Abstract
Tuberculosis (TB) is a deadly bacterial infectious disease caused by intra-cellular pathogen Mycobacterium tuberculosis (Mtb). There were an estimated 1.4 million TB deaths in 2015 and an additional 0.4 million deaths resulting from TB among individuals with HIV. Drug-discovery for its cure is very slow in comparison with the causative organism’s fast pace of mutations conferring drug resistance. Moreover, the field of drug-discovery of anti-TB drugs is constantly being challenged by the drug resistant strains of Mtb. Several molecules/inhibitors are being tested across the pharmaceutical industry and research centres for their suitability as drug candidate. It takes immense effort, high costs and a whole lot of screening to bring a single molecule to the clinics for patient cure. In last 60 years, hundreds of molecules have been patented for their probable use to develop drug for treatment of TB. However, only one drug has been successfully approved that is bedaquiline (1-(6-bromo-2 -methoxy-quinolin-3-yl)-4-dimethylamino-2-naphtalen-1-yl-1-phenyl-butan-2-ol). This is a brief review about bedaquiline (BDQ), the only drug in last 45 years approved for curing drug-resistant pulmonary TB, its development, action mechanism and development of resistance against it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis is the most widespread and deadly disease of mankind since centuries accounting for millions of deaths every year [1]. There were an estimated 1.4 million TB deaths in 2015 and an additional 0.4 million deaths among individuals with HIV co-infected with TB. However, the TB incidence rate and the number of TB deaths continue to fall and there was a 22% decrease in the rate of mortality by TB between 2000 and 2015 but TB remained one of the top 10 causes of deaths worldwide in 2015 [1]. The World Health Organization (WHO) compiles yearly reports about TB and simultaneously provides guidelines to clinicians for its treatment. Earlier WHO guidelines (2011) directed that the choice of drugs for TB should be based on efficacy and toxicity in the step-down manner, from group 1 to group 5. Group 1 included first line drugs and groups 2–5 included second-line drugs (Table 1). In the year 2016, WHO has provided an important and useful evidence-based new classification of anti-TB drugs to help clinicians design anti-TB drug regimen more accurately. The new classification of anti-TB drugs exclusively targets management of the drug-resistant TB (DR-TB) cases and not all TB cases (Table 1) [2, 3].
Over the time Mtb has developed resistance to many drugs and based on the susceptibility of its strains to the available drugs they have been classified into four categories (Fig. 1). The drug-susceptible (DS) strains of Mtb are susceptible to first-line drugs of anti-TB regimen, the multi-drug resistant (MDR) strains are resistant to first-line drugs rifampicin and/or isoniazid, the extensively-drug resistant (XDR) strains are the one harbouring resistance genes for a number of drugs (Isoniazid, Rifampicin and fluoroquinolones and to at least one of the three injectable second line drugs) and the totally drug resistant (TDR) Mtb strains are resistant to almost all available anti-TB drugs [4, 5]. Although the drug-resistance in the Mtb occurs due to poor patient compliance towards the drug-regimen suggested for them but there are cases of primary infection with drug-resistant Mtb strains as well [6]. Due to the emergence of a multitude of strains with resistance, the patient suffering is increasing and research for development of new anti-TB drugs is protracted [7, 8]. Several proteins/molecular targets have been identified which are essential for survival and virulence of Mtb [9, 10] and they prove to be potential drug targets. There has always been need for new drugs that could help design a better, shorter, cost-effective, safer and less toxic drug regimen to reduce patient suffering and mortality. Keeping in line with these two problems of drug resistance and urgency to improve anti-TB regimen, a single drug has come up in the market after four decades (since 1971 when rifampicin was approved) namely bedaquiline (Sirturo™) [11]. Like-wise there have been consistent efforts in the research laboratories to develop the antibiotics having better activity against Mtb. One such lead was achieved with the production of rifamycin B natural analog, 24-desmethylrifamycin B by manipulating the rifamycin polyketide synthase gene cluster in the producer organism Amycolatopsis mediterranei S699 [5, 12,13,14]. Notably, no new drug could be added to the first line regimen in the last 60 years even after thousands of publications and hundreds of patents related to the anti-TB drugs [15]. From these entire patented molecules only one novel drug, BDQ, could be included into anti-TB regimen [15].
