Abstract
In this work, acetic acid was found as one promising substrate to improve xylose utilization by Gluconacetobacter xylinus CH001. Also, with the help of adding acetic acid into medium, the bacterial cellulose (BC) production by G. xylinus was increased significantly. In the medium containing 3 g l−1 acetic acid, the optimal xylose concentration for BC production was 20 g l−1. In the medium containing 20 g l−1 xylose, the xylose utilization and BC production by G. xylinus were stimulated by acetic acid within certain concentration. The highest BC yield (1.35 ± 0.06 g l−1) was obtained in the medium containing 20 g l−1 xylose and 3 g l−1 acetic acid after 14 days. This value was 6.17-fold higher than the yield (0.21 ± 0.01 g l−1) in the medium only containing 20 g l−1 xylose. The results analyzed by FE-SEM, FTIR, and XRD showed that acetic acid affected little on the microscopic morphology and physicochemical characteristics of BC. Base on the phenomenon observed, lignocellulosic acid hydrolysates (xylose and acetic acid are main carbon sources present in it) could be considered as one potential substrate for BC production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC), synthesized by many bacteria, has great potential in the application on many fields such as materials, food, medicine, and etc. [1]. Among various bacteria, Gluconacetobacter xylinus is the most common one used for BC production [1]. Many factors would influence the BC productivity and fermentation substrate cost is a significant one. In order to reduce its production cost, different substrates such as molasses [2], konjac powder hydrolysate [3], various fruit juices [4] and etc. have been utilized for BC production already.
To further reduce the cost of BC production, lignocellulosic biomass seems to be a promising raw material because of its highest availability and renewable characteristics [5]. Simply hydrolyzed by diluted acid, lignocellulosic biomass could be turned into the hydrolysate with xylose as the dominant sugar present in it. Thus, the capability of using xylose as carbon source for BC production decides the possibility of using lignocellulosic acid hydrolysates for BC production.
To evaluate the possibility of using lignocellulosic acid hydrolysates as substrate for BC production, xylose had been used as carbon source for BC production already [6]. However, it was shown that xylose might not be an optimal carbon source for BC production when compared with glucose in this work. Although adding d-xylulose into medium could stimulate the xylose utilization, but this effect was not obvious [6]. Base on this, it is critical to find some simple ways to improve the capability of using xylose in order to make the bioconversion of lignocellulosic acid hydrolysates to BC possible.
Besides sugars, acetic acid is one common by-product generated during the hydrolysis process [5]. Interestingly, acetic acid was shown to be beneficial for BC production within certain concentration [7]. Thus, if acetic acid could stimulate the xylose utilization, it is possible that lignocellulosic acid hydrolysates could be used as one promising substrate for BC production. However, to our knowledge, no work has studied the effect of acetic acid on the xylose utilization and BC production by G. xylinus. In this work, for the first time, the effect of acetic acid on the xylose utilization and BC production by G. xylinus was evaluated.
Materials and Methods
Microorganism for BC Production
Gluconacetobacter xylinus CH001 (Laboratory of Energy and Biochemical Engineering, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences) was used as the microorganism for BC production in this work.
Effect of Xylose Concentration on BC Production by G. xylinus in the Medium with Acetic Acid
In the fermentation medium (g l−1, peptone 5, yeast extract 5, Na2HPO4 2.7) with natural pH (7.30), 3 g l−1 acetic acid and different concentration of xylose (g l−1, 10, 15, 20, 25, 30) was added into, and then the pH was adjusted to 7.30 ± 0.04 by NaOH. Then, the medium was sterilized at 121 °C for 20 min.
The pre-culture was performed on the pre-cultivation medium (g l−1, mannitol 25, peptone 5, yeast extract 3, initial pH 6.60) at 28 °C and 150 rpm for 48 h. Then, 8 % (v/v) seed culture was translated into the fermentation medium. After that, the static fermentation was carried out at 28 °C for 14 days.
