Abstract
Citric acid (CA) is the most important commercial product which is produced by using various sugar substrates in the terrestrial environment. The present study made an attempt to produce citric acid by the fungal strain Aspergillus niger from red seaweed Gelidiella acerosa is the best alternative to sugar substrate in the marine environment. In this study three types of production media were prepared including control (sucrose) by following standard fermentation conditions. The acid production was indicated by the reduction of pH levels. The control medium gave the highest yield of 80 g/l at pH 1.5 and the medium containing crude seaweed powder and other compositions gave the yield of 30 g/l at pH 3.5 whereas the medium containing crude seaweed and 10% sucrose gave the yield of 50 g/l at pH 3.0. When calculating the benefit cost ratio, crude seaweed powder and 10% sucrose yielded 50 g of citric acid at the lower cost of Rs. 35, whereas the other two media gave the yield of 80 and 30 g respectively with the cost of Rs. 77 and 28. In economic point of view, the medium containing seaweed and 10% sucrose showed more benefit with lower cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid) is the most important commercial product, which is found in almost all plant and animal tissues. It exists widely in the nature and present as a kind of fruit acid in lemon, orange, pine apple, plum, peas, peach and in animal bones, muscles and blood. It has many applications in food, pharmaceutical and cosmetic industries as an acidulant, flavour enhancer, preservative, antioxidant, emulsifier and chelating agent [5]. In recent years, citric acid has been commercially produced by fungal fermentation mainly by Aspergillus. niger [2]. Citric acid is also produced commercially using mutant fungal strains of Saccharomycopsis lipolytica, [3] Penicillium simplicissimum and Aspergillus. foeitidus [4]. A. niger are principally used to produce citric acid, however in culturing these fungi, the growth of Aspergillus in pellet form is desirable and this can be achieved by process optimization [5, 6]. Yarrowia lipolytica has the ability to produce citric acid from raw glycerol [7], a by-product of biodiesel production from rapeseed oil. Citric acid is produced commercially employing various inexpensive and readily available raw materials like molasses, carob pod extract, rape seed oil, corn-cobs, apple and grape pomace, kiwi-fruit peel, mandarine orange and brewery wastes have been used as carbon substrates [8] in terrestrial environment. Most reports are devoted to various types of citric acid producing yeasts and to experimental conditions governing production of citric acid, essentially based on shake flasks assays under undefined fermentation conditions. But there has been no report was found in use of marine sources, there is a need for finding the alternative carbon substrate from the marine environment. Marine environment has more and more number of beneficial sources in it, in this environment, seaweeds is easily available as well as renewable marine resources, which have enormous uses both in industrial and medicinal fields. Mostly all the living resources are rich in proteins, but the red seaweed, Gelidiella acerosa is highly rich in carbohydrates and very less in protein content [9]. G. acerosa is a red alga, which is commonly found in tide pools, high intertidal rocky areas and shallow sub-tides. This study was undertaken to explore alternate marine carbon sources and reports successful higher yields of citric acid using seaweed powder in shake flask fermentation at comparatively lower costs.
Materials and Methods
Aspergillus niger was isolated from the marine soil sample and identified by lactophenol cotton blue (LCB) mount method. The seaweed was collected from Rameswaram coastal area and brought to the laboratory and identification was made using the keys [10]. It was shade-dried for several days and then powdered mechanically. Seaweed powder was tested for its anti fungal activity against A. niger. Carbohydrate estimation [11] was carried out by following standard method. From the 7-days-old fungal culture, a loop-full of fungal spores was transferred to 5 ml of the sterile Potato dextrose broth and kept in rotary shaker for the formation of pellets. The production medium was prepared and the initial pH was adjusted to 6.5. Then the medium was sterilized at 15 lbs pressure and 121°C for 15 min. After sterilization, the medium was allowed to cool at room temperature. Then the pellet form of fungal culture was transferred and mixed in the sterilized production medium. The inoculated flask was placed into a water bath shaker at 30°C and 200 rpm for 10 days for proper aeration. The pH was checked in aseptic conditions at every 2 days of interval.
