Abstract
Beer is a complex beverage. Beer flavour is a multisensory experience in which, in addition to aroma volatiles, CO2, ethanol, bitterness (hop acids) and sweetness all contribute. To investigate the interactions between these fundamental components, a model beer system was developed using representative ingredients. Samples, selected according to a D-optimal design, were assessed by sensory profiling techniques by a trained panel. Predictive polynomial models generated from mean panel data described variations in the attributes as a function of design factors. Results show that CO2 significantly impacted on all discriminating attributes, including suppression of sweetness and modification of bitterness. A number of complex interactions with design factors showed the effects of CO2 to be dependent upon component concentration and level of carbonation. CO2 interacted with hop acids to increase carbonation and tingly perception, which increased linearly with hop acid addition but only at low levels of CO2. Ethanol was the main driver of warming perception and complexity. In agreement with other studies, ethanol enhanced sweet perception and also formed some complex interactions with hop acids and CO2 to modify various attributes, illustrating its ability to interact with both gustatory and trigeminal stimuli. Whether the mechanisms behind these interactions originate at the gustatory periphery or at higher centres in the brain is an area for further investigation. This study provides an in-depth assessment of important flavour components in beer and advances the limited data available on the effects of CO2 on sensory perception using a commonly carbonated beverage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beer continues to be a popular beverage, worth more than any other drink type (in sales value), despite a reduction in the consumption of alcohol across the UK population (Mintel 2009). A maturing market reveals a need to develop products to attract new consumers, and understanding their perception is paramount to success. Factors affecting beer quality include ingredients, processing parameters and packaging. Factors influencing consumer perception are much more complex and include interactions between the main flavour components. Beer flavour is a combination of a large number of volatile components and the contribution of carbonation, ethanol, bitterness (from hop acids) and sweetness (Meilgaard 1982), which also influence its mouthfeel and appearance. Flavour is perceived by the detection and integration of stimuli from the gustatory, olfactory and trigeminal systems, and the interactions between these stimuli can considerably modify sensory perception (Verhagen and Engelen 2006), resulting in a demand for research in this domain. Beer presents an ideal system to investigate multimodality, as some of the main flavour components are sensed by multiple sensory systems.
Limited instrumental data concerning the impact of individual components such as ethanol and carbonation on aroma release exist. Results from experiments investigating volatile release from the matrix into the headspace at static equilibrium (Aznar et al. 2004; Hewson 2007) differ considerably from those under dynamic conditions (where the headspace is diluted by air at a constant rate) (Pozo-Bayon et al. 2009; Saint-Eve et al. 2009) and from the breath during consumption (Clark et al. 2011). Sugars (Friel et al. 2000; Hewson et al. 2008; Saint-Eve et al. 2009) and isomerised hop acid products (Clark et al. 2011) have not been shown to have an effect on volatile partitioning at the concentrations commonly found in beer and other beverages.
CO2 perception is complex, involving excitatory and inhibitory processes in the oral somatosensory system (Green 1992). It is well accepted that CO2 acts on oral trigeminal receptors via a dual mechanism of action. The presence of bubbles bursting in the mouth activates mechanoreceptors (McEvoy 1998), whilst the conversion of CO2 to carbonic acid via carbonic anhydrase elicits a tingly response activating nociceptors (Simons et al. 1999; Dessirier et al. 2000). These mechanisms have been decoupled via the use of carbonic anhydrase inhibitors to block the conversion of CO2 and reduce the intensity of carbonation (Simons et al. 1999; Dessirier et al. 2000) and by inhibiting bubble formation via the use of a hyperbaric chamber. Subjects under hyperbaric conditions still described the tingle of CO2 even though no bubbles were being formed (McEvoy 1998). The taste of CO2 is usually described as sour due to the activation of sour-sensing cells by carbonic acid (Chandrashekar et al. 2009). There are limited sensory studies published investigating carbonation at levels representative of those found in carbonated soft drinks and lager-style beer (∼2.5–4 volumes) and currently none including alcohol such as beer. Studies investigating carbonated milk beverages (Yau et al. 1989; Lederer et al. 1991), juices (McLellan et al. 1984; Prescott et al. 2004; Hewson et al. 2009) and simple taste solutions (Cometto-Muniz et al. 1987; Cowart 1998) found conflicting data regarding the effects of CO2 on taste, aroma and flavour. The effect of CO2 seems to be beverage specific and Cowart (1998) suggested that results may be dependent upon the combination and levels of tastants present in the specific beverage. Consequently, further research with commonly carbonated beverages at appropriate CO2 levels is important to advance understanding in this area.