Effect of Acetic Acid Concentration on Xylose Utilization and BC Production by G. xylinus
In the fermentation medium (g l−1, xylose, 20, peptone 5, yeast extract 5, Na2HPO4 2.7) with natural pH (7.47), different concentration (g l−1, 1, 3, 5, 7, 9, 11) of acetic acid was added into, and then the pH was adjusted to 7.47 ± 0.04 by NaOH. Then, the medium was sterilized at 121 °C for 20 min. To test the effect of acetic acid, an experiment on the control medium without adding acetic acid (pH 7.47) was also carried out.
The pre-culture was performed on the pre-cultivation medium (g l−1, mannitol 25, peptone 5, yeast extract 3, initial pH 6.60) at 28 °C and 150 rpm for 48 h. Then, 8 % (v/v) seed culture was translated into the fermentation medium with and without acetic acid. After that, the static fermentation was carried out at 28 °C for 14 days.
Measurement of BC dry Weight, Sugar and Acetic Acid Concentration
After fermentation, the fermentation broth and BC was separated by vacuum filtration. Then, the BC was treated with 1.5 % (w/v) NaOH at 80 °C for 2 h. After that, the BC was repeatedly washed by distilled water. Finally, the BC was dried to constant weight at 105 °C and weighed.
Sugar and acetic acid concentrations (d-glucose, d-xylose, and l-arabinose) in the medium were analyzed by HPLC (Waters 2685 systems, Waters Corp., USA), with a RI detector (Waters 2414), and on Aminex HPX-87H column (300 × 7.8 mm, Bio Rad Corp., USA) using 5 mM H2SO4 solution at a flow rate of 0.55 ml min−1 at 50 °C.
The xylose and acetic acid utilization (% w/w) was defined as the ratio of xylose or acetic acid utilized (g l−1) to the initial concentration of xylose or acetic acid (g l−1).
Analysis of the Physical and Chemical Structure of BC
Field Emission Scanning Electron Microscopy (FE-SEM)
The morphologies of oven-dried BC samples were observed by a Hitachi S-4800 high resolution Field Emission Scanning Electron Microscope (FE-SEM, Hitachi, Japan) operated at 2.0 kV and 10 μA.
Fourier Transform Infrared Spectroscopy (FTIR)
BC samples were mixed with spectroscopic-grade potassium bromide powder (1 % w/w) and Fourier Transform Infrared Spectroscopy (FTIR) spectroscopy was carried out in the range of 400–4,000 cm−1 wavelength with a Perkin-Elmer Spectrum one FTIR Spectrometer (US). The kind of crystallite allomorph was determined with shifting FTIR bands related to specified groups and bonds [8].
X-ray Diffraction (XRD)
Structure, size, and percentage of crystals of BC samples were analyzed by D/max-RA X-ray diffractometer (Rigaku, Japan) with Cu Kα radiation (λ = 0.154 nm) operated at 40 kV and 100 mA. Samples were scanned from 10° to 40° (2θ range) at a scan speed of 0.008°/step. To determine the sample crystallinity, profile analysis was carried out with a peak fitting program using Gaussian line shapes. The crystal size of cellulose was calculated using Debye–Scherrer’s equation [9]. Crystallinity index (CI) was calculated from the related intensity data by the Segal method (Eq. 1) [10].
where I200 is the maximum intensity of crystalline region at 2θ (about 22.8°), Iam is the intensity of the amorphous region of the wide-angle X-ray diffraction curves at 2θ (about 18°).
Results and Discussion
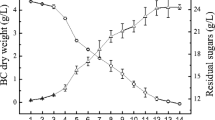
Different from other fermentation, BC production is usually a bioconversion process could not tolerate high sugar concentration. Namely, substrate inhibition is easy to happen during BC fermentation. Thus, we initially measured the effect of different xylose concentration on BC production in the medium containing 3 g l−1 acetic acid. As shown in Fig. 1A, the highest BC yield was obtained on the medium with initial xylose concentration of 20 g l−1. Also, the xylose utilization (41.6 %) was highest in the medium containing 20 g l−1 xylose. Base on this, 20 g l−1 concentration was chosen for further study in this work.
