Abstract
Background and aims
Metabolic dysfunction-associated fatty liver disease (MAFLD) establishes new criteria for diagnosis of fatty liver disease independent of alcohol intake. We aimed to describe the prevalence and compare characteristics and mortality outcomes of persons with nonobese and obese MAFLD.
Methods
Using data from 13,640 participants from the third National Health and Nutrition Examination Survey (NHANES III) 1988–1994, we identified participants with fatty liver on ultrasound who had MAFLD and analyzed them by the presence of obesity.
Results
Overall prevalence of MAFLD was 19%; amongst those, 54% were obese and 46% were nonobese. Nonobese MAFLD was more common in participants older than 65 than in younger participants (56.8% vs. 43.2%, p < 0.0001). Nonobese MAFLD was more common in males (63.2% vs. 48.3%, p < 0.0001). Obese MAFLD was more common in females (51.7% vs. 48.3%, p < 0.0001). After adjusting for several demographic factors and alcohol use, older age [adjusted odds ratio (aOR) 1.02, 95% CI 1.00–1.02, p = 0.003] and being male (aOR: 1.65, 95% CI 1.25–2.17, p = 0.001) were independent risk factors for nonobese MAFLD. Nonobese MAFLD participants had a higher 20-year cumulative incidence for all-cause mortality compared to obese MAFLD participants (33.2% vs. 28.8%, p = 0.0137). However, nonobese MAFLD was not independently associated with mortality after adjusting for relevant confounders, while FIB-4 > 1.3 and cardiovascular disease were the strongest risk factors associated with increased mortality [adjusted hazard ratio (aHR) > 2.7 for both, p < 0.0001 for both].
Conclusions
Nonobese MAFLD constitutes about half of the MAFLD in the United States, especially among males and the elderly. Notably, nonobese MAFLD carries higher mortality than obese MAFLD. Screening and diagnosis of MAFLD should be considered in nonobese populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) was recently proposed in place of non-alcoholic fatty liver disease (NAFLD), which is defined as the presence of significant hepatic steatosis in the absence of significant alcohol consumption and alternate causes of hepatic steatosis such as viral hepatitis or other chronic liver diseases and a leading cause of liver disease globally and in the US [1,2,3,4]. As fatty liver disease unrelated to alcohol is associated with type 2 diabetes, obesity, hyperlipidemia, and hypertension [1,2,3,4], it has been referred to as the liver manifestation of metabolic syndrome (MetS) [1]. Although there is a consistent and strong relationship between increasing body mass index (BMI) and the risk for metabolic fatty liver, it is important to acknowledge that NAFLD is found in nonobese individuals [4,5,6].
Given the intricate relationship between fatty liver that is not primarily due to alcohol and metabolic diseases, in March 2020, an expert opinion paper proposed changing the name of this fatty liver disease from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) [7], as evidence has shown that MAFLD would better represent this liver disease as a hepatic manifestation of metabolic syndrome. Currently, the established criteria for NAFLD diagnosis exclude those with significant alcohol use, which many feel does not capture the full spectrum of the disease since some could have both [7]. The proposed term “MAFLD” would represent a multisystem disorder that warrants a positive diagnosis instead of a “non”-disease rubric, as currently used by NAFLD [7]. Recent professional society guideline has also been proposed for MAFLD [8].
As with NAFLD, previous studies have reported the risk factors of MAFLD to include body composition [9, 10]. However, while nonobese NAFLD has been well recognized, little is known about the distinction between those with obese and nonobese MAFLD especially in the multiethnic US population. Therefore, we aim to investigate the prevalence, clinical characteristics, and mortality outcomes and associated factors of obese and nonobese individuals with MAFLD using the population-based NHANES (National Health and Nutrition Examination Survey) data.
