacting chicken immunity have been partly worked out. The chicken immune system includes both innate and adaptive responses. The gut of chicken has a robust mucosal layer with a dense layer of intestinal epithelial cell (IEC), secretory IgA and antimicrobial peptides (AMPs). Chicken gut microbiome has been reported to modulate the regulation of both types of the immune mechanism via host-microbial interactions. The innermost surface of the chicken gut is composed of a specific glycoprotein called mucin, secreted by epithelial cell linings [78]. Presence of this glycoprotein is linked with the stimulation of the production of antimicrobial peptides for preventing pathogen invasion. The conventionally reared chicken (mostly wild type) having diverse and healthy microbiota were found to have an abundance of mucin and sulfates as compared to chickens reared in protected or laboratory conditions [28]. The microbial community assumes a vital job in the production of antibacterial peptides present on the linings of intestinal epithelial and have a potential activity for killing or suppressing the activity of pathogens [79]. Pan and Yu [57] have reviewed this characteristic in chicken epithelial cells and confirmed the active role of the gut community in chicken innate immunity. There is a significant difference in count of lymphocytes and lymphoid cellular components in intestinal cell lines in germ-free chickens to normal chickens.
The role of the gut community-based immunity in chicken was analyzed in depth by Oakley et al., [37] where T cell stimulation and control of immunity mediator secretions were highlighted. Production of cytokines and B cell responses have also been studied in addition to T cell proliferation using chicken as a model [78, 80]. Secretory immunoglobulins (IgA) which play a vital role in pathogen elimination from the gut, was found dependent on the host gut community in germ-free chickens [81]. The gut community also control the production of immunity effector cells (IECs) such as angiopoietin 4 and S100, ribonucleases, C-type lectins, which are the defense cells against intruders [82]. Even defensins proteins have been reported to be induced by microbial community of the gut [83].
Thus, homeostasis of the microbial community of chicken gut is directly linked with the chicken immune homeostasis. Along with the constitutive expression of IECs, there is a significantly induced expression pattern observed in correlation with the gut microbiota of the chicken raised in germ-free or conventional conditions. With common IECs found in the human system, a chicken system can be used to study immune modeling for resident phagocytes, immune cells and their regulation with bacterial compositions.
Integrated Gene Catalogues: Identifying the Functional Potential of Gut Microbiomes
The total number of genes in higher organisms including humans, chicken, and mice is in the range of 19,000–55,000 [84,85,86]. The myriad functions carried out by the cells of these organisms cannot be justified with such a low number of genes. Estimation has shown that there are an almost similar number of bacterial cells (3.8 × 1013) as human cells (3.0 × 1013) [87] and there are 1011 obligate anaerobe bacterial cells g−1 chicken cecum [53]. These bacteria contribute significantly to genes that perform a specific function to maintain the daily life processes in higher organisms. Apart from exploring the microbiota, attempts are being made in identifying the entire gene pool of these commensal gut bacteria in chicken guts. The nearly complete set of genes for most gut microbes of an organism is called an integrated gene catalogue (IGC). Gene catalogues are generated after studying gut microbiome of a large number of individuals of the same species. The updated gene catalogue of human gastrointestinal (GI) microbiome had 9.87 million genes [88]. Recently a comprehensive microbial gene catalogue consisting of 9.04 million genes from bacteria residing in different compartments of the intestine of chicken has been published by Huang et al. [29]. They also mapped the overall functional potential of the GI microbiome and demonstrated the impact of a plant alkaloid (Macleaya cordata extract; MCE) on the diversity and health of the chicken. The chickens that were raised on a diet supplemented with MCE and without the antibiotic, showed increase abundance of probiotic bacteria predominantly Lactobacillus and there was also a significant upregulation of biosynthetic pathways involved in the formation of amino acids, vitamins, and secondary bile acids. Interestingly, antibiotic-resistant genes had a minimum presence in the gene pool of the bacterial community. On the contrary, the diet supplemented chlortetracycline showed similarly enriched nutrient biosynthetic pathway in the foregut microbiota that can suppress the pathogenic load and increase the meat production but provoked an upsurge in antibiotic-resistance genes and antibiotic-producing bacteria [29]. In the future, these IGC will be expanded in order to elucidate the mechanisms of growth promotion and help us to identify strains that suppress the growth of harmful pathogenic bacteria.