Then, the effect of acetic acid on BC production was systematically carried out on the medium containing 20 g l−1 xylose. As shown in Fig. 1B, without adding acetic acid, it is difficult for G. xylinus to utilize xylose for BC production. In fact, in the medium without acetic acid, no obvious BC pellicle could be observed in the flask. Also, the BC yield on the medium without acetic acid was merely about 0.2 g l−1, which was much lower than that in the medium containing acetic acid. This again showed that xylose might not be a suitable carbon source for BC production by G. xylinus in medium without adding acetic acid [6]. Surprisingly, when adding acetic acid into the xylose-containing medium, the situation was completely changed. Even small concentration of acetic acid (1 g l−1) could stimulate the xylose utilization and BC production. Compared with the control medium (without adding acetic acid), in the medium containing 1 g l−1 acetic acid, the BC yield increased from 0.20 g l−1 to about 0.66 g l−1, this value continued to increase with higher acetic acid concentration. The BC yield maintained at about 1.1–1.2 g l−1 when the acetic acid concentration ranged from 5 to 11 g l−1.
As shown in Fig. 1B, in the medium without acetic acid, the xylose utilization ratio was merely about 21.8 % (the xylose utilization was 4.4 g l−1), indicating that xylose might not be a suitable carbon source for the metabolism of G. xylinus. However, when certain concentrations of acetic acid were added into the medium, the xylose utilization became much better. When the acetic acid was 1 g l−1, the xylose utilization ratio increased to about 30 % (the xylose utilization was about 6.0 g l−1). This value continued to increase to about 40 % when 3–5 g l−1 acetic acid was added. However, with more than 7 g l−1 acetic acid, this value decreased. This indicates that the optimal acetic acid concentration for the xylose utilization of G. xylinus was about 3–5 g l−1.
Besides xylose utilization, the situation of acetic acid utilization by G. xylinus in the medium containing different concentration of acetic acid was further measured. When the acetic acid concentration was 1–3 g l−1, all the acetic acid was exhausted by G. xylinus, namely, the acetic acid utilization could reach 100 % in these mediums. However, when the acetic acid concentration increased to 5 g l−1, it could not be completely utilized by G. xylinus and its utilization ratio became lower as the acetic acid concentration to be higher. When the acetic acid concentration is 11 g l−1, there was still about 9 g l−1 acetic acid presented in the medium after fermentation by G. xylinus that the acetic acid utilization was merely about 15 % (Fig. 1B). As a result of this study, significant improvements in xylose utilization and BC dry weight were developed. In the medium containing 20 g l−1 xylose and 3 g l−1 acetic acid, the BC dry weight reached its highest point (1.35 ± 0.06 g l−1) after 14 days’ fermentation, which was apparently higher than that (about 0.1 g l−1) on the medium containing d-xylose and without acetic acid in the literature [6]. Also, this value was 6.17-fold higher than the BC yield in the medium containing only 20 g l−1 xylose and without acetic acid (control medium). Overall, in this medium, the amount of xylose and acetic acid utilization were 8.19 and 3.00 g l−1, respectively, namely the utilization ratio of xylose and acetic acid were 40.95 and 100.00 %, respectively.
It is worth noting that the pH value after fermentation by G. xylinus would increase on the medium containing different concentration of acetic acid. In the control medium without acetic acid, the pH value after fermentation was about 4.2. However, this value would increase to 5.8 when the initial acetic acid concentration was 1 g l−1. And this value increased when initial acetic acid concentration increased. This phenomenon was also observed in our previous work with other fermentation in lignocellulosic acid hydrolysates (acetic acid was present in it) [11, 12]. The initial pH value of the medium containing acetic acid was adjusted to around 7.5 by NaOH before fermentation. Thus, the utilization of acetic acid during fermentation by G. xylinus might lead to increasing pH value due to the existence of NaOH in the medium. Based on the above discussion, it could be concluded that acetic acid was not only a buffer component, but also worked as a carbon source.
Finally, the morphology and structure of BC samples produced using individual xylose, xylose mixed with 3 and 5 g l−1 of acetic acid and individual acetic acid as carbon source were characterized by FE-SEM, FTIR and XRD (Figs. 2, 3). It could be seen in Fig. 2 that the BC produced from xylose showed a porous network structure constructed by a large number of microfibril ribbons, macro-characteristically exhibiting a layer of transparent gel film. When xylose mixing with 3 g l−1 of acetic acid was used as carbon resources, the morphology was not influenced. With the addition of acetic acid reached up to 5 g l−1 and even individual acetic acid as carbon source, some rice-like particles deposited on the surface of microfibril ribbons that might be the alkali not completely washed off from the BC membrane, but the BC still kept a porous network structure, indicating that acetic acid did not affect the main morphology of BC from xylose.