Ethanol is a complex stimulus which acts on multiple modalities (Green 1988; Kiefer and Morrow 1991; Mattes and DiMeglio 2001; Cometto-Muniz and Abraham 2008). In sensory studies, the taste of ethanol has been found to include both sweet and bitter components depending on the concentration (Wilson et al. 1973; Scinska et al. 2000; Mattes and DiMeglio 2001). Neuronal taste response of ethanol, investigated in vitro using the rhesus monkey (Hellekant et al. 1997), rats (Lemon et al. 2004) and mice (Brasser et al. 2010), supports evidence that ethanol stimulates sweet-best fibres and that central processing follows a similar pathway to sucrose. Ethanol’s stimulation of the trigeminal system seems to be multifaceted, evoking both chemical irritation pathways and mechanoreceptors (Green 1991; Trevisani et al. 2002; Ellingson et al. 2009; Goldner et al. 2009). However, it appears that oral stimulation is the predominant cue for ethanol detection as taste thresholds were not affected when nasal stimulation (olfaction and irritation) was reduced using nose clips (Mattes and DiMeglio 2001). Cross-modal interactions between ethanol and other beverage components have been reported to modify sensations such as the sweetness of sucrose and the bitterness of quinine (Martin and Pangborn 1970), the astringency and bitterness of tannins (Fontoin et al. 2008), irritation (Prescott and Swain-Campbell 2000), hotness (Jones et al. 2008), perceived complexity (Meillon et al. 2010) and aroma (Goldner et al. 2009).
Sweet and bitter tastes are mediated by the G protein-coupled receptors. The similarities in sweet and bitter transduction have lead to considerable research regarding interactions between the two tastes; for review, see Margolskee (2002). Recent research has focussed on specific taste receptor cells and their role in sweet and bitter taste interactions, indicating peripheral gustatory integration (Talavera et al. 2008).
Literature suggests that the main components of beer flavour are capable of complex interactions. However, there is limited literature available on beer itself, which is surprising considering its commercial importance. The objective of this paper was to investigate interactions between sweetness, bitterness, alcohol content and carbonation levels in beer. Due to the complexity of the product, it was necessary to take a scientifically controlled approach which would allow independent manipulation of each component. This was achieved by development of a model beer system varying in its alcohol content (ethanol), sweetness (dextrose), bitterness (isomerised hop acid products) and carbonation level (CO2). These components were chosen to elicit the correct taste and flavour profile expected in a beer. Aroma, colouring and soluble fibre were also added at constant levels throughout to generate a model system representative of beer.
Material and Methods
Subjects
Ten assessors (two men and eight women) from the University of Nottingham external sensory panel volunteered to take part in the study after completing appropriate screening tests using the samples under investigation. Subsequent training sessions (40 × 2 h) were dedicated to attribute generation, definition, discussion, agreement and protocol development, followed by practice ratings and re-training where necessary. Full approval of a local ethics committee was obtained before the study commenced. Informed consent was obtained from all assessors after the nature of the methods, alcohol content and nutritional consumption per session was fully explained.
Samples
Experimental design software (Design Expert, Stat-Ease Inc., Minneapolis, MN, USA) was used to create a design space varying in four factors at three levels: ethanol (0%, 2.25% and 4.5%), sweetener (0, 15 and 30 g/L), hop acids (0, 300 and 600 μl/L) and carbonation (none, low and high). None corresponded to uncarbonated samples, low to ∼2 volumes and high to ∼3.6 volumes. A ‘volume’ is the industry-recognised unit of CO2 measurement and is dependent upon temperature and pressure (Smith and Hui 2004). Levels were chosen to be perceivably different and representative of levels found in beer. The reduced isomerised hop acid products used in this study have been processed in such a way to only impart intended bitterness to beer (O’Rourke 2002) and no hop aroma. Dextrose was chosen as the sweetener as it delivered a taste profile similar to that found in lager-style beers. A D-optimal design was selected to minimise the sample number for sensory assessment whilst maintaining the ability to produce reliable predictive models resulting in 31 samples, (including five replicate samples), which are detailed in the left panel of Table 2. A further set of 10 independent samples (validation set) were chosen from the design space and were evaluated in triplicate to allow the predictive models generated by the original data to be validated.
Sample Preparation
Model beer samples were manufactured using 70 g/L polydextrose (soluble fibre) (Litesse® Ultra powder, Danisco Sweeteners, New Century, KS, USA), water-soluble food colouring (Dr. Oetker, Leeds UK) comprising of 600 μl/L yellow, 50 μl/L green and 40 μl/L red and a beer flavouring. The beer flavouring was made by dissolving ethyl acetate, isoamyl acetate, dimethyl sulphide, phenethyl alcohol and isoamyl alcohol (2-methylbutanol) in a 60:40 mix of propylene glycol (Fisher Scientific, Loughborough, UK) and Evian water (Danone, Paris, France). The beer flavouring was added to obtain final volatile concentrations of ethyl acetate 3.2 μl/L, isoamyl acetate 0.024 μl/L, dimethyl sulphide 0.02 μl/L, phenethyl alcohol 13.2 μl/L and isoamyl alcohol 24 μl/L (Sigma Aldrich, Dorset, UK). Where appropriate, ethanol (VWR International, Lutterworth, UK), dextrose (MyProtein, Manchester, UK) and hop acids (Botanix, Kent, UK) were added at levels detailed in the experimental design in Table 2. The hop acids were made by dissolving isomerised hop acid products Tetrahop (tetrahydroiso-alpha-acids 9% w/w) and Redihop (rho-iso-alpha-acids 30% w/v) with a mix of propylene glycol and water (60:40) to create two stock solutions, (1) 600 μl/L and (2) 300 μl/L. The final hop acid concentration of (1) 600 μl/L comprised of 480 μl/L Tetrahop and 120 μl/L Redihop (∼80 International Bitterness Units (IBU)) and (2) 300 μl/L comprised of 240 μl/L Tetrahop and 60 μl/L Redihop (∼40 IBU). Where the hop acids level was 0 μl/L, an equivalent volume of propylene glycol and water (60:40 mix) was added to ensure consistency. Samples were made up to 1 L with water and left to solubilise on a roller bed for >6 h, then refrigerated (5 ± 1°C) before carbonation. All materials were food grade quality.