Citric Acid Production Using Seaweed Powder
Three experiments were carried out to find out the possibilities of using seaweed as an alternate substrate for CA production. Control production medium (Table 1) was maintained in the first experiment, in the second experiment, sucrose was replaced by the equal volume of crude seaweed powder and in the third experiment, 10% of sucrose was added to the crude seaweed powder in the fermentation medium for observing the enhanced activity of seaweed. The fermentation conditions maintained carefully throughout the process.
Recovery Process
After the completion of fermentation process, the incubated broth was filtered for the separation of pellet form of fungal culture and the fermentation broth, which acts as the source of citric acid. Lime (Ca(OH)2) was added to the fermentation broth to allow precipitation of CA in the form of calcium citrate. Again, the precipitate was treated with dil. H2SO4 to precipitate insoluble calcium sulphate, and then filtered. The precipitated solution containing CA was purified by passing through column of carbon granules. Before using the carbon granules, they were treated with heat. The obtained solution was evaporated under vacuum condition for getting the purified citric acid in crystal form [12]. The crystallized CA powder was determined titrimetrically using 0.1 NaOH and phenolphthalein as indicator [13].
Results
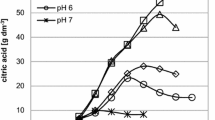
Fermentation process was carried out for CA production. During the fermentation process, the temperature was maintained at 30°C up to the end of the process. There was a gradual reduction (Fig. 1) of pH noticed in all the experiments and it indicated the production of citric acid. After recovery process, a purified citric acid was obtained and the amount of yield was given in Table 2. The percentage of CA produced in the different production media is given in the Fig. 2. The economic view (benefit/cost ratio and cost/benefit ratio) of the citric acid production is given in Fig. 3 and Table 3 respectively.
Discussion
In the present study, considerable amount of citric acid is produced using G. acerosa as a carbon substrate by shake flask fermentation. This seaweed contains 60% of carbohydrates and their sugar compositions are not identified. When the seaweed is added with the production medium, the pH gets slowly reduced at regular intervals, from the initial pH of 6.5 reduced to 3.5 on tenth day. This change of pH indicates the production of citric acid in the production medium.
Earlier studies report that the factors affecting CA production by fermentation includes the nutrient composition of the media, environmental conditions, deficiency of manganese [2], types and concentration of sugars [14], chelating effect on metal ions [15], ammonium nitrate and aeration [16]. The optimum time of incubation for maximal citric acid production varies with the organism and fermentation conditions used. In batch-wise fermentation of citric acid, the production starts after a lag phase of 1 day and reached maximum at the onset of stationary phase or late-exponential phase. Further increase in incubation period does not enhance the citric acid production. It may be due to the age of fungus used and depletion of sugar contents in the culture broth [17]. Actually, the acid production starts with the initial stage idiophase (between 80 and 120 h) of fungal growth. In this stage, the pH is generally stated at five. The yield is gradually increased and it reaches to maximum rate at the late idiophase (180–220 h) [18]. In this stage, the pH must be below three in order to suppress oxalic acid and gluconic acid formation. The acceleration of fermentation can be explained by the higher starting biomass concentration in fermenter and by the adaptation of biomass to very high osmotic pressures [19]. In this study, the biomass of fungal culture has not been calculated, because, the culture has inoculated in the form of pellets, due to this the biomass is not meaningful. Temperature plays an important role in citric acid production. Temperature between 25 and 30°C is usually employed for culturing of Aspergillus niger. The optimum temperature for citric acid production is 30°C, but temperature of the medium increases above 30°C and thus the biosynthesis of citric acid are decreased. It may be due to high temperature that can cause denaturation of enzyme-citrate synthase and accumulation of other by-product acids such as oxalic acid and enzyme catabolite repression [20] and it also inhibits the culture development.