According to the FTIR results in Fig. 3A, a weak and broad band centered at 898.54 cm−1 and a strong band centered at 1,428.55 cm−1 (CH2 scissoring) were present in the spectra of all the BC samples, defining them as cellulose I [13]. XRD analysis could be used for qualitative determination of the crystal structure and quantitative determination of the crystalline contents in composite samples. From Fig. 3B, it could be seen that these BC samples produced from different carbon source showed similar characteristic diffraction profiles of cellulose I, with peaks at 2θ angles of 14.5°, 16.7° and 22.6° corresponding to the (1Ī0), (110) and (200) crystallographic planes, respectively. Crystallinity, CI, and size of crystals of BC samples were calculated by the characteristic peaks intensities, and showed in Table 1. The BC sample using xylose mixed with 5 g l−1 of acetic acid as carbon source showed the highest crystallinity and CI, and the lowest crystallite size of crystal planes. Previous research [14] have shown that the peak corresponding to the (200) lattice plane showed the highest intensity in the diffraction patterns of native BC. The phenomenon is similar to our produced BC, indicating that the hydrogen band role during crystallization promoted the preferred orientation growth to (200) crystal plane and resulted in higher crystallinity and smaller crystallite size. Therefore, xylose mixed with 5 g l−1 of acetic acid could be used as an ideal carbon source for BC production by G. xylinus.
In spite that xylose might not be a suitable carbon source for BC production by G. xylinus, acetic acid was found that could significantly improve xylose utilization and BC production by G. xylinus. This undoubtedly offers the possibility for using lignocellulosic acid hydrolysate for BC production since xylose and acetic acid are two main carbon sources present in it.
References
Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47:107–124
Bae S, Shoda M (2004) Bacterial cellulose production by fed-batch fermentation in molasses medium. Biotechnol Prog 20:1366–1371
Hong F, Qiu K (2008) An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr Polym 72:545–549
Kurosumi A, Sasaki C, Yamashita Y, Nakamura Y (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr Polym 76:333–335
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Ishihara M, Matsunaga M, Hayashi N, Tisler V (2002) Utilization of d-xylose as carbon source for production of bacterial cellulose. Enzym Microb Technol 31:986–991
Toda K, Asakura T, Fukaya M, Entani E, Kawamura Y (1997) Cellulose production by acetic acid-resistant Acetobacter xylinum. J Ferment Bioeng 84:228–231
Shen W, Chen S, Shi S, Li X, Zhang X, Hu W, Wang H (2009) Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr Polym 75:110–114
Shezad O, Khan S, Khan T, Park JK (2010) Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym 82:173–180
Cheng KC, Catchmark JM, Demirci A (2009) Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 16:1033–1045
Chen XF, Huang C, Xiong L, Chen XD, Ma LL (2012) Microbial oil production from corncob acid hydrolysate by Trichosporon cutaneum. Biotechnol Lett 34:1025–1028
Huang C, Chen XF, Xiong L, Chen XD, Ma LL (2012) Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour Technol 110:711–714
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J Appl Polym Sci 8:1311–1324
Yudianti R, Indrarti L, Azuma J (2007) Structure and physical properties of natural gellous materials. J Appl Sci 7:580–584
Acknowledgments
The authors acknowledge the financial support of Project of National Natural Science Foundation of China (51303181, 51378486, U1261116), Project of Jiangsu Province Science and Technology (BE2013083), Foundation of Director of Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (y407pb1001, y107rf1001), Guangzhou Science and Technology (2013J4300031), the Support Plan Project of National Science and Technology (2012BAD32B07) and Natural Science Foundation of Guangdong Province (S2012040007546).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, XY., Huang, C., Guo, HJ. et al. Beneficial Effect of Acetic Acid on the Xylose Utilization and Bacterial Cellulose Production by Gluconacetobacter xylinus . Indian J Microbiol 54, 268–273 (2014). https://doi.org/10.1007/s12088-014-0450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-014-0450-3