Abstract
Hexavalent chromium reduction and accumulation by Acinetobacter AB1 isolated from Fez tanneries effluents were tested. The effects of some environmental factors such as pH, temperature, and exposure time on Cr(VI) reduction and resistance were investigated. We found that this strain was able to resist to concentrations as high as 400 mg/l of Cr(VI). Moreover, pH 10 and the temperature 30°C constitute favourable conditions to the growth and reduction of Acinetobacter AB1. Complete reduction of Cr(VI) was observed at low initial Cr(VI) concentrations of 50 mg/l after 72 h of incubation. Furthermore, Transmission electron microscope (TEM) analysis showed morphological changes in AB1 strain due 48H exposure to 100 mg/l chromate concentration and revealed circular electron dense (dark black point) inclusion within the cell cytoplasm suggesting chromium deposition within the cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contributing up to 53% to employment, the leather tanning industry is considered as a major socio-economic sector in Fez City (Morocco). Unfortunately, it generates a great deal of toxic products like chromium [1].
As a transition metal, chromium exists in a wide range of oxidation states from −2 to +6 while the dominant species in nature are hexavalent chromium Cr(VI) and trivalent chromium Cr(III). Cr(VI) is more toxic and soluble than Cr(III) [2]. Reduction of Cr(VI) to Cr(III) is one of the most useful methods to limit chromium toxicity [3].
In the carcinogenic behavior of chromium, chromate (CrO 2-4 ) (a strong oxidizing agent) is reduced intracellularly to Cr5+ which in turn reacts with nucleic acids and other cell components triggering mutagenic and carcinogenic effects in biological systems [4].
Although, there are several conventional methods for removing metals from wastewater, these approaches prove to be less cost effective, inconvenient for practical use and present many limitations. Alternative methods of metal removal and recovery based on biological materials have been considered [5–9].
In this study we have studied the Cr(VI) reduction and bio-accumulation of Acinetobacter sp. Strain AB1, an environmental isolate from Fez tanneries. Many strains belonging to Acinetobacter genus were known by their chromium bio-accumulation and biosorption activities [8, 10, 11].
Materials and Methods
Screening of Bacteria Resistant to Chromate
Leather treated with chromium and wastewater of tanneries effluent was collected from Fez tanneries. These leather and tanneries effluents (containing chromium) were added at different plates of BN contained: bacteriotriptone (10.0), sodium chloride (5.0), yeast extract (5.0) supplemented with Cr(VI) at final concentration of 10 mg/l and incubated at 30°C for 7 days. The bacterial colonies which appeared on the plates were isolated, purified and characterized. Among the 25 bacterial strains isolated from different tanning effluents in Fez city, AB1 strain was selected for further studies.
Strain Identification
The taxonomic identity of the strain AB1 was confirmed by 16S rRNA gene sequencing. DNA was extracted using Power Soil DNA Isolation Kit (Mo Bio, Lab. Inc., CA, USA) according to the manufacturer instructions.
PCR amplification of the 16S rDNA was performed at 100 μl reaction mixture containing 3 μl of template DNA, 1× PCR Taq polymerase buffer (Promega), 1.5 mM MgCl2, 0.1 mM of each dNTPs (Amersham Biosciences), 2.5 U of Taq DNA polymerase (Promega) and 0.5 μM of each primer (Isogen) specific for the bacteria domain: 27f (AGAGTTTGATCMTGGCTCAG) and 1492r (TACGGYTACCTTGTTACGACTT) [12]. PCR was carried out by Thermal Cycler AB 2720 (Applied Biosystems) at the following conditions: initial denaturation one cycle at 94°C for 10 min; 30 cycles of 94°C, 1 min; 52°C, 1 min; 72°C, 3 min and one final cycle of 72°C, 10 min and maintaining the temperature of 4°C following the final cycle.
Phylogenetic Analysis
Nucleotide sequences were compared to sequences in the National Center for Biotechnology Information GenBank database using the BLASTn program (http://www.ncbi.nlm.nih.gov/BLAST). Moreover, databases and matrices of evolutionary distance were constructed using Clustal X [13], while the topology distance and probability of phylogenetic tree were determined with MrBayes program [14]. The phylogenetic trees were constructed from the evolutionary distances by TreeView software [15]. Nucleotide sequence accession numbers: GenBank and EMBL accession number for reference 16S rDNA sequence used in this analysis are GU124474.