Overview of TB-Treatment and the New Drugs
The standard treatment regimen for DS-TB is a 2 month regimen of isoniazid, rifampicin, ethambutol and pyrazinamide; it is followed by a 4 month treatment with isoniazid and rifampicin. The MDR-TB treatment is intensive, prolonged, comparatively more toxic and complex as it comprises of six second line drugs including injectables (Table 1). The total treatment duration of MDR-TB continues to 20 months to 2 years for most patients. The field of TB drug development has gained some success in last decade and some novel drug candidates are entering phase III trials for treatment of DR-TB, including BDQ and delamanid. Other repurposed drugs include linezolid, amoxicillin, clofazimine meropenem and imipenem/cilastin. They have shown good in vitro and in vivo activity against MDR-TB but are not yet approved for its treatment [16].
BDQ (Bedaquiline, Sirturo™, TMC 207 or R207910) most recently developed and FDA approved drug for treatment of pulmonary tuberculosis. It was discovered and developed by the team lead by Koen Andries at Janssen Therapeutics, pharmaceutical division of Johnson & Johnson and was granted accelerated approval on 28th December 2012 by the Food and Drug Administration (United States-FDA) based on the phase IIb clinical trial data [17]. BDQ is a diarylquinoline that specifically inhibit ATP synthase of the bacteria and interfere with its energy metabolism. In the following years it gained approval in different parts of the world considering the urgency of a drug for DR-TB [18]. Another drug named Delamanid (DLM) (Deltyba®, OPC-67683) by Otsuka pharmaceutical has received conditional approval by European Medicines Agency (EMA) for the treatment of MDR-TB in November 2013 in the Europe, Japan and South Korea and the FDA approval is still pending. Delamanid is a nitroimidazole that predominantly acts on the synthesis of mycolic acid and stops cell wall production. It increases rates of culture conversion thus improving outcome in adult studies [19]. BDQ and DLM are increasingly being used to treat MDR- and XDR-TB. WHO recommends their use under specific conditions and not in combination because of the lack of evidence [20]. BDQ has gained importance after the FDA approval and the revised TB drug classification by WHO [3].
BDQ: Structure, Function and Mechanism of Action
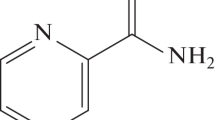
BDQ showed up as the most active compound among a series of molecules (diarylquinolines) tested for antimicrobial activity against M. smegmatis, a non-pathogen [11]. BDQ is chemically named as 1-(6-bromo-2 -methoxy-quinolin-3-yl)-4-dimethylamino-2-naphtalen-1-yl-1-phenyl-butan-2-ol and has a molecular weight of 555.51 daltons. BDQ structure has a quinolinic heterocyclic nucleus with alcohol and amine side chains that act as key effectors for the antimycobacterial activity (Fig. 2). It kills both actively replicating and dormant mycobacteria as it target adenosine triphosphate (ATP) synthase inhibiting the mycobacterial cell’s energy production and disrupt their metabolism leaving them in energy starvation condition [11, 18]. BDQ particularly interferes with the proton transfer chain [18]. It was found that mutation in the atpE gene that encodes the c subunit, of the mycobacterial ATP synthase, confers resistant to BDQ, suggesting that it binds crucially to this target (although almost certainly other components of the complex are required for a competent binding site), inhibiting the proton pump of Mtb and therefore interfering with the rotation properties of the transmembrane disk, leading to ATP depletion (Fig. 2) [21]. Study further suggested that human mitochondrial ATP synthase was ~ 20,000 times less sensitive than its mycobacterial counterpart to BDQ [18] and can be used as TB drug. This drug shows good bactericidal activity against both DS and DR Mtb with minimum inhibitory concentrations (MICs) ranging from 0.03 to 0.12 µg/ml and similar effect was against MDR strains with MICs from 0.004 to 0.13 µg/ml [18]. It acts on most of the non-tuberculous mycobacteria as well except some with intrinsic resistance to BDQ [11, 18]. In some, like M. avium, BDQ induced ATP synthase inhibition is not bactericidal but bacteriostatic [18]. The initial activity of BDQ is delayed till ATP concentration is depleted. It works at very low MICs and has been found equally effective for treatment of Mycobacterium avium Complex (MAC) in in vitro, in vivo and susceptibility studies [22]. Thus, like rifamycin derived drugs, BDQ is proving to be the next wonder drug against mycobacterial infections.