Co-culturing Probiotic Bacteria for Pathogen Exclusion
Various bacterial communities start occupying the gut of newly hatched birds as soon as they come in contact with the environment. As discussed, these genera are sporadic and hence the abundant genera compete with the colonizing pathogenic bacteria. This exclusion is perhaps due to the physical occupation, competition for resources and production of toxins compounds that interfere with the colonizing process of pathogenic bacteria [28]. Successful attempts have been made for controlling Salmonella outspread using the probiotic model in broiler chickens [65]. Different species of Lactobacillus and Bacillus have been already commercialized into products like Aviguard®, Interbac® and Primalac® [89,90,91]. The usage of these cultures as feed additives led to a reduction in colonization of pathogens. Restriction of Salmonella is also correlated with weight gain and improved FCR leading to increased meat yield [92]. Although these findings using few bacterial genera are promising, there are several improvements that can be taken forward in the development and manufacture of more products from healthy microbiome with better effectiveness and quality. The majority of the probiotic compounds had strains that can be cultured separately, but this limits our ability to use strains that have a better probiotic effect and have limited culturing capacity. Co-culturing or mix culturing can be adopted for generating bacterial mix with better effect. Currently, most of the strains in probiotic mixes are facultative anaerobes. Futuristic methods can implement obligatory anaerobes to design the novel probiotic consortium using wild type pathogen-free chickens. These methods will effectively reduce the invasiveness of pathogenic bacteria.
Conclusions
In recent years, the core GI microbiome of chicken has been primarily linked with the overall health and development of the chicken. The GI tract of chicken harbour probiotic Lactobacillus and Bacillus strains along with various pathogenic strains of Salmonella, Campylobacter and E. coli that can be present sporadically. Dysbiosis of the core genome is linked to increased growth of pathogenic strains leading to low quality and contaminated poultry products. To restrict the growth of these pathogens, the feed is supplemented with sub-therapeutic doses of antibiotics in the poultry industry. Their use increases the chance of the spread of antibiotic resistance not only among chickens but also can be passed on to humans and directly impacts on the One Health concept. This seeks the immediate involvement and development of new tools to further investigate chicken microbiome for their functional contributions. The generation of IGC is a milestone achievement that can provide the requisite knowledge for designing the co-culture experiments for generating more efficient probiotic mix. In particular, this can be used as a forecasting tool to devise strategies for improving gut healthiness in livestock industrial processes. Microbial interaction in the chicken gut and its immunity modulations can be predicted for better control of human pathogens and management of poultry diseases, thus improving the overall health of the poultry.
References
Poultry Trends-2018. Watt Global Media. http://www.poultrytrends.com/201811/. Accessed 05 Jan 2019
Bedford D, Claro J, Doro E, Lucarelli L, Marocco E, Milo M, Mustafa S, Yang D, Fisheries Statistical Team (2018) Food outlook-biannual report on global food markets. Food and Agriculture Organization of the United Nations. http://www.fao.org/3/CA2320EN/ca2320en.pdf. Accessed 05 Jan 2019
World population prospects: the 2017 revision. United Nations Department of Economic and Social Affairs. https://www.un.org/development/desa/publications/world-population-prospects-the-2017-revision.html. Accessed 05 Jan 2019