Effect of pH and Temperature
The influence of pH and temperature on bacterial growth and chromate reduction were assessed with the NB medium and culture conditions described for Cr(VI) reduction by the isolates. For the effect of pH, autoclaved culture medium was adjusted to pH 4.0, 6.0, 8.0, and 10.0 with predetermined amounts of filter-sterilized (0.22 m) 1 M HCl or 1 M NaOH and incubated at 30°C. Effect of temperature was studied by measuring absorbance and Cr(VI) reduction at various incubation temperatures (20, 25, 30, 37, and 40°C). The inoculums used was 5% of the total volume and was the logarithmic-phase the bacterial cultures were prepared in NB broth plus 25 mg/l of Cr(VI). For cell density determination, the absorbance of adequate volumes diluted with water was measured.
Effect of Chromate Concentration
The effect of varying concentrations of Cr(VI) on bacteria tolerance was examined for 72 h at 30°C. Stock Cr(VI) solution was prepared by dissolving 2.829 g K2Cr2O7 (294.18/gmol) in 1 l of deionised water. The initial pH was adjusted to 10 by using 0.1 M NaOH or 0.1 M HCL before filter-sterilized using a 0.45 μm Whatman filter paper. The Cr(VI) reduction and growth of the isolate were evaluated after 24, 48, 72, and 96 h incubation.
Control sets consisted of bacterial cells in NB medium only and Cr(VI) in NB medium. Resistant level of bacteria to Cr(VI) was evaluated by measuring percentages of cell survival at OD600 (IC 6400 spectrophotomètre, InforLab chimie, France) after a 72 h of incubation period. Any possible chemical reduction due to components of the medium was checked by performing the experiment in the absence of bacteria (abiotic control).
Estimation of Chromium
Cr(VI) in aqueous solution was estimated by a colorimetric method using 1,5-diphenyl carbazide (DPC) reagent. Solution containing 4 ml sulfuric acid (2 N), 0.2 ml diphenyl carbazide reagent and 0.8 ml of deionised water, was added to 5 ml of appropriately diluted sample. DPC reacts with chromate forming a purple complex with a maximum absorbance at 540 nm. Samples were periodically collected under sterile conditions and, when necessary, centrifuged before dilution to remove suspended cells and avoid turbidity. Absorbance was performed with (IC 6400 spectrophotomètre, InforLab chimie, France) [16, 17].
Transmission Electron Microscope (TEM) Analysis
Transmission electron microscopy was performed in Acinetobacter AB1 cells grown in 100 mg/l of Cr(VI) for 48 h and fixed in glutaraldehyde (1% solution) and paraformaldehyde (2%) buffered with sodium phosphate buffer saline (0.1 M, pH 6.8). Fixation lasts for 12–18 h at 4°C temperature, after which the cells were washed in fresh buffer, and post fixed for 2 h in osmium tetraoxide (1%) in the same buffer at 4°C. After several washes in buffer, the specimens were dehydrated in graded acetone solutions. The ultrathin section were cut using an ultracute, ultramicrotome and the sections were stained in alcoholic uranyl acetate (10 min) and lead citrate (10 min) before examining the grids in a transmission electron microscope.
Results and Discussion
Identification of Cr(VI) Resistant Strains
AB1 was previously isolated from several tanneries effluents in Fez, Morocco. BLASTN analysis of 16S rRNA gene of strain AB1 compared to known sequences in NCBI showed close relation to Acinetobacter (Fig. 1). In fact phylogenetic tree revels that, this bacteria clustered with Acinetobacter haemolyticus AY586400 (99% of similitude) (Fig. 1), which is a alkane-degrading bacteria [18]. Furthermore AB1 exhibited 99% of similitude to Acinetobacter calcoaceticus (FJ459985) which has a bioremediation activity by its ability to degrade TPH.