a Chemical structure of BDQ, b slanted view of the ion-binding side showing the interaction of BDQ with the c-ring and Two-dimensional (2D) plot of the BDQ/c-ring interactions. (figure courtesy: Laura Preiss et al. Sci Adv 2015;1:e1500106) and c diagrammatic representation and depiction of the drug resistance mutations in mycobacterial ATP synthase (figure courtesy: http://chembl.blogspot.in/2013/01/)
Although BDQ has been approved by the health organizations worldwide for use to cure pulmonary tuberculosis but necessary studies for assessing the after-effects, side-effects and treatment failure with BDQ are still underway. As reported previously BDQ side-effects include nausea, increase in the QTcF interval, drug interactions with cytochrome oxidase (CYP) 3A4 inducers/inhibitors, including rifamycin and some anti-retrovirals, it has long terminal half-life in humans [23] and relapses have been reported with resistant Mtb strains [6].
Drug Interactions
In the murine model, only BDQ for 4 months proved as effective as first line drugs administered for 6 months [24]. But for humans single drug is never recommended for TB treatment and thus BDQ was also tested for its pharmacokinetic interactions with other TB drugs. There were no significant pharmacokinetic interactions with isoniazid, pyrazinamide, ethambutol, kanamycin, ofloxacin or cycloserine [4]. In mice model, BDQ in combination with pyrazinamide, pyrazinamide and isoniazid, pyrazinamide and rifampin or pyrazinamide and moxifloxacin for 2 months resulted in 70–100% culture negative results, whereas same combinations lacking BDQ remained culture positive [24]. BDQ shows synergistic effect with pyrazinamide and clofazimine unless particular mutations for each of them are present (Tables 2 and 3). For example, cross resistance between clofazimine and BDQ is reported through upregulation of MmpL5 efflux system [25]. Rifampin, rifapentine and other CYP3A4 inducers reduced the BDQ effectiveness whereas CYP3A4 inhibitors such as ketoconazole, aid in BDQ function [18]. Lopinavir/ritonavir reduces clearance of BDQ and its metabolites significantly [18]. Efavirenz minimally affected BDQ pharmacokinetics. Some other drugs like meropenem/clavulanate have also been tested for their effectiveness in treatment of MDR- and XDR-TB [26]. They showed promising results but there is no data available for their interactions with BDQ if used for combined TB therapy. The combination of delamanid and BDQ and eventually of other QT interval-prolonging drugs (e.g. fluoroquinolones, clofazimine) is predicted to show adverse effects and potentially harmful QT prolongation based on the data present till 2016 [3]. Thus, it is recommended that only specialized centres undertake the responsibility of managing the patients with delamanid-BDQ combined treatment [3]. It is suggested that good pharmacokinetic profile (drug-like) inclusive of better drug absorption, distribution, metabolism and excretion, is necessary for new anti-TB drug candidates [27] and the studies so far affirms BDQ clearing this criteria.
The Cases of BDQ Failure
There have been two recent reports about a TB patient from Tibet who migrated to Switzerland in December 2010 and treated at a Swiss hospital [6, 28]. The sputum culture showed the prevalence of a M. tuberculosis strain showing mutation in nine genes conferring resistance to seven TB drugs (Table 1). Subsequently in January 2011, a mutation was found in the rpoC gene confirming it to be pre-extensively drug-resistant tuberculosis. The patient treatment began with four antibiotics namely ethambutol, cycloserine, aminosalicylic acid and intravenous capreomycin. BDQ was added to this drug regimen in September 2011. After treatment with these five drugs patient was deemed to be clinically cured of TB in March 2013. Symptoms showed up again with a confirmed relapse of TB in August 2013. The Mtb strain isolated after relapse in August 2013 showed mutation in mmpR gene providing resistance towards BDQ (Table 2) and additional resistance to other drugs (Table 3).
This reports the failure of DR-TB treatment even when individualistic approach was adopted. Such cases indicate towards the risk of drug resistance that can develop due to unobserved discriminate use of newly developed anti-TB drugs as the causative organism mutates rapidly making the drug ineffective [16].
However, the interim results among the patients with MDR-TB in Belarus who started treatment in the year 2012 (BDQ included into their regimen in 2015) showed encouraging results with only two deaths of which only one was related to MDR-TB drug therapy [29].