Patients and methods
Study population and study design
The NHANES is conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) in 2-year cycles in the US. This is a retrospective study of a population-based sample of people with MAFLD using NHANES III, the survey conducted from 1988 to 1994. NHANES III is a comprehensive dataset that uses a stratified, clustered, and multistage probability sample design to obtain a representative sample of the noninstitutionalized civilian US population [11]. Answers to the questionnaires, such as medical history and demographics are self-reported. NHANES is designed to monitor health and nutritional status in the US through the collection of demographic, dietary, physical examination, laboratory and questionnaire data from adults and children [11]. Hepatic ultrasound was also performed in NHANES III. Therefore, we elected to use NHANES III to utilize ultrasound to define fatty liver, as there is no validated blood test-based biomarker for MAFLD. In addition, as NHANES III was conducted between 1988 and 1994, we were able to robust long-term mortality outcome for the cohort. All participants gave written informed consent.
Inclusion/exclusion criteria
We included participants aged 20 years and older with a complete set of relevant laboratory data and physical measurements to allow for an accurate diagnosis of MAFLD as detailed below. We excluded participants with missing ultrasound data. We also excluded patients if they were pregnant at the time of examination due to different waist circumference and BMI measurements caused by pregnancy.
Definition of hepatic steatosis
Hepatic steatosis was determined in NHANES III participants using the Hepatic Steatosis Ultrasound Examination (HSUE). Adults underwent hepatic ultrasound at a mobile examination center using a Toshiba Sonolayer SSA-90A ultrasound machine (Toshiba America Medical Systems, Inc., Tustin, California) [12]. Board-certified radiologists used five different parameters to assess hepatic steatosis: parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition [13]. The ultrasonographic assessments were reported as normal, mild, moderate, or severe hepatic steatosis. Abiding by quality control procedures, reliability results (intra-rater and inter-rater) were calculated. The intra-rater reliability was found to be 91.3% (kappa 0.77) and the inter-rater reliability was found to be 88.7% (kappa 0.70) [13].
Definition of MAFLD
MAFLD was defined as the presence of hepatic steatosis by liver ultrasound plus the presence of one of the following conditions: (1) overweight (BMI ≥ 25 and < 30 kg/m2, or obese (BMI ≥ 30 kg/m2), (2) presence of type 2 diabetes mellitus (T2DM), or (3) at least two metabolic risk abnormalities [7]. Metabolic risk abnormalities consisted of: (1) waist circumference ≥ 102 cm in male and ≥ 88 cm female, (2) blood pressure ≥ 130/85 mmHg or specific drug treatment, (3) plasma triglycerides ≥ 150 mg/dl (≥ 1.70 mmol/L) or specific drug treatment, (4) plasma HDL-cholesterol < 40 mg/dl (< 1.0 mmol/L) for male and < 50 mg/dl (< 1.3 mmol/L) for female or specific drug treatment, (5) prediabetes (fasting glucose levels 100–125 mg/dl [5.6 to 6.9 mmol/L] or hemoglobin A1c [HbA1c[ 5.7–6.4% [39 to 47 mmol/mol]), (6) homeostasis model assessment of insulin resistance (HOMA-IR) score ≥ 2.5, (7) and/or plasma high-sensitivity C-reactive protein level > 2 mg/L [7].
Other definitions and measurements
We defined obese participants as those with BMI ≥ 30 kg/m2. Nonobese participants were defined as having a BMI under 30 kg/m2 and includes normal-weight (BMI ≤ 25 kg/m2) and overweight (25 ≤ BMI < 30 kg/m2). T2DM is defined as fasting plasma glucose levels ≥ 126 mg/dl or HbA1c ≥ 6.5%. Advanced fibrosis was defined as Fibrosis-4 index (FIB-4) > 2.67 [14, 15]
General demographic and socioeconomic characteristics such as age, sex, race and ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other [Asian was not oversampled by NHANES until 2012]), birthplace (US-born, foreign-born), military service, marital status (single, married, divorced/separated, other/widowed), education level (≤ high school, > high school), poverty income ratio (< 1.0), and insurance (public, private) were obtained. All these variables were self-reported as per the design of NHANES. Comorbidities such as stroke, heart attack, and congestive heart failure were all self-reported by participants. Excessive alcohol intake was defined as having > 2 drinks/day for male or > 1 drink/day for female.