Mottet A, Tempio G (2017) Global poultry production: current state and future outlook and challenges. Worlds Poult Sci J 73:245–256. https://doi.org/10.1017/S0043933917000071
FAO edible insects—future prospects for food and feed security. http://www.fao.org/docrep/018/i3253e/i3253e.pdf. Accessed 05 Jan 2019
Onsongo VO, Osuga IM, Gachuiri CK, Wachira AM, Miano DM, Tanga CM, Ekesi S, Nakimbugwe D, Fiaboe KKM (2018) Insects for income generation through animal feed: effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J Econ Entomol 111:1966–1973
Abudabos AM, Okab AB, Aljumaah RS, Samara EM, Abdoun KA, Al-Haidary AA (2013) Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Ital J Anim Sci 12:e28. https://doi.org/10.4081/ijas.2013.e28
Rezaei M, Yngvesson J, Gunnarsson S, Jonsson L, Wallenbeck A (2018) Feed efficiency, growth performance, and carcass characteristics of a fast- and a slower-growing broiler hybrid fed low- or high-protein organic diets. Org Agric 8:121–128. https://doi.org/10.1007/s13165-017-0178-6
Dwivedi V, Kumari K, Gupta SK, Kumari R, Tripathi C, Lata P, Niharika N, Singh AK, Kumar R, Nigam A, Garg N, Lal R (2015) Thermus parvatiensis RL(T) sp. nov., isolated from a hot water spring, located atop the Himalayan ranges at Manikaran, India. Indian J Microbiol 55:357–365. https://doi.org/10.1007/s12088-015-0538-4
Sharma A, Kohli P, Singh Y, Schumann P, Lal R (2016) Fictibacillus halophilus sp. nov., from a microbial mat of a hot spring atop the Himalayan range. Int J Syst Evol Microbiol 66:2409–2416. https://doi.org/10.1099/ijsem.0.001051
Zhang X, Xu W, Liu Y, Cai M, Luo Z, Li M (2018) Metagenomics reveals microbial diversity and metabolic potentials of seawater and surface sediment from a hadal biosphere at the Yap Trench. Front Microbiol 9:2402. https://doi.org/10.3389/fmicb.2018.02402
Be NA, Avila-Herrera A, Allen JE, Singh N, Sielaff AC, Jaing C, Venkateswaran K (2017) Whole metagenome profiles of particulates collected from the International Space Station. Microbiome 5:81. https://doi.org/10.1186/s40168-017-0292-4
Negi V, Singh Y, Schumann P, Lal R (2016) Corynebacterium pollutisoli sp. nov., isolated from hexachlorocyclohexane-contaminated soil. Int J Syst Evol Microbiol 66:3531–3537. https://doi.org/10.1099/ijsem.0.001228
Kumari R, Singh P, Schumann P, Lal R (2016) Tessaracoccus flavus sp. nov., isolated from the drainage system of a lindane-producing factory. Int J Syst Evol Microbiol 66:1862–1868. https://doi.org/10.1099/ijsem.0.000958
Ellis RJ, McSweeney CS (2016) Animal gut microbiomes. In: Yates MV, Nakatsu CH, Miller RV, Pillai SD (eds) Manual of environmental microbiology, 4th edn. American Society of Microbiology, Boston, pp 4.4.3-1–4.4.3-7
Singh P, Kumari R, Mukherjee U, Saxena A, Sood U, Lal R (2014) Draft genome sequence of rifamycin derivatives producing Amycolatopsis mediterranei strain DSM 46096/S955. Genome Announc. https://doi.org/10.1128/genomeA.00837-14
Lal R, Pandey G, Sharma P, Kumari K, Malhotra S, Pandey R, Raina V, Kohler HP, Holliger C, Jackson C, Oakeshott JG (2010) Biochemistry of microbial degradation of hexachlorocyclohexane and prospects for bioremediation. Microbiol Mol Biol Rev 74:58–80. https://doi.org/10.1128/MMBR.00029-09
Sood U, Singh Y, Shakarad M, Lal R (2017) Highlight on engineering Mycobacterium smegmatis for testosterone production. Microb Biotechnol 10:73–75. https://doi.org/10.1111/1751-7915.12466
Acevedo-Rocha CG, Gronenberg LS, Mack M, Commichau FM, Genee HJ (2019) Microbial cell factories for the sustainable manufacturing of B vitamins. Curr Opin Biotechnol 56:18–29. https://doi.org/10.1016/J.COPBIO.2018.07.006
Hira P, Sood U, Gupta V, Nayyar N, Mahato NK, Singh Y, Lal R, Shakarad M (2017) Human microbiome: implications on health and disease. In: Rawal L, Ali S (eds) Genome analysis and human health. Springer, Singapore, pp 153–168
