Abstract
Rhizobium–legume symbiotic interaction is an efficient model system for soil remediation and reclamation. We earlier isolated an arsenic (As) (2.8 mM arsenate) tolerant and symbiotically effective Rhizobium strain, VMA301 from Vigna mungo and in this study we further characterized its efficacy for arsenic removal from the soil and its nitrogen fixation capacity. Although nodule formation is delayed in plants with As-treated composite when the inoculum was prepared without arsenic in culture medium, whereas it attains the significant number of nodules compare to plant grown in As-free soil when the inoculum was prepared with arsenic supplemented medium. Arsenic accumulation was higher in roots than root nodules. Nitrogenase activity is reduced to almost 2 fold in plants with As-treated soil but not abolished. These results suggest that this strain, VMA301, has been able to establish an effective symbiotic interaction in V. mungo in As-contaminated soil and can perform dual role of arsenic bioremediation as well as soil nitrogen improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Millions of people in the world are suffering with skin lesions, cancers and other related diseases due to consumption of arsenic (As) contaminated underground water in Bangladesh, Taiwan, India, Japan, Poland, Hungary, Belgium, Chile, Argentina, North Mexico, Mongolia, and China [1, 2]. Arsenic is accumulated in human beings from drinking water as well as from agricultural crops and vegetables [3]. As in irrigation water can result in land degradation and adversely affecting on agro-ecosystem services for sufficient and safe foods. The continuous accumulation of As in soil by irrigation is a growing threat to the nutritional status of food product. Still to date, the risks of using As-contaminated groundwater resources for irrigation and decontamination of agricultural field have not received enough attention yet. Earlier several researchers have been evaluated the adverse effect of arsenic in crop field [4–6].
It is essential to remove arsenic from groundwater and contaminated soil. Several chemical methods have been established for decontamination of arsenic from ground water supply [7] including biological treatments [8]. Sequestration of arsenic by biological material is receiving attention in a biotechnological context as microbe based technologies [9] and phytoremediation [10] is cost effective and environment friendly. Phytoremdiation is also an emerging technology that uses plants to clean up pollutants from the environment. A number of arsenic hyper accumulating plants [10, 11] have been reported but of limited use because of their small biomass and slow growth rate.
Recently, an increasing interest has risen on the use of legume plants because of its bioremediation potential as well as capacity of biological nitrogen fixation through root nodule formation [12, 13]. Root nodule is a unique and highly organized structure developed as a result of the symbiotic relationship between leguminous plants and bacteria of the genus Rhizobium. This system has some advantages because of microorganism’s ability to affect metals solubility, bioavailability, mobility and use of plants, legumes for phytoremediation. Finally, Rhizobium–legume symbiosis as an efficient system for soil nitrogen improvement. A few Rhizobium strains tolerant to arsenic toxicity and symbiotically effective have been reported [14, 15]. We have previously isolated one Rhizobium strain, VMA301, from the root nodules of Vigna mungo, grown in arsenic contaminated field and characterized its several aspects to arsenic toxicity [16]. Our aim is to evaluate the phytotoxic effects of arsenic on nodulation of blackgram using the strain, VMA301.
Materials and Methods
Plant growth and Nodulation Condition
Seeds of V. mungo were surface sterilized in 1% mercuric chloride solution for 15 min followed by 70% ethanol wash for 5 min. Seeds were then washed thrice in sterile distilled water, taken in Petri dishes containing sterile half strength of Murashige and Skoog (MS) medium [17] and allowed to germinate under 16 h photoperiod at 30°C. Seedlings were planted in Leonard jars filled with a composite, mixture of perlite and vermiculite (1:1) in a N-free medium [18] and inoculated with Rizobium sp. VMA301. Seedlings were allowed to grow in a plant chamber with 16 h photoperiod and arsenate (2.8 mM) containing water was applied to seedlings at an interval of 2 days. After each week roots and root nodules were counted and harvested for biochemical characterization. A control experiment was also performed by applying arsenate free water.
Determination of N2-Fixing Area in Root Nodule
Microscopic investigations were made to determine the N2-fixing area in arsenate treated and untreated root nodule. Young and fresh nodules were pulled, sections were prepared and viewed under optical microscope (Olympus, PM-10ADS) using an optical lens.
Nitrogenase Assay
Nitrogenase activity was measured by the acetylene reduction assay following Hardy et al. [19]. Isolated nodules bearing root was assayed at atmospheric partial pressures of oxygen by transferring into a serum vial (total volume, 8.0 ml). The vial was sealed with a serum stopper, the acetylene was added to a final concentration of 10% (v/v), and the reaction mixture was incubated at 25°C with constant shaking. At different time intervals, samples were removed for analysis with a gas chromatograph (Perkin-Elmer 8600 gas chromatograph equipped with a Poropak-R column) to determine the amount of ethylene produced. The nanomoles of ethylene produced per time unit were standardized to total cell protein. Total protein was measured following the method of Bradford [20] after samples were solubilized by heating for 15 min at 90°C in 1.0 N NaOH.