Physical and blood measurements including BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), albumin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, plasma fasting glucose, creatinine, bilirubin, and homeostatic model assessment of insulin resistance (HOMA-IR) were obtained from the laboratory tests obtained by highly trained medical personnel using a standard protocol.
Mortality follow-up
The NHANES data are linked to death records from the National Death Index. Vital status was ascertained through probabilistic matching and death certificate review. International Classification of Diseases, Ninth Revision (ICD-9) was used to define the cause of death prior to 1999 and ICD-10 codes for deaths from 1999 to 2015. The follow-up period for each study participant was the length of time between the NHANES III baseline examination date and the participant’s death date or last date of follow-up (December 31, 2015), whichever came first.
Statistical analyses
Weighted analyses were performed using NHANES survey weights, which account for the survey design, survey nonresponse, post-stratification, and oversampling [16]. We used weights in our statistical analysis so that the estimates are representative of the US civilian, noninstitutionalized population.
Descriptive statistics were reported as a weighted proportion (%) for categorical variables and mean ± standard deviation for continuous variables. Chi-square tests were performed to compare the distribution of categorical variables such as sex, race and ethnicity, education level, and comorbidities among MAFLD patients with and without obesity. The Student t-tests were performed to compare the distribution of continuous variables such as age, blood biochemical lab data, and physical measurements.
We calculated the prevalence of nonobese MAFLD among the overall cohort of patients with MAFLD as well as in relevant subgroups such as by age, sex, and FIB-4 categories. We also performed univariable and multivariable logistic regression to determine factors associated with nonobese MAFLD. We reported the univariate and multivariate odd ratios (OR) as 95% confidence intervals (CI).
Next, we estimated mortality rates in patients with MAFLD overall and in subgroups using the Kaplan–Meier methods. We compared mortality between groups using the log-rank test. In addition, we performed univariable and multivariable Cox proportional hazards regressions to estimate hazard ratios (HR) of all-cause mortality in obese MAFLD individuals compared to nonobese MAFLD individuals. Variables were included if there was a clinical concern for possible association with survival. These were sex, race and ethnicity, current smoking status, viral hepatitis, FIB-4 category, alcohol use, cardiovascular disease, lung disease, and non-cutaneous cancer. We tested all variables for the proportional hazards assumption by graphical comparison of the Kaplan–Meier survival curves with the Cox predicted curves for the same variable. If the predicted and observed curves were close together and did not intersect, the proportional-hazards assumption was considered to have been met [17].
The p-values for all statistical analyses were two-tailed, and statistical significance was defined with p < 0.05. We analyzed the data using Stata version 16.1 (Stata Corp., College Station, TX, USA).
Results
Study population
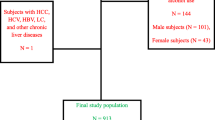
A total of 33,199 participants with sufficient data for study analysis were identified in the NHANES database between 1988 and 1994. We then excluded participants under the age of 18 (n = 13,581), patients with missing ultrasound data (n = 6557), and pregnant patients (n = 216); after which a total of 13,640 participants remained and were included in the analyses (Fig. 1).
Prevalence, clinical characteristics, and factors associated with nonobese MAFLD
Overall, the prevalence of MAFLD was 19.2% (2619/13,640). Amongst the MAFLD population, 53.8% (1410/2619) were obese and 46.2% (1209/2619) were nonobese; and among the nonobese, the majority (85%, 1027/1209) were overweight. The demographic and clinical characteristics of participants with obese MAFLD and nonobese MAFLD are summarized in Table 1A and B. Participants in the nonobese MAFLD group were older (50.1 vs. 47.5, p = 0.001), more likely to be male (63.2% vs. 48.3%, p < 0.0001), more likely to have smaller mean waist circumference (96.7 vs. 114.0 cm, p < 0.0001), lower BMI (26.8 vs. 35.9 kg/m2, p < 0.0001), lower ALT levels (24.6 vs. 27.2 U/L, p = 0.033), and lower platelet levels (268.3 vs. 281.4 × 103, p = 0.018). The obese MAFLD group had a higher prevalence of advanced fibrosis (2.2% vs. 1.5%, p = 0.0046), diabetes (20.0% vs. 12.9%, p = 0.0003), and HOMA-IR (7.1 vs. 4.3, p < 0.0001). Cardiovascular disease prevalence was similar between the obese and nonobese MAFLD group (7.8% vs. 9.0%, p = 0.38) (Table 1).