Carbonation of Samples
Forty millilitres of the sample, measured by volume, was aliquoted into a 100-ml Schott bottle (Fisher Scientific, Loughborough, UK). The cap was tightly secured using a silicone sealing ring (RS Components, Corby, UK). A schematic of the batch carbonation system, developed and manufactured in-house (Medical Engineering Unit, University of Nottingham, UK), is detailed in Fig. 1. Schott bottle caps (Fisher Scientific, Loughborough, UK) were modified to incorporate a one-way connecting valve (RS Components, Corby, UK), which allows the flow of CO2 into the sample vessel when connected but is isolated on disconnection. All samples were purged with CO2 before carbonation commenced. The samples were carbonated using food grade CO2 (BOC, Guilford, UK). The flow of CO2 was isolated my means of a shut off valve, allowing pressure in the sample bottle to be monitored by a pressure gauge. Samples were carbonated by setting the delivered gas pressure to the desired level, opening the isolation switch and gently shaking the sample bottle to speed the dispersion of CO2 into the liquid. Once equilibrium was achieved, the shut off switch was closed to isolate the sample bottle and the pressure within the bottle was monitored using a second pressure gauge to ensure that the correct pressure was attained. The sample was disconnected from the carbonated equipment and stored at 4–6°C until sampling commenced.
Sample Presentation and Evaluation
Samples (40 ml) were served at 5 ± 1°C and presented monadically, with 10-min breaks between each sample. A maximum of eight samples were evaluated per 2-h session to ensure no carryover effects or intoxication of alcohol. Each sample was evaluated in triplicate by each panellist, resulting in 12 sessions. Samples were presented in a balanced, blocked and randomised presentation order. Unsalted crackers (Rakusens, Leeds, UK), green apple (Asda, Leeds, UK) and Evian mineral water (Danone, Paris, France) were provided for palate cleansing. All tests were performed over a 3-month period from April to June 2009 at room temperature in an air-conditioned room, under Northern Hemisphere daylight and in individual booths. Data were collected using FIZZ software (Biosystems, Cergy-Pontoise, France). Modified quantitative descriptive analysis (Stone and Sidel 2004) was used to profile the sensory attributes of the samples. This method uses the panellist’s own vocabulary to generate consensus attributes and definitions, which were fully discussed to remove any uncertainty of meaning. Attribute references were the samples themselves and were used in combination with attribute definitions to standardise language and minimise misunderstanding. This method was chosen to be the most appropriate as it benefits from reducing errors associated with ‘dumping’ of sensations into inappropriate attribute ratings when response alternatives are limited (Clark and Lawless 1994). Only attributes which the panel agreed on by consensus and which discriminated between the samples were used. The final set of attributes and their lexicons can be found in Table 1. A continuous, unstructured line scale was used for each attribute. Marks were converted to a score of 10 for data analysis purposes. All scales were study-specific and labelled with verbal ‘anchors’ for scale ends, which were discussed and agreed upon by the panel. Practice rating sessions were carried out until the panel could repeatedly quantify between samples for generated attributes. Panel performance during these sessions was monitored by analysing the coefficient of variance (CV) between replicates.
Data Analysis
Repeatability and discrimination ability of the panel were monitored by assessment of replicate scores. A repeatability index was calculated by FIZZ sensory software (Biosystems, Cergy-Pontoise, France) using CV subjected to analysis of variance (ANOVA) Two-factor (judge and product) ANOVA with interaction was conducted for each attribute to identify significant differences between the samples for each of the attributes assessed. Where appropriate, Tukey’s honestly significant difference multiple comparison tests were used to determine where samples and attributes were significantly different (α = 0.05). Predictive polynomial models from panel means were generated using Design Expert to explain variations in perception of each attribute as a function of sweetener, hop acids, ethanol and carbonation levels. Non-significant terms, as determined by ANOVA, were removed. After examination of model statistics (R 2, adjusted R 2 [Adj R 2], predicted R 2 [Pred R 2] and adequate precision [Adeq Precision]), a mathematical model was selected which best represented the data (Table 3). R 2 is a measure of the amount of variation about the mean explained by the model; a value close to 1 shows little variation. The Adj R 2 should be close to the R 2 value to signify that there are only terms in the model which add value. Pred R 2 explains variation in the model and should be close to the adjusted R 2 values if there is little variation. Adeq Precision measures signal to noise ratio, a value >4 indicates adequate model discrimination. The predictive ability of the models was validated by the evaluation of a separate set of 10 samples (validation set), which were taken from within the design space, representing the full range of compositional factors, but were not part of the original model data set. Interaction plots generated by the predictive models were used to visualise key interactions between the design factors. These are not plots of the data points themselves but instead they give a visual representation of the predictive model and are considered more illustrative than the predictive model equations.