Phylogenetic tree resulting from comparison of 16S rRNA gene sequences of selected isolates with reference sequences extracted from the GenBank Database, using MrBayes program [14].The numbers at the nodes represent Bayesian posterior probability, the GeneBank accession numbers of the sequences are given next to the species name
Factors Affecting Hexavalent Chromium Reduction and Bacterial Growth
Effect of Initial Concentration Cr(VI)
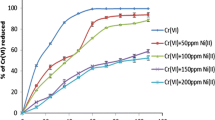
The effect of initial Cr(VI) concentration on Cr(VI) reduction and bacterial growth was investigated at different initial chromium concentrations after 3 days of incubation. The time course of Cr(VI) reduction by the AB1 isolate at different Cr(VI) concentrations is shown in Fig. 2. In our study, isolate AB1 rapidly reduced 25 mg/l of Cr(VI) to a non-detectable level within 24 h. Acinetobacter AB1 was able to completely reduce Cr(VI) at a concentration of 50 mg/l (Fig. 2). The rate of Cr(VI) reduction decreased with time, and between 50 and 200 mg/l. Significant reduction rate was observed after 24 h incubation but it stabilized without reaching complete Cr(VI) reduction. Increasing concentrations beyond 200 mg/l of Cr(VI) showed weak reduction rates (Fig. 2).
Furthermore, the rate of chromate was determined by estimating the reduction percentage of Cr(VI). Influence of initial Cr(VI) concentration was observed Fig. 3, At initial Cr(VI) concentration of 50 mg/l, 93.049% reduction of Cr(VI) was observed. However, this value decreased to 56.875% at 200 mg/l Cr(VI) after a 72 h reaction time (Fig. 3). The reduction rate decreased gradually beyond 200 mg/l concentration. Above 400 mg/l, Cr(VI) reduction percentage was as low as 10% (Fig. 3). On another hand regular chromium concentration increase had a negative effect on bacterial growth. Absorbance reached a very high level of 0.49 at 25 mg/l concentration but decreased continuously with increasing chromium concentrations. Bacterial growth was totally inhibited by Cr(VI) concentrations over 400 mg/l (Fig. 4). These data show that in contrast with other reported strains, Cr(VI) resistance of Acinetobacter AB1 is observed [8; 11].
Temperature
Temperature is also an important factor for bacterial growth and microbial Cr(VI) reduction. The optimization of this parameter was studied in NB culture 20–40 range. The optimum of the tested temperatures was 30–37°C for Cr(VI) reduction. Maximum chromium reduction was observed after 24 h incubation at 30 and 37°C temperatures but decreased to contrasting a very weak or absence chromium reduction levels at 25 and 20°C temperatures. In addition, bacterial growth was very high at 30°C temperature after 3 days of incubation (Fig. 5). Incubation at 40°C severely decreased bacterial growth and chromate reduction (Fig. 5). Our results are in agreement with previously published data. Indeed, Zakaria et al. [11] have already reported an optimal growth of Acinetobacter haemolyticus at temperature of 30°C. Sarangi and Krishnan [19] also found that Bacillus KCH3 growth was stimulated at temperature of 30°C and inhibited at temperature below 15°C and over 40°C. Moreover, Wang et al. [20] reported that no chromate reduction was observed at 4 and 60°C by Enterobacter cloacae.
pH
The effect of pH on Cr(VI) reduction capacity in the NB medium is presented in Fig. 6. Reduction rate is dependent on pH value, being maximal at pH 10 (Fig. 6). After 24 h incubation at this pH value, bacterial growth showed a larger rate (absorbance of 0.654) than in all other tested pH values. This increase was threefold than in pH six and four. While very low bacterial growth was observed at pH 4, but the considerable reduction rate was obtained. This could be explained by a stabilization of Cr III by the acid medium favoring Cr(VI) reduction due to the positive value of the reduction potential standard of the Cr(VI)/Cr(III) Redox couple [21].
The same trend has been described by several other authors such as He et al. [22] which showed optimal chromium reduction and Ochrobactrum sp. (strain CSCr-3) growth in an initial pH of 10. Furthermore, Wani et al. [23] reported that Burkholderia cepacia MCMB-821 present an optimum reduction at pH between 9 and 10. Srivastava and Thakur have found higher chromium removal at pH 7 by Acinetobacter sp. PCP2 [8].