In subgroup analysis, participants older than 65 were more likely to have nonobese MAFLD vs. obese MAFLD (56.8% vs. 43.2%, p < 0.0001; Fig. 2a). By sex, nonobese MAFLD was more common in male compared to female (52.4% vs. 37.4%, p < 0.0001) while obese MAFLD was more common in female compared to male (62.6% vs. 47.6%; p < 0.0001, respectively; Fig. 2b). By race and ethnicity, there was no statistically significant difference in the distribution of obese versus nonobese MALFD with about 46% of the non-Hispanic white group having nonobese MAFLD, 39% of the non-Hispanic blacks, 47.5% among the Mexican Americans, and 50.2% among other race and ethnicity (p = 0.24; Fig. 2c).
Compared to the obese MAFLD group, nonobese MALFD participants were less likely to have advanced fibrosis (FIB-4 > 2.67) (36.2% vs. 63.8%, p = 0.0046; Fig. 3a), with similar and consistent findings among the male subgroup and the female subgroup (Fig. 3b).
In multivariable analysis (Table 2A), factors independently associated with a higher risk of nonobese MAFLD (as compared to obese MAFLD) were older age (aHR: 1.02, 95% CI 1.00–1.02, p = 0.003), being male (aHR: 1.65, 95% CI 1.25–2.17, p = 0.001), and being widowed/separated (aHR: 1.66, 95% CI 1.09–2.54, p = 0.015).
Long-term mortality in obese versus nonobese MAFLD and factors associated with mortality
We found higher 20-year cumulative all-cause mortality among participants in the nonobese MAFLD group as compared to the obese MAFLD group (log-rank test p = 0.0137) (Fig. 4a). There was no significant difference in all-cause mortality by the presence of significant alcohol use among the obese MAFLD group (log-rank p = 0.762, Fig. 4b) as well as the nonobese MAFLD population (log-rank p = 0.092, Fig. 4c). There was also no statistically significant difference in cardiovascular mortality, cancer-related mortality and other cause mortality between the obese and nonobese MAFLD (p = 0.116, p = 0.250, p = 0.117, respectively, Supplemental Figure S1).
On multivariable Cox regression analysis, we found no significant difference in the risk of all-cause mortality between the nonobese MAFLD and obese MAFLD groups (aHR: 1.11, 95% CI 0.88–1.40, p = 0.360) after adjusting for sex, race and ethnicity, smoking, viral hepatitis, alcohol use, cardiovascular diseases, lung disease, non-cutaneous cancer, and FIB-4 index (which includes age, AST, ALT, and platelet) (Table 2B).
In addition, among participants with MAFLD, cardiovascular disease, lung disease, and non-cutaneous cancer were significantly associated with over 3 times (aHR: 3.19, 95% CI 2.25–4.53, p < 0.0001), over 1.3 times (aHR: 1.38, 95% CI 1.04–1.82, p = 0.027), and 1.8 times the risk of all-cause mortality (aHR: 1.79, 95% CI 1.12–2.85, p = 0.016), respectively. FIB-4 index within the range of 1.3–2.67 and greater than 2.67 were also significantly associated with over 2.7 times (aHR 2.73, 95% CI 2.16–3.44, p < 0.001) and over 3.6 times the higher risk of mortality (aHR 3.69, 95% CI 2.37–5.74, p < 0.0001), respectively. In an alternative model without the inclusion of nonliver comorbidities such as cardiovascular diseases, lung disease, and non-cutaneous cancer (Supplemental Table S1), the finding of nonobese MAFLD not independently associated with all-cause mortality remained consistent (adjusted HR 1.07, 95% CI 0.86–1.33, p = 0.53).