Results
The aim of this research was to investigate the perceptual interactions of the major flavour components in beer: sweetness, bitterness, alcohol and carbonation. Panellists developed their own attribute lexicons as described in Table 1 encompassing taste, mouthfeel and flavour. One-way ANOVA performed on CV data and assessment of probability values demonstrated that the panel were able to repeatedly discriminate between samples for all attributes (p < 0.05) except sweaty/cheesy aroma, floral aroma, sweaty/cheesy flavour and floral flavour. The non-discriminating attributes will not be discussed further. Consequently, the increases in volatile release caused by ethanol and carbonation as previously found in our laboratory (Clark et al. 2011) do not appear to result in perceivable differences in these samples. Viscosity was also a non-discriminating attribute, which is not surprising as it is very difficult for the human palate to significantly discriminate between Newtonian fluids (i.e. lager beer) within such a narrow range (Ragot et al. 1989). Furthermore, CO2 adds a level of complexity to this measurement when it is made in the mouth, increasing turbulence which will impact on sheering stresses and consequently sensory assessment of beer viscosity (Ragot et al. 1989). As a result, the attribute ‘viscosity’ was rejected as a term by the beer flavour wheel discussion group (Langstaff and Lewis 1993).
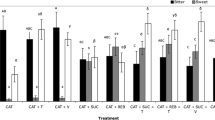
The panel means, standard deviations (SD) and results of Tukey’s multiple comparison analysis are shown in Table 2. The Tukey’s test indicates that samples could be split into between 7 and 16 groups (Table 2), indicating a good level of discrimination between the samples across the attributes. ANOVA (judge and product factors) were performed on the panel mean data (three replicates). Using the global mean of the panellists, polynomial predictive models were generated using multiple linear regression (Design Expert, Stat-Ease Inc., Minneapolis, MN, USA), which described the perceptual results in terms of the design factors (sweetener, hop acids, carbonation and ethanol) for each attribute assessed. The resulting model equations, along with associated statistics describing the model fit (adequate precision) and predictive capability (Adj and Pred R 2 values) can be found in Table 3. The independent set of validation samples showed good agreement with model data. Average differences between values predicted by the model and actual values from the validation set for each attribute and across all 10 samples were <0.6 points on the sensory scale. There was excellent correlation between the experimental mean panel sensory intensity values and predicated values generated by the models for all attributes (R 2 < 0.92). Figure 2 shows an example of this correlation for the sweetness attribute (R 2 = 0.96). The validation sample set (closed squares) have been overlaid onto the predicted versus actual correlation in Fig. 2 and is typical of the pattern for the other attributes.
Mouthfeel Attributes
The attribute ‘carbonation’ relates to the presence of bubbles in the mouth, activated by the mechanoreceptors and predictably driven by increasing CO2 level. As indicated in the interaction plot in Fig. 3, hop acids interacted with CO2 to increase perception of carbonation at the low CO2 level; this effect was not found to be significant at high CO2 levels. Tingly was used by the panel to describe the painful, chemogenic response from the conversion of carbon dioxide to carbonic acid, and as a result, CO2 was the main driver of tingly perception. As with carbonation perception, hop acids interacted with CO2 to increase tingly perception at low CO2 levels. Mean panel results for tingly and carbonation attributes were significantly correlated (p < 0.01), suggesting that even though the attributes are describing a different action of CO2 on the trigeminal system, they are unsurprisingly related. This was also found in other studies where attributes such as sting, tingly, fizziness, bubble size and total CO2 were significantly correlated (Langstaff et al. 1991; Hewson et al. 2009).
Astringency perception was driven by hop acids. However, it is unlikely that the hop acid products used contained any active astringent ingredients, such as polyphenols, as the fractionation process used to create the hop acids leaves the polyphenols with the spent hops (O’Rourke 2003). It is possible that the panel confused astringency with bitterness or that the two attributes are closely related, as suggested by a significant Pearson’s correlation coefficient that reveal the mean between bitterness intensity and astringency (p < 0.01). A significant interaction between CO2 and hop acids exists and indicates a positive effect of CO2 on astringency perception at 0 μl/L hop acids and a decrease in astringency perception due to CO2 at 600 μl/L. However, the effect of CO2 on astringency perception was very small, <1 unit on the sensory scale (1–10).
Warming describes the mouthfeel of ethanol, whilst alcohol flavour was a separate attribute used to discriminate between the samples for the flavour of ethanol. It is likely that the panel were unable to decouple the flavour and trigeminal components of ethanol, resulting in significant correlation (p < 0.01) and generation of similar predictive models. As a result, the two attributes will be discussed together hereafter. Warming/alcohol perception was primarily driven by ethanol addition in a concentration-dependent manner. Hop acids interacted with ethanol to suppress warming perception at 4.5% (ethanol) but contributed slightly when no ethanol was in the system (0%). CO2 also interacted with ethanol to modify warming perception. At low levels of ethanol, CO2 contributed to warming perception but detracted from it at high levels (Fig. 4). The addition of the sweetener brought about a small, but albeit significant (p < 0.001) increase.
Taste and Flavour Attributes
Not surprisingly, sweetness was driven by the sweetener, increasing linearly with sweetener concentration. Ethanol also linearly increased sweetness perception with the greatest effect at 4.5% ethanol. This result supports other work on a range of different sweeteners which found an increased sweetness perception with 10% ethanol addition (Hoopman et al. 1993) and also with sucrose at ethanol concentrations of 4%, 8%, 12% and 24% compared to other tastants in water (Martin and Pangborn 1970) and presumably relates to the gustatory response of ethanol. Hop acids significantly suppressed sweetness perception exponentially with the decrease reaching a plateau at ∼300 μl/L hop acids. An interesting interaction with CO2 reveals that carbonation significantly reduced sweetness perception (Fig. 5), which is in agreement with previous studies (McLellan et al. 1984; Lederer et al. 1991; Cowart 1998; Hewson et al. 2009), but in conflict with others studies who reported no such effect (Cometto-Muniz et al. 1987; Yau et al. 1989; Prescott et al. 2004).