Maji A, Misra R, Dhakan DB, Gupta V, Mahato NK, Saxena R, Mittal P, Thukral N, Sharma E, Singh A, Virmani R, Gaur M, Singh H, Hasija Y, Arora G, Agrawal A, Chaudhary A, Khurana JP, Sharma VK, Lal R, Singh Y (2018) Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol 20:402–419. https://doi.org/10.1111/1462-2920.14015
Sood U, Bajaj A, Kumar R, Khurana S, Kalia VC (2018) Infection and microbiome: impact of tuberculosis on human gut microbiome of Indian cohort. Indian J Microbiol 58:123–125. https://doi.org/10.1007/s12088-018-0706-4
Wang WL, Xu SY, Ren ZG, Tao L, Jiang JW, Zheng SS (2015) Application of metagenomics in the human gut microbiome. World J Gastroenterol 21:803–814. https://doi.org/10.3748/wjg.v21.i3.803
Schulz MD, Atay Ç, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, Salinas-Riester G, Bock A, Alpert C, Blaut (2014) High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514:508–512. https://doi.org/10.1038/nature13398
Luo Y, Chen H, Yu B, He J, Zheng P, Mao X, Tian G, Yu J, Huang Z, Luo J, Chen D (2017) Dietary pea fiber increases diversity of colonic methanogens of pigs with a shift from Methanobrevibacter to Methanomassiliicoccus-like genus and change in numbers of three hydrogenotrophs. BMC Microbiol 17:17. https://doi.org/10.1186/s12866-016-0919-9
Hoving LR, Katiraei S, Heijink M, Pronk A, van der Wee-Pals L, Streefland T, Giera M, Willems van Dijk K, van Harmelen V (2018) Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol Nutr Food Res 62:1700942. https://doi.org/10.1002/mnfr.201700942
Borda-Molina D, Seifert J, Camarinha-Silva A (2018) Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput Struct Biotechnol J 16:131–139. https://doi.org/10.1016/J.CSBJ.2018.03.002
Clavijo V, Flórez MJV (2018) The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult Sci 97:1006–1021. https://doi.org/10.3382/ps/pex359
Huang P, Zhang Y, Xiao K, Jiang F, Wang H, Tang D, Liu D, Liu B, Liu Y, He X, Liu H, Liu X, Qing Z, Liu C, Huang J, Ren Y, Yun L, Yin L, Lin Q, Zeng C, Su X, Yuan J, Lin L, Hu N, Cao H, Huang S, Guo Y, Fan W, Zeng J (2018) The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 6:211. https://doi.org/10.1186/s40168-018-0590-5
Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NP, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahão J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier PE, Levasseur A, Raoult D (2016) Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1:16203. https://doi.org/10.1038/nmicrobiol.2016.203
Medvecky M, Cejkova D, Polansky O, Karasova D, Kubasova T, Cizek A, Rychlik I (2018) Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom 19:561. https://doi.org/10.1186/s12864-018-4959-4
Choi KY, Lee TK, Sul WJ (2015) Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens—a review. Asian Australas J Anim Sci 28:1217–1225. https://doi.org/10.5713/ajas.15.0026
Zou A, Sharif S, Parkinson J (2018) Lactobacillus elicits a “Marmite effect” on the chicken cecal microbiome. NPJ Biofilms Microbiomes 4:27. https://doi.org/10.1038/s41522-018-0070-5
Wei S, Morrison M, Yu Z (2013) Bacterial census of poultry intestinal microbiome. Poult Sci 92:671–683. https://doi.org/10.3382/ps.2012-02822
Lillehaug A, Bergsjø B, Schau J, Bruheim T, Vikøren T, Handeland K (2005) Campylobacter spp., Salmonella spp., verocytotoxic Escherichia coli, and antibiotic resistance in indicator organisms in wild cervids. Acta Vet Scand 46:23–32. https://doi.org/10.1186/1751-0147-46-23
Chinivasagam HN, Estella W, Rodrigues H, Mayer DG, Weyand C, Tran T, Onysk A, Diallo I (2016) On-farm Campylobacter and Escherichia coli in commercial broiler chickens: re-used bedding does not influence Campylobacter emergence and levels across sequential farming cycles. Poult Sci 95:1105–1115. https://doi.org/10.3382/ps/pew003
Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, Lee MD, Collett SR, Johnson TJ, Cox NA (2014) The chicken gastrointestinal microbiome. FEMS Microbiol Lett 360:100–112. https://doi.org/10.1111/1574-6968.12608
Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P (2014) Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 5:e01364-14. https://doi.org/10.1128/mBio.01364-14
Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P (2011) Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol 2:200. https://doi.org/10.3389/fmicb.2011.00200
Meade KG, Narciandi F, Cahalane S, Reiman C, Allan B, O’Farrelly C (2009) Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics 61:101–110. https://doi.org/10.1007/s00251-008-0346-7
Man SM (2011) The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. https://doi.org/10.1038/nrgastro.2011.191
Swaminathan B, Gerner-Smidt P (2007) The epidemiology of human listeriosis. Microbes Infect 9:1236–1243. https://doi.org/10.1016/J.MICINF.2007.05.011
Piérard D, De Greve H, Haesebrouck F, Mainil J (2012) O157:H7 and O104:H4 Vero/Shiga toxin-producing Escherichia coli outbreaks: respective role of cattle and humans. Vet Res 43:13. https://doi.org/10.1186/1297-9716-43-13
Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA (2012) Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. https://doi.org/10.1016/S0140-6736(11)61752-2
Zhao S, White DG, McDermott PF, Friedman S, English L, Ayers S, Meng J, Maurer JJ, Holland R, Walker RD (2001) Identification and expression of cephamycinase bla (CMY) genes in Escherichia coli and Salmonella isolates from food animals and ground meat. Antimicrob Agents Chemother 45:3647–3650. https://doi.org/10.1128/AAC.45.12.3647-3650.2001
Winokur PL, Vonstein DL, Hoffman LJ, Uhlenhopp EK, Doern GV (2001) Evidence for transfer of CMY-2 AmpC beta-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother 45:2716–2722. https://doi.org/10.1128/AAC.45.10.2716-2722.2001
Blake DP, Hillman K, Fenlon DR, Low JC (2003) Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J Appl Microbiol 95:428–436. https://doi.org/10.1046/j.1365-2672.2003.01988.x
Mathew AG, Liamthong S, Lin J, Hong Y (2009) Evidence of class 1 integron transfer between Escherichia coli and Salmonella spp. on livestock farms. Foodborne Pathog Dis 6:959–964. https://doi.org/10.1089/fpd.2009.0263
Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, Gavryushkin A, Carlson JM, Beerenwinkel N, Ludington WB (2018) Microbiome interactions shape host fitness. Proc Natl Acad Sci 115:E11951–E11960. https://doi.org/10.1073/PNAS.1809349115
Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651. https://doi.org/10.1126/science.1155725
Groussin M, Mazel F, Sanders JG, Smillie CS, Lavergne S, Thuiller W, Alm EJ (2017) Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nat Commun 8:14319. https://doi.org/10.1038/ncomms14319
Xiao Y, Xiang Y, Zhou W, Chen J, Li K, Yang H (2016) Microbial community mapping in intestinal tract of broiler chicken. Poult Sci 96:1387–1393. https://doi.org/10.3382/ps/pew372
Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD (2003) Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 69:6816–6824. https://doi.org/10.1128/AEM.69.11.6816-6824.2003
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. https://doi.org/10.1194/jlr.R036012
Rinttilä T, Apajalahti J (2013) Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J Appl Poult Res 22:647–658. https://doi.org/10.3382/japr.2013-00742
Yegani M, Korver DR (2008) Factors affecting intestinal health in poultry. Poult Sci 87:2052–2063. https://doi.org/10.3382/ps.2008-00091
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. https://doi.org/10.4161/gmic.26945
Diarra MS, Malouin F (2014) Antibiotics in Canadian poultry productions and anticipated alternatives. Front Microbiol 5:282. https://doi.org/10.3389/fmicb.2014.00282
Chattopadhyay MK (2014) Use of antibiotics as feed additives: a burning question. Front Microbiol 5:334. https://doi.org/10.3389/fmicb.2014.00334
Lee KW, Ho Hong Y, Lee SH, Jang SI, Park MS, Bautista DA, Ritter GD, Jeong W, Jeoung HY, An DJ, Lillehoj EP, Lillehoj HS (2012) Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status. Res Vet Sci 93:721–728. https://doi.org/10.1016/J.RVSC.2012.01.001