Discussion
As research continues to investigate the use of MAFLD as a more encompassing term and definition to better describe what appears to be a metabolically based fatty liver disease, our study investigated the prevalence, clinical characteristics, and long-term mortality of obese and nonobese MAFLD in the multi-ethnic US population over a 6-year period from 1988 to 1994. In total, we found the overall prevalence of MAFLD was 19.2%, 53.8% were obese and 46.2% were nonobese. Those with MAFLD were more frequently male, non-Hispanic white, married, had less than a high school degree, and had private insurance. Notably, 78.4% have mild fibrosis and only 1.9% with advanced fibrosis, and with over 60% having hypertension.
We found that there were distinct differences between individuals with MAFLD who were obese and nonobese. The obese MAFLD group was younger, more frequently female, more frequently had diabetes or insulin resistance. Among the nonobese MAFLD group, 85% were overweight and 15% had normal weight. Nonobese MAFLD participants were less likely to have advanced liver fibrosis via FIB-4 criteria as compared to the obese MAFLD group. However, there was no difference in the prevalence of obese and nonobese MAFLD among the different racial and ethnic groups. These findings were highlighted in our multivariate analysis where we found that the predictors for having nonobese MAFLD included being older and being male, but not race and ethnicity.
We also examined the mortality and found that over a follow-up time of 20 years, the overall mortality rate was higher among nonobese participants as compared to obese participants (p = 0.0137). However, nonobese MAFLD was not independently associated with higher mortality as compared to obese MAFLD after adjusting for confounders. Notably, the strongest and most significant predictor of all-cause mortality was having FIB-4 > 2.67 where those with MAFLD and FIB-4 > 2.67 were almost four times more likely to die compared to those without severe fibrosis, followed by having cardiovascular disease or FIB-4 between 1.3 and 2.67 where the risk was about three times higher.
Together, these descriptions of those with MAFLD help to paint a more complete picture of patients using the MAFLD definition. While obesity is often associated with fatty liver not related to alcohol use, close to half of the participants with MAFLD in our study were not obese and major criteria of MAFLD is the presence of obesity or overweight. Our study expands the results of other studies in that we have taken the concepts of obese and nonobese from what we have learned from the study of NAFLD and applied them to those with MAFLD. Like what has been indicated in the NAFLD studies, having cardiovascular disease is one of the strongest predictors for all-cause mortality among those with MAFLD [18,19,20,21]. However, we found no difference in cardiovascular-related mortality between those with obese MAFLD and those with nonobese MAFLD which is most likely due to there being no difference in the cardiometabolic risk factors between the two groups.
It is also of interest to note, that being at high risk for advanced fibrosis may play a role in increasing the risk of mortality among those with MAFLD. Such an indicator would also be in line with what has been reported to be the most significant predictor of mortality among those with NAFLD—the presence of fibrosis as well as recently in the MAFLD population [22,23,24,25,26,27,28,29]. Therefore, we suggest that mortality is an area that will require much more in-depth study and whether the current treatment interventions (diet and exercise) have been advocated for those with NAFLD will have the same impact for those with MAFLD [29, 30].