Bitterness was driven by hop acids as expected, whilst addition of the sweetener reduced bitter perception, which is likely to be due to mixture suppression (Walters 1996). CO2 interacted with hop acids to significantly reduce bitter perception at high levels but contribute bitter perception at low levels (up to ∼150 μl/L or ∼20 IBU) (Fig. 6). The ‘double opposite’ effect of CO2 on bitterness perception has been previously found with quinine sulphate (Cometto-Muniz et al. 1987). Whilst the contributory effect of CO2 on bitterness perception was relatively small, it is of significance to brewers as the bitterness level of most lagers falls within this range. The suppression effect began at ∼300 μl/L and was most significant between 450 and 600 μl/L. Ethanol was a significant model term which interacted with hop acids in the predictive model. However, closer examination of data points shows that ethanol does not significantly modify the bitter perception elicited by hop acids. This highlights the fact that predictive models should be used with caution and raw data must be scrutinised before conclusions are drawn.
Complexity is a term which is commonly used to describe wine (Meillon et al. 2010). In this study, it was an all encompassing term used to describe the balance of flavour and mouthfeel attributes. Complexity of flavour was the only attribute which was significantly increased by all design factors. Ethanol was the main driver, followed by hop acids, carbonation and, to a lesser extent, the sweetener. An interaction between ethanol and hop acids shows that hop acids have a more pronounced effect on complexity when ethanol is not present. These results indicate the importance of ethanol on perceived complexity in beer which may result from the multiple receptors it stimulates and the associated complex transduction pathways.
Discussion
Attributes were similar to those previously used to describe alcoholic and soft beverages (Lyman and Green 1990; Keast and Breslin 2003; Kappes et al. 2006; Bajec and Pickering 2008; Hewson et al. 2009) and some are detailed on the beer and whisky flavour wheels (Meilgaard et al. 1979; Shortreed et al. 1979). Complexity is a term which is frequently used to describe wine (Meillon et al. 2010) and is said to encompass eight sensory dimensions: familiarity, homogeneity, harmony, balance, the number of perceived aromas, the ability to indentify sensations and the strength and persistence of flavour perception (Medel et al. 2009).
Mouthfeel Attributes
Figure 3 illustrates the interaction between hop acids and CO2 for carbonation perception. Modelling of tingly data produced a similar interaction plot (not shown). It seems that increasing hop acid concentration is capable of increasing both mechanical and nociceptive response at the low CO2 level, an effect which is not seen at high CO2 level. Informal discussions with the panel revealed that carbonated samples with high hop acid content seemed to have a larger quantity of smaller ‘more tingly’ bubbles than those without. It is possible that the hop acids interact with the CO2 to create an increased number of smaller bubbles, increasing surface area and filling the mouth, resulting in increased carbonation perception and activating more nociceptors providing an increased tingly response. This seems possible as the isomerised hop acid products used to elicit bitterness could alter surface tension (Briggs et al. 2004). It is likely that this effect is not seen at the high CO2 level because of CO2 saturation of both mechanoreceptors and nociceptors, thus any increased effect of the hop acids is not perceivable. With hindsight, it would have been beneficial to include bubble size as an attribute in this sensory profile in order to investigate this mechanism further.
Hop acids were the main driver of astringency perception. Considerable time was spent during training to ensure that the panel could differentiate between bitterness and astringency. However, this attribute has been described as a ‘complex, multifaceted sensation’ (Bajec and Pickering 2008) and assessment is made difficult by a number of variables. Bajec and Pickering (2008) concluded in their review paper that astringency is sensed by both taste and tactile sensations, suggesting physiological and psychological mechanisms underlying its perception. It is not surprising, therefore, that, in the absence of the main tactile trigger of astringency in beer (polyphenols), the bitter taste component would contribute to this attribute and supports the theory that astringency can be sensed by the taste receptors (Bajec and Pickering 2008).
Ethanol stimulates multiple modalities: the gustatory, olfactory and trigeminal systems (Mattes and DiMeglio 2001), resulting in polymodal sensation. Activation of ethanol by the trigeminal system has been found to be (in part) due to the vanilloid receptor-1 (VR1) (Brasser et al. 2010), which is the nociceptor responsible for the burning sensation elicited by capsaicin and a wide variety of mechanical, thermal and physical chemical stimuli. It is feasible that this is also the receptor responsible for detecting the warming perception of ethanol. However, the origin of warming perception is currently unknown and could be a result of a non-capsaicin-sensitive pathway (Green 1991; Brasser et al. 2010).