Lin J, Hunkapiller AA, Layton AC, Chang YJ, Robbins KR (2013) Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog Dis 10:331–337. https://doi.org/10.1089/fpd.2012.1348
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738. https://doi.org/10.1128/AEM.72.3.1729-1738.2006
Card RM, Cawthraw SA, Nunez-Garcia J, Ellis RJ, Kay G, Pallen MJ, Woodward MJ, Anjum MF (2017) An in vitro chicken gut model demonstrates transfer of a multidrug resistance plasmid from Salmonella to commensal Escherichia coli. MBio 8:e00777-17. https://doi.org/10.1128/mBio.00777-17
Dibner JJ, Richards JD (2005) Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 84:634–643. https://doi.org/10.1093/ps/84.4.634
Park YH, Hamidon F, Rajangan C, Soh KP, Gan CY, Lim TS, Abdullah WN, Liong MT (2016) Application of probiotics for the production of safe and high-quality poultry meat. Korean J food Sci Anim Resour 36:567–576. https://doi.org/10.5851/kosfa.2016.36.5.567
Crisol-Martínez E, Stanley D, Geier MS, Hughes RJ, Moore RJ (2017) Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl Microbiol Biotechnol 101:4547–4559. https://doi.org/10.1007/s00253-017-8193-9
Shang Y, Kumar S, Oakley B, Kim WK (2018) Chicken gut microbiota: importance and detection technology. Front Vet Sci 5:254. https://doi.org/10.3389/fvets.2018.00254
Azad MAK, Sarker M, Li T, Yin J (2018) Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int 2018:9478630. https://doi.org/10.1155/2018/9478630
Metges CC (2000) Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr 130:1857S–1864S. https://doi.org/10.1093/jn/130.7.1857S
Ruas-Madiedo P, Gueimonde M, Fernández-García M, de los Reyes-Gavilán CG, Margolles A (2008) Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl Environ Microbiol 74:1936–1940. https://doi.org/10.1128/AEM.02509-07
Hooper LV, Midtvedt T, Gordon JI (2002) How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22:283–307. https://doi.org/10.1146/annurev.nutr.22.011602.092259
Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM (2008) The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol 74:1646–1648. https://doi.org/10.1128/AEM.01226-07
Mehdi Y, Létourneau-Montminy MP, Gaucher ML, Chorfi Y, Suresh G, Rouissi T, Brar SK, Côté C, Ramirez AA, Godbout S (2018) Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr 4:170–178. https://doi.org/10.1016/J.ANINU.2018.03.002
Gonçalves-Tenório A, Silva B, Rodrigues V, Cadavez V, Gonzales-Barron U (2018) Prevalence of pathogens in poultry meat: a meta-analysis of European published surveys. Foods 7:69. https://doi.org/10.3390/foods7050069
Chai SJ, Cole D, Nisler A, Mahon BE (2017) Poultry: the most common food in outbreaks with known pathogens, United States, 1998–2012. Epidemiol Infect 145:316–325. https://doi.org/10.1017/S0950268816002375
Kirk M, Glass K, Ford L, Brown K, Hall G (2014) Foodborne illness in Australia: annual incidence circa 2010. National Centre for Epidemiology and Population Health, Australian National University, Canberra
Sudershan RV, Naveen Kumar R, Kashinath L, Bhaskar V, Polasa K (2012) Microbiological hazard identification and exposure assessment of poultry products sold in various localities of Hyderabad, India. Sci World J 2012:736040. https://doi.org/10.1100/2012/736040
Brisbin JT, Gong J, Sharif S (2008) Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Heal Res Rev 9:101–110. https://doi.org/10.1017/S146625230800145X
Forder REA, Howarth GS, Tivey DR, Hughes RJ (2007) Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult Sci 86:2396–2403. https://doi.org/10.3382/ps.2007-00222
Mwangi WN, Beal RK, Powers C, Wu X, Humphrey T, Watson M, Bailey M, Friedman A, Smith AL (2010) Regional and global changes in TCRαβ T cell repertoires in the gut are dependent upon the complexity of the enteric microflora. Dev Comp Immunol 34:406–417. https://doi.org/10.1016/j.dci.2009.11.009