The concentration and type of sugar influence the yield of citric acid production by A. niger [21]. In control production medium, the initial pH 6.5 is gradually reduced to 1.5 during fermentation. Sucrose is the substrate for citric acid production in this medium. The fungus A. niger utilizes the sugar compound and produces 80 g l−1 (57% citric acid) whereas in the second experiment seaweed used as a sugar substrate in the production medium which yields only 30 g l−1 (22% citric acid). Sucrose has relatively low molecular weight and it is readily transported into microbial cells for hydrolysis by intracellular enzymes [13]. This is the main reason for using sucrose-based substrate in citric acid production. The chemical nature of sugar source has a marked effect on citric acid production by A. niger [14]. Glucose, fructose and maltose have also been used as substrates for citric acid production [22]. The combination of two sugars in the medium at 50% of each improves the yields of citric acid [23, 24]. In the third experiment, when the crude powder of seaweed is added with 10% of sucrose instead of 50% and produced 50 g l−1 as evident by the pH reduction from 6.5 to 3.0. Sucrose combined with crude seaweed powder and enhances the utilization of sugar substrate from seaweed powder and induces the production of citric acid. A maximal citric acid production rate is obtained at a range from 14 to 22% of the sugar in the control medium whereas the combination of the two sugars such as sugar from seaweed powder and 10% sucrose improve the yields of citric acid up to 36%.
Nitrogen is a limiting factor in the citric acid production. Nitrogen is usually supplied in the form of ammonium nitrate, which is completely metabolized during fermentation periods. Citric acid starts to appear when the nitrogen concentration falls below a low limiting value. It appears that the citric acid is produced by carbon-storing cells. Trace elements are the one of the most important factors, affecting the yield of citric acid fermentation. In particular, the levels of manganese, iron, copper and zinc are quite critical. If the levels of these trace elements are at optimal level, other factors have less pronounced effects. Conversely, medium will not allow high production unless trace element content is controlled carefully.
It is found from the study, using seaweed alone as a sugar substrate for citric acid production by fermentation is lower (30 g l−1) when compared with seaweed and 10% sucrose (50 g l−1). Maximum amount (80 g l−1) of citric acid is produced from control medium. During the process of making the seaweed powder using mixy, enormous amount of heat is liberated, this also might interfere with the yield of citric acid when it is used as substrate. It is suggested that maintaining a specific concentration and type of sugars probably is useful to increase the citric acid production. Further, a separate or combined effect of critical environmental parameters and the strain of A. niger for citric acid production need to be studied carefully. In economic point of view, the cost of chemically produced and commercially available citric acid is Rs. 700/kg (Sd-fine Chemicals Pvt. Ltd.) and the main substrate sucrose is normally high expensive (Rs. 545/kg). When sucrose and other compositions used in the control medium yields 80 g l−1 of citric acid at the cost of Rs. 77. At the same time, in the second experiment crude seaweed powder (Rs. 200/kg) in the production medium gives 30 g l−1 of yield at the cost of Rs. 21. But, the media containing seaweed powder and 10% sucrose in the third experiment provides 50 g l−1 at the cost of Rs. 35. This shows more benefit of CA production with lower cost when compared to commercial citric acid (Table 3). Thus seaweed supplementation for citric acid production is economically viable exercise.
Summary and Conclusion
Citric acid is commercially produced from various sources by Aspergillus niger in terrestrial environment. In marine environment, the red seaweed Gelediella acerosa which contains 60% carbohydrate is used for citric acid production in this study. This seaweed shows resistance against A. niger. Three types of production media prepared including control (sucrose) and the fermentation process was carried out for citric acid production by following standard conditions. After the process, the control medium gave highest yield about 80 g l−1 and the medium containing the crude seaweed powder gave 30 g l−1. But the medium containing crude seaweed powder and 10% sucrose with other compositions gave the yield of about 50 g l−1 with the cost of Rs. 35. In the economic point of view, the media containing the seaweed powder and 10% sucrose gives more benefit at lower cost when compared to control media. The seaweed has been proved to have the ability to produce citric acid and it can be used as alternate source of sugar substrate for citric acid production.