An interaction between ethanol with CO2 would suggest competition between the trigeminal aspects of both stimuli thus suppressing warming perception (Fig. 4). The contributory effect of CO2 to warming perception at 0% ethanol levels is supportive of this hypothesis. Research focussing on cross-desensitization with capsaicin on ethanol (30%) found a significant decrease in perceived irritation and intensity of ‘burning’, ‘stinging’ and ‘prickling’ sensations of ethanol after treatment of the tongue with capsaicin (Green 1991), suggesting a suppressive effect when two trigeminal stimuli are presented sequentially. However, further research is needed to determine if this effect is seen when the two stimuli are presented simultaneously.
A minor interaction of hop acids with ethanol suppressed warming perception in a concentration-dependent manner, with the greatest effect seen at the highest level of hop acid addition eliciting a high level of bitterness to the system. Lim and Green (2007) investigated the relationship between bitter taste and burning sensations and found that bitterness was qualitatively similar to ‘burning’ despite being mediated by different sensory modalities. The interaction found in the present study could be due to this similarity.
Complexity
The significant impact of all four factors on complexity perception illustrates the importance of each in beer flavour perception and also the complex nature of each in a beverage. The ability of ethanol to impact on an array of stimuli means that it is not surprising that it is the main driver of complexity and supports other studies which have also linked increased complexity due to ethanol with liking (Meillon et al. 2010). Carbonation adds a further level of complexity which is a vital characteristic of beer and has been found to increase thirst-quenching character and drinkability (Guinard et al. 1998). Additional work could focus on the contribution of each stimulus on complexity and liking.
Taste Attributes
From current knowledge regarding sweet–bitter taste interactions, it is not surprising that hop acids suppressed sweetness perception. Multiple mechanisms have been proposed for this interaction, with the more recent studies narrowing the locus to the gustatory periphery as opposed to higher central neural processing due to similarities between taste transduction mechanisms (Walters 1996; Margolskee 2002). A large body of research exists on bitter–sweet taste interactions with almost exclusive use of quinine as the bitter stimulus. In one such study, quinine was reported to directly inhibit the sweet taste transduction cation channel, TRPM5 (Talavera et al. 2008). Whilst sweet perception is not exclusively TRPM5-dependent, this work suggests that other bitter compounds may affect the perception of the sweet taste by altering TRPM5 function and significantly suppressing sweet perception, as suggested by the results in the present study. However, research of the pathways responsible for hop acid transduction is required before conclusions about the mechanism of this interaction can be made. Furthermore, the above mechanism does not support the suppression of bitter perception with sweetener addition because bitter signal detection and processing was found to be unaltered by four different sweeteners: sucrose, fructose, saccharin and SC-45647 (Talavera et al. 2008). Due to the focus of the gustatory periphery on sweet and bitter interactions, it is possible that the mechanism of bitter–sweet suppression is a result of gustatory integration. Further investigation into both TRPM5-dependent and TRPM5-independent sweet and bitter transduction pathways are required to determine the source of the interaction.
The additive effect of ethanol to sweetness perception has been previously reported (Martin and Pangborn 1970) and ethanol itself has been described as sweet (Wilson et al. 1973). The mechanism for this seems to be a taste–taste interaction as ethanol has been found to stimulate sweet-best fibres in the rhesus monkey (Hellekant et al. 1997) and neuronal response to ethanol was similar to that evoked by sucrose in rats (Lemon et al. 2004). This mechanism has also been extended to humans where ethanol has been suggested to activate some nerve fibres sensitive to sugar (Scinska et al. 2000), which could explain the source of the interaction as found in this study.
The effect of CO2 on taste perception is interesting. CO2 is able to significantly decrease the sweetness of dextrose and modify the bitterness of hop acids. Whether the mechanism of these interactions is peripheral or as a result of higher central processing requires further investigation. However, similarities can be drawn from the study of capsaicin as different classes of oral irritants have been suggested to be mediated partly by a common population of capsaicin-sensitive fibres (Carstens et al. 1998). Physiological interactions between oral irritation by capsaicin and various tastants have been investigated in electrophysiological experiments with rats and also in human sensory studies (Lawless and Stevens 1984; Simons et al. 2003). Results found significant suppression of capsaicin on taste, which is in agreement with the present study using CO2. Gustatory neuronal stimulation recordings in the nucleus tractus solitarius of rats before and after capsaicin application provide strong evidence that the mechanism of this suppression is peripheral, acting directly on the taste receptor to alter gustatory response (Simons et al. 2003). However, this does not explain the contributory effect of CO2 on bitterness perception when no bitterness is in the system or at low levels of hop acid addition. Green and Hayes (2003) investigated the relationship between bitter taste and chemesthesis using capsaicin and suggested two mechanisms: (1) capsaicin may stimulate the taste neurons which express VR1 and thus stimulate bitterness or (2) capsaicin may stimulate one or more members of the heterogeneous family of T2Rs that encode for bitter taste. Conversely, CO2 is also mediated by non-capsaicin-sensitive pathways (Carstens et al. 1998), which may interact with gustatory stimuli via centrally mediated integration. The effect of CO2 on taste perception requires further study as a comprehensive understanding is important for many food and beverage industries.