Arsenic Content in Roots and Root Nodules
Total arsenic content in the roots and root nodules were determined by digesting with concentrated nitric acid according to the method of Quaghebeur and Rengel [21]. In brief, dry and ground plant material (0.5 g) was weighed into a 50 ml conical flask to which 10 ml of concentrated nitric acid was added. The mixture was then heated at 90°C for 30 min, after which the temperature was increased to 140°C. When the volume of acid had decreased to 1 ml, the flasks were allowed to cool at room temperature and made up to a final volume (10 ml) with deionized water. In each analytical batch, at least 2 reagent blanks and two standard (known concentration) solutions were included. 1 ml of digest product was mixed with 9 ml of reducing solution consisting of 1.5% (w/v) potassium iodide, 1.5% (w/v) ascorbic acid and 10% (v/v) hydrochloric acid. This mixture was then heated at 50°C for 1 h. Total arsenic in digested sample was determined by a flame atomic absorption spectrophotometer (Perkin Elmer, 100 analyst system) and repeated three times.
Results and Discussion
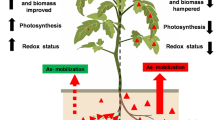
Arsenic accumulation occurs in human beings from drinking water as well as from agricultural crops and vegetables [3] and enhances human risk of arsenic poisoning through the food chain. Therefore, arsenic decontamination is vital from drinking water and agricultural land. Rhizobium–legume symbiosis in leguminous plants is considered as an alternative procedure for arsenic removal from contaminated lands as well as increase of soil fertility. The legume crops grown in various agrochemical conditions and cropping systems with diverse cultural practices, carry traits that have allowed them to adapt the adverse environmental conditions. A number of microorganisms including Rhizobium tolerant to arsenic have been isolated and identified [14]. In this study, we have used Rhizobium sp. VMA301 strain tolerant to a concentration of 2.8 mM arsenate [16].
In addition to arsenic remediation, we have performed some tests to evaluate its symbiotic properties in arsenic stressed condition. Although, the arsenic concentrations used in this work was much higher to create extreme stress than those generally observed in contaminated crop land [22]. The Rhizobium sp. VMA301, showed its ability to form fully effective nodules which were pink in colour and globule to oval in shape similar to root nodules from the plants grown on non-contaminated field. But microscopic observations of the cross section of root nodules revealed that the effective areas of N2-fixing zone were reduced in plants grown in arsenic contaminated field (Fig. 1). Effects on nodulation and growth were recorded in each week after seedling. We observed that the number of nodules and growth of the plants were affected by the presence of arsenic in composite. In first and second week nodule number was highly affected by arsenic (Table 1). It is clear that arsenic produced a drastic effect in nodule number in early stage of nodulation when the inoculum was prepared without arsenic supplemented medium but it recovers when the inoculum was prepared with arsenic supplemented YEM medium. This is may be due to the effect of arsenic on Rhizobium survivability and initial tolerance. So, when the bacterium was adapted with arsenic during inoculum preparation it overcomes the initial stress for root nodule establishment. Earlier Broos et al. [23] noticed that contaminated soil not only affects the legume growth but also microbial survivability. Furthermore, recent investigation by Pajuelo et al. [15] demonstrated that a decreased number of root hairs and infection thread were affected drastically in the early step of nodulation.
We also observed that the nitrogenase activity was consistent in the root nodules of plants grown in arsenic contaminated field though it is lower than root nodules from plants grown in arsenic free composite (Fig. 2). This may be due to the decrease of nitrogen fixing area in root nodules from As-contaminated composite. The root nodules of As-grown plants showed a symptom of toxicity, such as inhibition of nitrogenase activity (Fig. 1). Furthermore, iron–molybdenum (Fe–Mo) is the cofactor of nitrogenase, arsenic has an affinity to iron molecules [24] and that may be another reason for the reduction of nitrogenase activity. Toxic effects of metals on nitrogen fixing area in root nodules of soybean plants have been reported earlier by Chen et al. [25]. Arsenic accumulation was 3.5 times higher in roots than root nodules (Fig. 3). In plants, arsenic can be bound to plant phytochelatins and subsequently stored in vacuoles or inert structures such as cell wall, lignin, etc. [26]. Arsenic content was higher in aged roots and root nodules due to its increasing effect of arsenic availability in soils. These results suggest that this arsenic tolerant strain, VMA301, is able to establish an effective symbiotic interaction with blackgram in arsenic contaminated field. This implies that this model system Rhizobium–legume symbiosis is more effective for arsenic contaminated soil reclamation. Thus, it can perform the dual role of arsenic bioremediation as well as soil nitrogen improvement by symbiotic nodulation.