Decreased bioreduction at low pH (acid) levels could be due, principally, to the relation of bio-accumulation with the number of surface negative charges resulting from dissociation of functional groups [24]. Because pH changes affect enzyme ionization rate, change protein’s conformation and consequently affects the enzyme activity, it has been concluded that Cr(VI) reduction is enzyme-mediated [25].
Transmission Electron Microscope (TEM) Analysis
Chromium accumulation in bacterial cells was observed by TEM in initial hexavalent chromium concentration of 100 mg/l after 48 h incubation as suggested by the identification of chromate accumulation within incubated cells.
TEM of Acinetobacter sp. AB1 was also performed in control (Fig. 7a) and exposed cells (Fig. 7b). TEM photograph of morphological changes in AB1 strain due to the exposure of chromate revealed circular electron dense (dark black point) inclusions within the cell cytoplasm indicating that Cr(III) was adsorbed on microbial surface cells, the presence of electron dense particles in the cytoplasmic region of the bacteria suggested deposition of chromium in these cells. These intracellular and extracellular electron-dense precipitates were confirmed by Middleton et al. as chromium through examination using electron energy loss spectroscopy (EELS) where Cr precipitated as Cr(III) and not Cr(VI) [26].
TEM analysis confirmed chromate accumulation within bacterial cells. This result is in agreement with similar findings reported by Srivastava and Thakur [8] in Acinetobacter sp. PCP3 strain. Furthermore, Zakaria et al. [11] indicated that when Acinetobacter haemolyticus was exposed to 100 mg Cr(VI)/l, the cells lost its shape and increased in size. At this concentration, cells appeared thicker than their non exposed counterparts [11].
Surface functional groups (e.g., carboxyl, phosphoryl, and hydroxyl) play a major role in bio-accumulation of metals.
More than that, Cr(VI) uptake is carried out by the sulphate transport pathway, hence competitively inhibited by sulphate [27]. This capacity can be attributed to the chemical similarity between CrO 2-4 and SO 2-4 ions [28].
Gadd et al. [29] and Brierley [30] have described many ways in which bacteria, fungi and algae can take up toxic metal ions. Heavy metal ions can be entrapped in the cellular structure and subsequently biosorbed onto the binding sites present in the cellular structure.
This method of uptake is independent of the biological metabolic cycle and is known as biosorption or passive uptake. The heavy metal can also pass into the cell across the cell membrane through the cell metabolic cycle [31]. This mode of metal uptake is referred as active uptake. The metal uptake by both active and passive modes can be termed as bioaccumulation.
In order to treat the Fez tannery effluents, several costly conventional physico-chemical methods are used. These methods present also difficulties in eliminating the high quantities of polluting substances found in these effluents [8; 3]. Fortunately, biological system seems to be a suitable approach for such treatment. Indeed, indigenous bacteria similar to Acinetobacter strain AB1 are able to remediate the chromate toxicity present in Fez tanneries thanks to its chromium reduction and accumulation abilities. The isolate was able to significantly reduce Cr(VI) under a wide range of temperatures and pHs, as well as at higher Cr(VI) concentrations.
References
PREM (2004) Projet Pérennité des Ressources en Eau du Maroc Rapport Final Janvier Royaume du Maroc Secrétariat d’Etat Chargé de l’Environnement Projet financé par l’USAID/Maroc
Anita I, Kalpana M, Bhavanath J (2004) Accumulation of hexavalent chromium by an exopolysaccharide producing marine Enterobacter cloaceae. Mar Pollut Bull 49:974–977
Amoozegar MA, Ghasemi A, Razavi MR, Naddaf S (2007) Evaluation of hexavalent chromium reduction by chromate-resistant moderately halophile, Nesterenkonia sp. strain MF2. Process Biochem 42:1475–1479
McLean J, Beveridge TJ (2001) Chromate reduction by a pseudomonad isolated from a site contaminated with chromate copper arsenate. Appl Environ Microbiol 67:1076–1084
Pino GH, Mesquita LMS, Torem ML, Pinto GAS (2006) Biosorption of cadmium by green coconut shell powder. Miner Eng 19:380–387