References
Rohr M, Kubicek CP, Kominek J (1996) Citric acid. In: Rehm HJ, Reed J (eds) Biotechnology, vol 6, 2nd edn. VCH Publishers, Weinheim, pp 308–345
Rohr M, Kubicek CP, Kominek J (1983) Citric acid. In: Rehm HJ, Reed J (eds) Biotechnology, vol 3, 1st edn. VCH Publishers, Weinheim, pp 331–373
Good DW, Droniuk R, Lawford GR, Fein JE (1985) Isolation and characterization of a Saccharomycopsis lipolytica mutant showing increased production of citric acid from canola oil. Can J Microbiol 31:436–440
Tran CT, Sly LI, Mitchel DA (1998) Selection of a strain of Aspergillus for the production of citric acid from pineapple waste in solid state fermentation. World J Microbiol Biotechnol 14:399–404
Rohr MA (1998) Century of citric acid fermentation and research. Food Technol Biotechnol 36:163–171
Wayman FM, Mattey M (2003) Simple diffusion is the primary mechanism for glucose uptake during the production phase of the Aspergillus niger citric acid process. Biotechnol Bioeng 67:451–456
Rymowicz W, Rywinska A, Żarowska B, Juszczyk P (2006) Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem Pap 6(5):391–394
Roukas T (1998) Fermentation of citric acid with cellulase hydrolite by Aspergillus niger. Appl Biochem Biotechnol 74:43–53
Chennubhotla VSK, Najmuddin M, Ramalingam JR, Kaliyaperumal N (1991) Biochemical composition of some marine algae from Mandabam coast, Tamilnadu. CMFRI Bull 44:442–445
Sahoo D, Nivedita, Debasish (2001) Seaweeds of Indian coast. In: Sahoo D, Pandey PC (eds). Published by S. B. Nangia, A. P. H. Publishing Corporation, New Delhi, p 283
Dubois M, Gills KA, Hamilton JK, Rober PA, Smith F (1956) Colorimetric method determination sugars and related substances. Anal Chem 28:350
Dubey RC (2003) Primary metabolites: organic acids. In: A text book of biotechnology. S. Chand and Company Ltd, New Delhi, pp 261–264
Elholi MA, Al-Delaimy KS (2003) Citric acid production from whey with sugars and additives by Aspergillus niger. Afr J Biotechnol 2(10):356–359
Hossain M, Brooks JD, Maddox IS (1984) The effect of sugar source on citric acid production by Aspergillus niger. Appl Microbiol Biotechnol 19:393–397
Roukas T, Kotzekiodou P (1997) Pretreatment of date syrup to increase citric acid production. Enzyme Microb Technol 21:273–276
Bayraktar E, Mehmetoglu U (2000) Production of citric acid using immobilized conidia of Aspergillus niger. Appl Biochem Biotechnol 87:117–125
Arzumanov TE, Shishkanova NV, Finogenova TV (2000) Biosynthesis of citric acid by Yarrowia lipolytica repeat-batch culture on ethanol. Appl Microbiol Biotechnol 53:525–529
Guebel DV, Darias NVT (2001) Optimization of the citric acid production by Aspergillus niger through a metabolic flux balance model. Electron J Biotechnol 4(1):1–17
Anastassiadis S, Rehm HJ (2006) Citric acid production from glucose by yeast Candida oleophila ATCC 20177 under batch, continuous and repeated batch cultivation. Electron J Biotechnol 9(1):26–39
Panda T, Kundu S, Majumdar SK (1984) Studies on citric acid production by Aspergillus niger using treated Indian cane molasses. J Microbiol 52(2):61–66
Peksel A, Kubicek CP (2003) Effect of sucrose concentration during citric acid production accumulation by Aspergillus niger. Turk J Chem 27:581–590
Xu DB, Madrid CP, Rohr M, Kubicek CP (1989) The influence of type and concentration of the carbon source on production of citric acid by Aspergillus niger. Appl Microbiol Biotechnol 30:553–558
Begum AA, Choudhury N, Islam MA (1990) Citric acid fermentation by gamma ray induced mutants of Aspergillus niger in different carbohydrate media. J Ferment Eng 70(4):286
Shu P, Johnson MJ (1947) Effect of the composition of the sporulation medium on citric acid production by Aspergillus niger in submerged culture. J Bacteriol 54:161–167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, T., Kalaiselvam, M. An Experimental Study on Citric Acid Production by Aspergillus niger Using Gelidiella acerosa as a Substrate. Indian J Microbiol 51, 289–293 (2011). https://doi.org/10.1007/s12088-011-0066-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-011-0066-9