Conclusion
The interaction between CO2 at low levels and hop acids on the enhancement of carbonation and tingly perception is interesting and requires further study especially at a time when the brewing industry is moving towards reducing CO2 levels in beers. It is possible that, with the right combination of ingredients, CO2 levels could be reduced without significant effect on carbonation and tingly perception. CO2 interacted with the other variable components in the system (ethanol, sweetener and hop acids) to suppress the perception of warming, sweetness and bitterness attributes, respectively, at the higher end of component concentration but contributed to perception at the lower end, showing a double and opposite effect. Ethanol was the main driver of complexity, which illustrates the importance of its presence in beer. This also supports the knowledge that ethanol is a complex stimulus acting on different receptors and capable of modifying flavour perception as well as aroma partitioning during consumption. Hop acids were found to act in a similar way to the much researched quinine on the suppression of sweetness perception, suggesting that hop acids can also act directly on the gustatory periphery to inhibit the sweet taste transduction pathway. Results support previously inconclusive evidence that CO2 addition suppresses sweetness perception. The mechanism of this is unknown and could be the result of suppression at the periphery or integration of higher central processing, and the next stage of this research is to use human functional magnetic resonance imaging techniques to investigate this in vivo.
References
Aznar M, Tsachaki M, Linforth RST et al (2004) Headspace analysis of volatile organic compounds from ethanolic systems by direct APCI-MS. Intl J Mass Spec 239(1):17–25
Bajec MR, Pickering GJ (2008) Astringency: mechanisms and perception. Crit Rev Food Sci Nutr 48(9):858–875
Brasser SM, Norman MB, Lemon CH (2010) T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Phys Gen 41(3):232–243
Briggs DE, Boulton CA, Brookes PA et al (2004) Brewing science and practice. Woodhead, Cambridge
Carstens E, Kuenzler N, Handwerker HO (1998) Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol 80(2):465–492
Chandrashekar J, Yarmolinsky D, von Buchholtz L et al (2009) The taste of carbonation. Science 326(5951):443–445
Clark CC, Lawless HT (1994) Limiting response alternatives in time-intensity scaling—an examination of the halo-dumping effect. Chem Senses 19(6):583–594
Clark RA, Linforth R, Bealin-Kelly F et al (2011) Effects of ethanol, carbonation and hop acids on volatile delivery in a model beer system. J Inst Brew 117(1) (in press)
Cometto-Muniz JE, Abraham MH (2008) Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pham Biochem Behav 89(3):279–291
Cometto-Muniz JE, Garcia-Medina MR, Calvino AM et al (1987) Interactions between CO2 oral pungency and taste. Perception 16(5):629–640
Cowart BJ (1998) The addition of CO2 to traditional taste solutions alters taste quality. Chem Senses 23(4):397–402
Dessirier JM, Simons CT, Carstens MI et al (2000) Psychophysical and neurobiological evidence that the oral sensation elicited by carbonated water is of chemogenic origin. Chem Senses 25(3):277–284
Ellingson J, Silbaugh B, Brasser S (2009) Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Gen 39(1):62–72
Fontoin H, Saucier C, Teissedre PL et al (2008) Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual Prefer 19(3):286–291
Friel EN, Linforth RST, Taylor AJ (2000) An empirical model to predict the headspace concentration of volatile compounds above solutions containing sucrose. Food Chem 71(3):309–317
Goldner MC, Zamora MC, Di Leo LP et al (2009) Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J Sens Stud 24(2):243–257
Green BG (1988) Spatial and temporal factors in the perception of ethanol irritation on the tongue. Percept Psychophys 44(2):108–116
Green BG (1991) Capsaicin cross-desensitization on the tongue-psychophysical evidence that oral chemical irritation is mediated by more than one sensory pathway. Chem Senses 16(6):675–689
Green BG (1992) The effects of temperature and concentration on the perceived intensity and quality of carbonation. Chem Senses 17(4):435–450
Green BG, Hayes JE (2003) Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol Behav 79(4–5):811–821
Guinard JX, Souchard A, Picot M et al (1998) Sensory determinants of the thirst-quenching character of beer. Appetite 31(1):101–115
Hellekant G, Danilova V, Roberts T et al (1997) The taste of ethanol in a primate model. 1. Chorda tympani nerve response in Macaca mulatta. Alcohol 14(5):473–484
Hewson L (2007) Multimodal interactions in a carbonated beverage system. The University of Nottingham, Nottingham. PhD Thesis
Hewson L, Hollowood T, Chandra S et al (2008) Taste-aroma interactions in a citrus flavoured model beverage system: similarities and differences between acid and sugar type. Food Qual Prefer 19(3):323–334
Hewson L, Hollowood T, Chandra S et al (2009) Gustatory, olfactory and trigeminal interactions in a model carbonated beverage. Chem Percept 2(2):94–107
Hoopman T, Birch G, Serghat S et al (1993) Solute-solvent interactions and the sweet taste of small carbohydrates. 2. Sweetness intensity and persistence in ethanol water mixtures. Food Chem 46(2):147–153
Jones PR, Gawel R, Francis IL et al (2008) The influence of interactions between major white wine components on the aroma, flavour and texture of model white wine. Food Qual Prefer 19(6):596–607