Park JM, Park D, Yun Y (2005) Use of dead fungal biomass for the detoxification of hexavalent chromium: screening and kinetics. Process Biochem 40(7):2559–2565
Zahoor A, Rehman A (2009) Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J Environ Sci 21(6):814–820
Srivastava S, Thakur IS (2006) Evaluation of bioremediation and detoxification potentiality of Aspergillus niger for removal of hexavalent chromium in soil microcosm. Soil Biol Biochem 38:1904–1911
Ma Z, Zhu W, Long H, Chai L, Wang Q (2007) Chromate reduction by resting cells of Achromobacter sp. Ch-1 under aerobic conditions. Process Biochem 42:1028–1032
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA (2006) Bioremediation of Cr(VI)-containing electroplating wastewater using Acinetobacter sp. International Conference on Environment (ICENV 2006), Penang, 13–15 November 2006
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA (2007) Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J Hazard Mater 146:30–38
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E; Goodfellow M (eds) Nucleic acid techniques in bacterial systematics, Wiley, Chichester, pp 115–175
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with clustal X trends. Biochem Sci 23:403–405
Holder M, Lewis PO (2003) Phylogeny estimation: traditional and Bayesian approaches. Nat Rev Genet 4:275–284
Page RDM (1996) T-REEVIEW: an application to display of phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Apha (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
CEAEQ (Centre d’expertise en analyse environnementale du Québec) (2008) Détermination du chrome hexavalent: méthode colorimétrique, MA. 200—CrHex 1.1, 2 July 2008
Bihari Z, Pettkó-Szandtner A, Csanádi G, Balázs M, Bartos P, Kesseru P, Kiss I, Mécs I (2007) Isolation and characterization of a novel n-alkane-degrading strain Acinetobacter haemolyticus AR-46. J Biosci 62(3–4):285–295
Sarangi A, Krishnan C (2008) Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour Technol 99(10):4130–4137
Wang PC, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172:1670–1672
Nragu JO, Nieboer E (1988) Chromium in the natural and human environments. Wiley, New York
He Z, Gao F, Sha T, Hu Y, He C (2009) Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater 163(2–3):869–873
Wani R, Kodam KM, Gawai KR, Dhakephalkar PK (2007) Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline Crater Lake. Appl Microbiol Cell Physiol 75:627–632
Yakup AM, Gulay B, Meltem Y, Sema B, Omer G (2004) Biosorption of Hg2+, Cd2+, and Zn2+ by Ca—alginate and immobilized wood-rotting fungus Funalia trogii. J Hazard Mater B 109(1–3):191–199
Farrell SO, Ranallo RT (2000) Experiments in biochemistry. A hands-on approach. Saunders College Publication, Orlando
Middleton SS, Latmani RB, Mackey MR, Ellisman MH, Tebo BM, Criddle CS (2003) Cometabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell-associated reduced chromium and inhibits growth. Biotechnol Bioeng 83:627–637
Ohtake H, Cervantes C, Silver S (1987) Decreased chromate uptake in Pseudomonas fluorescens carrying a Cr(VI) resistance plasmid. J Bacteriol 169:3853–3856
Mabbett AN, Macaskie LE (2001) A novel isolate of Desulfovibrio sp. With enhanced ability to reduce Cr(VI). Biotechnol Lett 23:683–687
Gadd GM (1988) Accumulation of metal by microorganisms and algae. In: Rehm H (ed) Biotechnology: a comprehensive complete treatise, vol 6B, special microbial process, vol 4. Verlagsgesellschaft, Weinheim, pp 401–403
Brierley CL (1990) Bioremediation of metal-contaminated surface and groundwater. Geomicrobiol J 8(3–4):201–223
Srivastava S, Thakur IS (2007) Evaluation of biosorption potency of Acinetobacter sp. For removal of hexavalent chromium from tannery effluent. Biodegradation 18:637–646
Acknowledgments
This study is supported by Programa de Cooperación Interuniversitaria e Investigación Científica entre España y Marruecos, y proyecto A/5712/06 Estudio de la biodiversidad bacteriana presente en el río Sebou mediante el uso de técnicas microbiologías clásicas y moleculares (DGGE Y FISH).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Essahale, A., Malki, M., Marín, I. et al. Hexavalent Chromium Reduction and Accumulation by Acinetobacter AB1 Isolated from Fez Tanneries in Morocco. Indian J Microbiol 52, 48–53 (2012). https://doi.org/10.1007/s12088-011-0187-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-011-0187-1