The Probable Reasons of BDQ Failure
Andries et al. carried out simultaneous studies to infer BDQ mechanism of action and possible resistance towards it [11, 30]. The results showed that BDQ mechanism of action is inhibition of ATP synthase F0 subunit impeding the proton pump. Resistance towards BDQ is of two types, target based and non-target based mutations in the Mtb genomes. Point mutations in the atpE gene resulted into resistance against BDQ by modification of its target, the atpE gene product i.e. membrane spanning domain region of the F0 subunit. The various point mutations identified were Ala63 → Pro (A63P) for M. tuberculosis and Asp32 → Val (D32 V) for M. smegmatis. Both of these mutations are in the membrane spanning domain region of the protein [11]. Later along with the phase IIb trial of the BDQ for treatment of DR-TB patients, the group did the Luria-Delbruck fluctuation assay for generating a larger set of strains with BDQ-resistance and found that target based mutations were prevailing only in 30% mutants [30]. This indicated that there must be other mechanisms that caused resistance to BDQ in non-atpE mutants. The subsequent analysis of these mutants showed that non-target resistance to BDQ and cross-resistance to clofazimine (CFZ) is because of the mutations in Rv0678, a transcriptional negative regulator of genes encoding the MmpS5–MmpL5 efflux pump. As Rv0678 is a repressor of MmpS5 and MmpL5, these mutations lead to the increased expression of the genes that encode the MmpS5–MmpL5 efflux pump proteins. MmpS5 and MmpL5 form the MmpS5–MmpL5 efflux pump complex that belongs to Resistance Nodulation Cell Division (RND) family of transporters of Mycobacterium. Four MmpL genes out of 13 are transcriptionally coupled with MmpS genes and function as a complex for transporting out many different substrates [18]. Unfortunately BDQ and CFZ also became substrates for one of this transporter (MmpS5–MmpL5). This reduces the effectiveness of BDQ as it goes below MIC and chances of development of drug-resistance are increased in this condition.
In another similar study, it was found that the non-target based determinant of the low-level BDQ and clofazimine cross-resistance are due to ‘loss of function’ mutations in a putative Xaa-Pro aminopeptidase, pepQ (Rv2535c) [31]. Although the mechanism is not known for this yet it holds important clinical implications about the reduced drug activity.
The Recent Concerns
Amongst the available TB drugs, BDQ is the only drug that has shown hopeful results for treatment of the DR-TB. Guglielmetti et al. has reported the treatment and follow up of a cohort of MDR-TB patients for a period of 3 years (January 1, 2011–December 31, 2013) using BDQ in combination with various drug regimen designed after carrying out susceptibility tests of the isolates [32]. This group of patients showed successful outcomes in 80% of the MDR-TB patients treated with BDQ containing drug regimens. The most important factors that proved positive were the individualistic approach towards patients and treatment at the specialised centres. To add to the benefits of including BDQ in the anti-TB regimens a study was done using the cohort-based Markov state transition model simulated for a period of 10 years. This assessed the health and economic effects of the background regimen (BR) with or without BDQ and concluded that adding BDQ to BR provides improvements in health outcomes and reductions in healthcare costs in high MDR-TB burden countries [33]. As per CDC guidelines, BDQ can be effectively included into the anti-TB drug regimen to treat MDR infected children when “an effective treatment regimen cannot otherwise be provided” [34]. It is very effective in shortening of the treatment time for DS-TB but as per the recommendations of WHO and other health agencies BDQ must be exclusively used for treating MDR- or XDR-TB for which other treatments are failing. Although BDQ proves to be a potential treatment option for DR-TB but development of the BDQ resistant strains in presumably successfully treated patient [6, 28] again pose the same question. The reason that can be attributed to treatment failure is the exceedingly long half-life of BDQ resulting in slow release that exposed Mtb to below MIC doses after other drugs were stopped leading to development of BDQ resistance [18]. Thus, it comes with a recommendation that BR should continue for at least 5–6 months after withdrawal of BDQ (https://www.sirturo.com/). Every possible measure needs to be taken into consideration while using any of the new drugs for treatment of TB as the causative organism is very good at developing fast and very accurate mutations to escape from their effects. The definition of BDQ resistance is currently not completely established [35].