Kappes SM, Schmidt SI, Lee SY (2006) Descriptive analysis of cola and lemon/lime carbonated beverages. J Food Sci 71(8):S583–S589
Keast SJR, Breslin PAS (2003) An overview of binary taste–taste interactions. Food Qual Prefer 14(2):111–124
Kiefer SW, Morrow NS (1991) Odor cue mediation of alcohol aversion learning in rats lacking gustatory Neocortex. Behav Neurosci 105(1):25–32
Langstaff SA, Lewis MJ (1993) The mouthfeel of beer—a review. J Inst Brew 99:31–37
Langstaff SA, Guinard JX, Lewis MJ (1991) Sensory evaluation of the mouthfeel of beer. Am Soc Brew Chem 49(2):54–59
Lawless H, Stevens DA (1984) Effects of oral chemical irritation on taste. Physiol Behav 32(6):995–998
Lederer CL, Bodyfelt FW, McDaniel MR (1991) The effect of carbonation level on the sensory properties of flavored milk beverages. J Dairy Sci 74(7):2100–2108
Lemon CH, Brasser SM, Smith DV (2004) Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol 92(1):536–544
Lim JY, Green BG (2007) The psychophysical relationship between bitter taste and burning sensation: evidence of qualitative similarity. Chem Senses 32(1):31–39
Lyman BJ, Green BG (1990) Oral astringency: effects of repeated exposure and interactions with sweeteners. Chem Senses 15(2):151–164
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277(1):1–4
Martin S, Pangborn RM (1970) Taste interaction of ethyl alcohol with sweet, salty, sour and bitter compounds. J Sci Food Agric 21(12):653
Mattes RD, DiMeglio D (2001) Ethanol perception and ingestion. Physiol Behav 72(1–2):217–229
McEvoy S (1998) Sensory evaluation of carbonated beverages utilizing a hyperbaric chamber or what would soda taste like if you could get inside the can? Chemical senses day XIV abstracts, Santa Rosa, CA
McLellan MR, Barnard J, Queale DT (1984) Sensory analysis of carbonated apple juice using response-surface methodology. J Food Sci 49(6):1595–1597
Medel M, Viala D, Meillon S et al (2009) A questionnaire for assessing the perceived complexity of wine: application to the study of the effect of expertise on perception of wine complexity. 8th Pangborn Sensory Science Symposium. Elsevier, Florence
Meilgaard MC (1982) Prediction of flavor differences between beers from their chemical-composition. J Agric Food Chem 30(6):1009–1017
Meilgaard MC, Dalgliesh CE, Clapperton JF (1979) Beer flavor terminology. J Inst Brew 85(1):38–42
Meillon S, Viala D, Medel M et al (2010) Impact of partial alcohol reduction in Syrah wine on perceived complexity and temporality of sensations and link with preference. Food Qual Prefer 21(7):732–740
Mintel Report (November 2009) Lager, UK
O’Rourke T (2002) Malt specifications & brewing performance. Brewer Int Tech Summ 2(10):27–30
O’Rourke T (2003) Hop and hop products. Brewer Int Tech Summ 3(1):21–25
Pozo-Bayon MA, Santos M, Martin-Alvarez PJ et al (2009) Influence of carbonation on aroma release from liquid systems using an artificial throat and a proton transfer reaction-mass spectrometric technique (PTR-MS). Flav Frag J 24(5):226–233
Prescott J, Swain-Campbell N (2000) Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem Senses 25(3):239–246
Prescott J, Soo J, Campbell H et al (2004) Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol Behav 82(2–3):459–469
Ragot F, Guinard JX, Shoemaker CF et al (1989) The contribution of dextrins to beer sensory properties. Part I. Mouthfeel. J Inst Brew 95(6):427–430
Saint-Eve A, Deleris I, Aubin E et al (2009) Influence of composition (CO2 and sugar) on aroma release and perception of mint-flavored carbonated beverages. J Agric Food Chem 57(13):5891–5898
Scinska A, Koros E, Habrat B et al (2000) Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend 60(2):199–206
Shortreed GW, Rickards P, Swan JS et al (1979) The flavour terminology of Scotch whisky. Brewers’ Guardian 11:55–62
Simons CT, Dessirier JM, Carstens MI et al (1999) Neurobiological and psychophysical mechanisms underlying the oral sensation produced by carbonated water (vol 19, pg 8134, 1999). J Neurosci 19(22):10191–10191
Simons CT, Boucher Y, Carstens E (2003) Suppression of central taste transmission by oral capsaicin. J Neurosci 23(3):978–985
Smith JS, Hui YH (2004) Food processing: principles and applications. Blackwell, Oxford
Stone H, Sidel JL (2004) Sensory evaluation practices. Elsevier Academic Press, California
Talavera K, Yasumatsu K, Yoshida R et al (2008) The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB J 22(5):1343–1355
Trevisani M, Smart D, Gunthorpe MJ et al (2002) Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 5(6):546–551
Verhagen JV, Engelen L (2006) The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev 30(5):613–650
Walters ED (1996) How are bitter and sweet tastes related? Trends Food Sci Technol 7(12):399–403
Wilson CWM, Obrien C, Macairt JG (1973) Effect of metronidazole on human taste threshold to alcohol. Brit J Add 68(2):99–110
Yau NJN, McDaniel MR, Bodyfelt FW (1989) Sensory evaluation of sweetened flavored carbonated milk beverages. J Dairy Sci 72(2):367–377
Acknowledgements
This work was carried out with the financial support from BBSRC and SABMiller.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, R.A., Hewson, L., Bealin-Kelly, F. et al. The Interactions of CO2, Ethanol, Hop Acids and Sweetener on Flavour Perception in a Model Beer. Chem. Percept. 4, 42–54 (2011). https://doi.org/10.1007/s12078-011-9087-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-011-9087-3