Ren C, Yin G, Qin M, Suo J, Lv Q, Xie L, Wang Y, Huang X, Chen Y, Liu X, Suo X (2014) CDR3 analysis of TCR Vβ repertoire of CD8+ T cells from chickens infected with Eimeria maxima. Exp Parasitol 143:1–4. https://doi.org/10.1016/j.exppara.2014.04.016
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516. https://doi.org/10.1038/nri3228
Cash HL, Whitham CV, Behrendt CL, Hooper LV (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. https://doi.org/10.1126/science.1127119
Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML (2014) Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum Mol Genet 23:5866–5878. https://doi.org/10.1093/hmg/ddu309
Burt DW (2005) Chicken genome: current status and future opportunities. Genome Res 15:1692–1698. https://doi.org/10.1101/gr.4141805
Bult CJ, Eppig JT, Blake JA, Kadin JA, Richardson JE, Mouse Genome Database Group (2016) Mouse genome database 2016. Nucleic Acids Res 44:D840–D847. https://doi.org/10.1093/nar/gkv1211
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. https://doi.org/10.1371/journal.pbio.1002533
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD, Bork P, Wang J, MetaHIT Consortium (2014) An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 32:834–841. https://doi.org/10.1038/nbt.2942
Nakamura A, Ota Y, Mizukami A, Ito T, Ngwai YB, Adachi Y (2002) Evaluation of aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult Sci 81:1653–1660. https://doi.org/10.1093/ps/81.11.1653
Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B, Chambers JR, Sharif S (2006) Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol 13:975–980. https://doi.org/10.1128/CVI.00161-06
Talebi A, Amirzadeh B, Mokhtari B, Gahri H (2008) Effects of a multi-strain probiotic (PrimaLac) on performance and antibody responses to Newcastle disease virus and infectious bursal disease virus vaccination in broiler chickens. Avian Pathol 37:509–512. https://doi.org/10.1080/03079450802356995
Chambers JR, Gong J (2011) The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res Int 44:3149–3159. https://doi.org/10.1016/J.FOODRES.2011.08.017
Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. https://doi.org/10.1128/CMR.00006-15
Cosby DE, Cox NA, Harrison MA, Wilson JL, Buhr RJ, Fedorka-Cray PJ (2015) Salmonella and antimicrobial resistance in broilers: a review. J Appl Poult Res 24:408–426. https://doi.org/10.3382/japr/pfv038
Temple ME, Nahata MC (2000) Treatment of listeriosis. Ann Pharmacother 34:656–661. https://doi.org/10.1345/aph.19315
Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 33:537–549. https://doi.org/10.1080/03079450400013162
Jones BL, Wilcox MH (1995) Aeromonas infections and their treatment. J Antimicrob Chemother 35:453–461
Momtaz H, Jamshidi A (2013) Shiga toxin-producing Escherichia coli isolated from chicken meat in Iran: serogroups, virulence factors, and antimicrobial resistance properties. Poult Sci 92:1305–1313. https://doi.org/10.3382/ps.2012-02542
Overview of Staphylococcosis in poultry. Poultry-Merck veterinary manual. https://www.merckvetmanual.com/poultry/staphylococcosis/overview-of-staphylococcosis-in-poultry. Accessed 7 Jan 2019
Acknowledgements
This manuscript was partly written when RL was on Executive Endeavour Fellowship at Murdoch University, Perth, Australia. RL is thankful to The National Academy of Sciences, India (NASI) for providing the NASI Senior Scientist Platinum Jubilee Fellowship. SL is grateful to Dr. Manoj Khanna, Principal, Ramjas College, University of Delhi, for providing sabbatical leave to undertake this work at Murdoch University, Perth, Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sood, U., Gupta, V., Kumar, R. et al. Chicken Gut Microbiome and Human Health: Past Scenarios, Current Perspectives, and Futuristic Applications. Indian J Microbiol 60, 2–11 (2020). https://doi.org/10.1007/s12088-019-00785-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-019-00785-2