Conclusions
Even though BDQ has a novel mechanism of action but this also could not escape development of resistance in Mtb. Thus, careful use is recommended and trials with various combinations of drugs can prove helpful in some way. Although, the 2013 WHO recommendations on BDQ use do not address the effectiveness of BDQ companion drugs and safety data is incomplete but the regimens should be planned cautiously. It is suggested that BDQ should be always associated with at least one drug with both bactericidal and sterilising activity for the full treatment duration in order to avoid selection of BDQ resistance. Fluoroquinolones, LNZ, DLM and possibly ETH may be considered in this category. Controlled and planned regimens of BDQ along with other drugs will result in better outcomes.
References
WHO-Global Tuberculosis Report (2016) http://www.who.int/tb/publications/global_report/en/
Tiberi S, Scardigli A, Centis R, D’Ambrosio L, Munoz-Torrico M, Salazar-Lezama MA et al (2017) Classifying new anti-tuberculosis drugs: rationale and future perspectives. Int J Infect Dis 56:181–184. doi:10.1016/j.ijid.2016.10.026
Migliori GB, Pontali E, Sotgiu G, Centis R, D’Ambrosio L, Tiberi S et al (2017) Combined use of Delamanid and Bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 18:E341. doi:10.3390/ijms18020341
Centres for Disease Control and Prevention (2016) http://www.cdc.gov/tb/topic/drtb/default.htm/. Accessed 23rd Nov 2016
Saxena A, Mukherjee U, Kumari R, Singh P, Lal R (2014) Synthetic biology in action: developing a drug against MDR-TB. Indian J Microbiol 54:369–375. doi:10.1007/s12088-014-0498-0
Bloemberg GV, Keller PM, Stucki D, Trauner A, Borrell S, Tsogyal L et al (2015) Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. N Engl J Med 373:1986–1988. doi:10.1056/NEJMx150041
Kalia VC (2014) Microbes, antimicrobials and resistance: the battle goes on. Indian J Microbiol 54:1–2. doi:10.1007/s12088-013-0443-7
Purohit HJ, Cheema S, Lal S, Raut CP, Kalia VC (2007) In search of drug targets for Mycobacterium tuberculosis. Infect Disord Drug Targets 7:245–250. doi:10.2174/187152607782110068
Sajid A, Arora G, Singhal A, Kalia VC, Singh Y (2015) Protein phosphatases of pathogenic bacteria: role in physiology and virulence. Ann Rev Microbiol 69:527–547. doi:10.1146/annurev-micro-020415-111342
Sharma AK, Dhasmana N, Dubey N, Kumar N, Gangwal A, Gupta M, Singh Y (2017) Bacterial virulence factors: secreted for survival. Indian J Microbiol 57:1–10. doi:10.1007/s12088-016-0625-1
Andries K, Verhasselt P, Guillemont J, Gohlmann H, Neefs J, Winkler H et al (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi:10.1126/science.1106753
Lal R, Khanna R, Dhingra N, Khanna M, Lal S (1998) Development of an improved cloning vector and transformation system in Amycolatopsis mediterranei (Nocardia mediterranei). J Antibiot B 51:161–169. doi:10.7164/antibiotics.51.161
Khanna M, Dua M, Lal R (1998) Selection of suitable marker genes for the development of cloning vectors and electroporation in different strains of Amycolatopsis mediterranei. Microbiol Res 153:205–211. doi:10.1016/S0944-5013(98)80002-5
Nigam A, Almabruk KH, Saxena A, Jongtae Y, Mukherjee U, Kaur H et al (2014) Modification of rifamycin polyketide backbone leads to improved drug activity against rifampicin resistant Mycobacterium tuberculosis. J Biol Chem 289:21142–21152. doi:10.1074/jbc.M114.572636
da Silva PB, Campos DL, Ribeiro CM, da Silva IC, Pavan FR (2017) New antimycobacterial agents in the pre-clinical phase or beyond: recent advances in patent literature (2001–2016). Expert Opin Ther Pat 27:269–282. doi:10.1080/13543776.2017.125368115
Nguyen TV, Cao TB, Akkerman OW, Tiberi S, Vu DH, Alffenaar JW (2016) Bedaquiline as part of combination therapy in adults with pulmonary multi-drug resistant tuberculosis. Expert Rev Clin Pharmacol 9:1025–1037. doi:10.1080/17512433.2016.1200462
United States Food and Drug Administration (2016) http://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm336115.htm/2012. Accessed on 23rd Nov 2016