References
Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, Bonorino G, Albouy R (2004) Ground water arsenic in the Chaco-Pampean Plain, Argentina: case study from Robles County, Santiago del Estero Province. Appl Geochem 19:231–243
Tseng CH, Huang YK, Hhung YL, Chung CJ, Yang MH, Chen CJ, Hsueh YM (2005) Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease—hyperendemic villages in Taiwan. Toxicol Appl Pharmacol 206:299
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentration in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30:383–387
Islam MR, Salminen R, Lahermo PW (2000) Arsenic and other toxic elemental contamination of groundwater, surface water and soil in Bangladesh and its possible effects on human health. Environ Geochem Health 22:33–53
Jahiruddin M, Islam MA, Islam MR, Islam S (2004) Effects of arsenic contamination on rice crop. Environtropica 1:204–210
Norra S, Berner ZA, Agarwala P, Wagner F, Chandrasekharam D, Stuben D (2005) Impact of irrigation with As rich groundwater on soil and crops: a geochemical case study in West Bengal Delta Plain, India. Appl Geochem 20:1890–1906
Mayo JT, Yavuz C, Yean S, Cong L, Shipley H, Yu W, Falkner J, Kan A, Tomson M, Colvin VL (2007) The effect of nanocrystalline magnetite size on arsenic removal. Sci Technol Adv Mater 8:71
Mandal SM, Mondal KC, Dey S, Pati BR (2007) Arsenic biosorption by a mucilaginous seed of H. suaveolens. J Sci Ind Res 66:577–582
Satishkumar M, Murugesan GS, Ayyasamy PM, Swaminathan K, Lakshmanaperumalsamy P (2004) Bioremediation of arsenic contaminated groundwater by modified mycelial pellets of Aspergillus fumigatus. Bull Environ Contam Toxicol 72:617–624
Ma LQ, Kumar KM, Tu C, Zhang W, Cai Y, Kennelly ED (2001) A fern that hyperacumulates arsenic. Nature 409:579
Francesconi K, Visoottiviseth P, Sridokchan W, Goessler W (2002) Arsenic species in an arsenic hyperacumulating fern, Pityrogramma calomelanos: a potential phytoremidiator of arsenic contaminated soils. Sci Total Environ 284:27–35
Pastor J, Hernandez AJ, Prieto N, Fernandez-Pascual M (2003) Accumulating behaviour of Lupinus albus L. growing in a normal and a decalcified calcic luvisol polluted with Zn. J Plant Physiol 160:1457–1465
Kamaludeen SPB, Ramasamy K (2008) Rhizoremediation of metals: harnessing microbial communities. Indian J Microbiol 48:80–88
Carrasco JA, Armario P, Pajuelo E, Burgos A, Caviedes MA, Lopez R, Chamber MA, Palomares AJ (2005) Isolation and characterization of symbiotically effective Rhizobium resistant to arsenic and heavy metals after the toxic spill at the Azanalcollar pyrite mine. Soil Biol Biochem 37:1131–1140
Pajuelo E, Rodrı′guez-Llorente ID, Dary M, Palomares AJ (2008) Toxic effects of arsenic on Sinorhizobiume–Medicago sativa symbiotic interaction. Environ Poll 154:203–211
Mandal SM, Pati BR, Das AK, Ghosh AK (2008) Characterization of an arsenic tolerant and nodulation effective Rhizobium isolate of V. mungo. J Gen Appl Microbiol 54:93–99
Chou LC, Chang DCN (2004) A symbiotic and symbiotic seed germination of Anoectochilus formosanus and Haemaria discolor and their F1 hybrids. Bot Bull Acad Sin 45:143–147
Serrano A, Chamber M (1990) Nitrate reduction in Bradyrhizobium sp (Lupinus) strains and its effects on their symbiosis with Lupinus luteus. J Plant Physiol 136:240–246
Hardy RWF, Filner P, Hageman RH (1975) Nitrogen input. In: Brown AWA (ed) Crop productivity—research imperatives. Charles F. Kettering Foundation, Yellow Springs, OH
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye-binding. Anal Biochem 72:248–254
Quaghebeur M, Rengel Z (2003) The distribution of arsenate and arsenite in shoots and roots of Holcus lanatus is influenced by arsenic tolerance and arsenate and phosphate supply. Plant Physiol 132:1600–1609
Chaterjee A, Das D, Mandal BK (1995) Arsenic in groundwater in six districts of west Bengal, India. The biggest arsenic calamity in the world. Prt 1. Arsenic species in drinking water and urine of affected people. Analyst 120:643–650
Broos K, Uyttebroek M, Mertens J, Smolders E (2004) A survey of symbiotic nitrogen fixation by white clover grown on metal contaminated soils. Soil Biol Biochem 36:633–640
Ahmad S, Kitchin KT, Cullen WR (2000) Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch Biochem Biophys 382:195–202
Chen XY, He YF, Yang Y, Yu YL, Zheng SJ, Tian GM, Luo YM, Wong MH (2003) Effect of cadmium on nodulation and N2-fixation of soybean in contaminated soils. Chemosphere 50:781–787
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, S.M., Gouri, S.S., De, D. et al. Effect of Arsenic on Nodulation and Nitrogen Fixation of Blackgram (Vigna mungo). Indian J Microbiol 51, 44–47 (2011). https://doi.org/10.1007/s12088-011-0080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-011-0080-y