Field SK (2015) Bedaquiline for the treatment of multidrug-resistant tuberculosis: great promise or disappointment? Ther Adv Chronic Dis 6:170–184. doi:10.1177/2040622315582325
Xavier AS, Lakshmanan M (2014) Delamanid: a new armor in combating drug-resistant tuberculosis. J Pharmacol Pharmacother 5:222–224. doi:10.4103/0976-500X.136121
Sotgiu G, Pontali E, Centis R, D’Ambrosio L, Migliori GB (2016) New anti-tuberculosis drugs for special populations: a difficult-to-address issue. Eur Respir J 48:957–958. doi:10.1183/13993003.01289-2016
The ChEMBL-og The organization of the drug discovery data (2017) http://chembl.blogspot.in/2013/01/. Accessed on 5th July 2017
Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ (2017) In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 61:e01798. doi:10.1128/AAC.01798-16
McLeay S, Vis P, van Heeswijk R, van Heeswijk R, Green B (2014) Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob Agents Chemother 58:5315–5324. doi:10.1128/AAC.01418-13
Ibrahim M, Andries K, Lounis N, Chaffour A, Truffot-Pernot C, Jarlier V et al (2007) Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother 51:1011–1015. doi:10.1128/AAC.00898-06
Nguyen L (2016) Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch Toxicol 90:1585–1604. doi:10.1007/s00204-016-1727-627
Tiberi S, Payen MC, Sotgiu G, D’Ambrosio L, Guizado VA, Alffenaar JW et al (2016) Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of MDR-and XDR-TB. Eur Respir J 47:1235–1243. doi:10.1183/13993003.02146-2015
Santos P, López-Vallejo F, Soto CY (2017) In silico approaches and chemical space of anti-P-type ATPase compounds for discovering new antituberculous drugs. Chem Biol Drug Des 90:175–187. doi:10.1111/cbdd.12950
Hoffmann H, Kohl TA, Hofmann-Thiel S, Merker M, Beckert P, Jaton K et al (2016) Delamanid and bedaquiline resistance in Mycobacterium tuberculosis ancestral Beijing genotype causing extensively drug-resistant tuberculosis in a tibetan refugee. Am J Respir Crit Care Med 193:337–340. doi:10.1164/rccm.201502-0372LE
Skrahina A, Hurevich H, Falzon D, Zhilevich L, Rusovich V, Dara M, Setkina S (2016) Bedaquiline in the multidrug-resistant tuberculosis treatment: belarus experience. Int J Mycobacteriol 5:S62–S63. doi:10.1016/j.ijmyco.2016.11.014
Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L et al (2014) Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS ONE 9:e102135. doi:10.1371/journal.pone.0102135
Almeida D, Ioerger T, Tyagi S, Li SY, Mdluli K, Andries K et al (2016) Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:4590–4599. doi:10.1128/AAC.00753-16
Guglielmetti L, Le Dû D, Veziris N, Caumes E, Marigot-Outtandy D, Yazdanpanah Y, Robert J, Fréchet-Jachym M (2016) Is bedaquiline as effective as fluoroquinolones in the treatment of multidrug-resistant tuberculosis? Eur Respir J 48:582–585. doi:10.1183/13993003.00411-2016
Lu X, Smare C, Kambili C, El Khoury AC, Wolfson LJ (2017) Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Serv Res 17:87. doi:10.1186/s12913-016-1931-3
Seddon JA, Schaaf HS (2016) Drug-resistant tuberculosis and advances in the treatment of childhood tuberculosis. Pneumonia 8:20. doi:10.1186/s41479-016-0019-5
Veziris N, Bernard C, Guglielmetti L, Le Du D, Marigot-Outtandy D, Jaspard M et al (2017) Rapid emergence of Mycobacterium tuberculosis bedaquiline resistance: lessons to avoid repeating past errors. Eur Respir J 49:1601719. doi:10.1183/13993003.01719-2016
Acknowledgements
The work at author’s laboratory is supported by funding from the Department of Biotechnology (DBT), Government of India, and University of Delhi/Department of Science and Technology Promotion of University Research and Scientific Excellence (DU-DST-PURSE). P.S. and R.K. gratefully acknowledge University Grants Commission (UGC) for research fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, P., Kumari, R. & Lal, R. Bedaquiline: Fallible Hope Against Drug Resistant Tuberculosis. Indian J Microbiol 57, 371–377 (2017). https://doi.org/10.1007/s12088-017-0674-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-017-0674-0