There are some limitations to this study. NHANES III did not include an over-sampling of Asian Americans and participants with race and ethnicities other than white, black, and Hispanic, so our data may not be generalizable to the Asian American and other race and ethnicity groups. Second, this study did not use liver biopsy to determine the presence of hepatic steatosis or liver fibrosis since it is invasive, not practical, and not ethical in a large epidemiological cohort of asymptomatic patients. Instead, our study used the noninvasive FIB-4 test with cutoff of 2.67 for advanced fibrosis, which has been proven to be an accurate measure of liver fibrosis among patients [16]. Regarding outcome, our dataset did not include liver-related mortality data as access to this data is more restricted, but part of the mortality related to liver causes may be reflected in the data for other-cause mortality. Data on cirrhosis and hepatocellular carcinoma development were also not available in this community-based epidemiology cohort. Additionally, the classification of obese and nonobese was only determined at baseline while people can transition between obese to nonobese status and vice versa, so future studies should examine outcomes in people who remained nonobese throughout follow-up time. Studies inclusive of participants from more recent time are also needed since our study cohort were recruited from over 20 years ago. Finally, many responses in NHANES such as demographic data and medical conditions were self-reported.
Conclusion
Using the data from NHANES 1988–1994, we found the overall prevalence of MAFLD to be 19%, with almost half (46%) of them having nonobese MAFLD, suggesting that about half of MAFLD patients had a significant metabolic disease in the absence of obesity. Nonobese individuals with MAFLD tended to be older and male had a higher incidence of all-cause mortality at 20-year follow-up as compared to participants with obese MAFLD. Notably, among participants with MAFLD, after adjustment for alcohol, viral hepatitis, liver fibrosis, cardiovascular disease, race/ethnicities and cancer, not only participants with FIB-4 indicative of advanced fibrosis with a value > 2.67 had a higher risk for mortality but those in the indeterminate FIB-4 range (between 1.3 and 2.67) also had three times the risk of mortality compared to those with FIB < 1.3. As such, the older nonobese population with metabolic derangement should also be targeted for early screening and diagnosis of MAFLD so that appropriate lifestyle and pharmacologic interventions to curtail their mortality risk.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- aOR:
-
Adjusted odds ratio
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- FIB-4:
-
Fibrosis-4
- GGT:
-
Gamma-glutamyl transferase
- HBA1C:
-
Hemoglobin A1C
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- MetS:
-
Metabolic syndrome
- NAFLD:
-
Nonalcoholic fatty liver disease
- NHANES:
-
National Health and Nutrition Examination Survey
- T2DM:
-
Type 2 diabetes mellitus
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84
Le MH, Devaki P, Ha NB, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS ONE. 2017;12(3): e0173499
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20
Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288(1):139–151
Younoussi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012;91:319–327
Loomis AK, Kabadi S, Preiss D, et al. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record propsective studies. J Clin Endocrinal Metab. 2016;101:945–952
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209
Eslam M, Sarin SK, Wong VWS, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. https://doi.org/10.1007/s12072-020-10094-2
Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017;112:102–110
Cheng YM, Kao JH, Wang CC. The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol Int. 2022;15:405–412
NCHS. National Health and Nutrition Examination Survey (NHANES). Available at: https://wwwn.cdc.gov/nchs/nhanes/Nhanes3/Default.aspx. Accessed Aug 18, 2021).
National Health and Nutrition Examination Survey, Third National Health and Nutrition Examination Survey: Hepatic/Gallbladder Ultrasound and Hepatic Steatosis (HGUHS). https://wwwn.cdc.gov/nchs/Data/Nhanes3/34A/HGUHS.htm. Accessed Aug 23, 2021.
National Health and Nutrition Examination Survey (NHANES) III, Hepatic Steatosis Ultrasound Images Assessment Procedures Manual 2010. https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf. Accessed Aug 23, 2021.
Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325
NCHS. Key Concepts About Weighting in NHANES. Available at: https://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/OverviewKey.htm. Accessed Aug 23, 2021.
StataCorp LP. Stata survival analysis and epidemiological tables reference manual; 1985. Accessed Aug 23, 2021.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-64. https://doi.org/10.1016/j.jhep.2014.12.012
Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650
Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343: d6891
Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71(4):1213–1227. https://doi.org/10.1002/hep.30908
Alam S, Eslam M, Skm Hasan N, Anam K, Chowdhury MAB, Khan MAS, et al. Risk factors of nonalcoholic fatty liver disease in lean body mass population: a systematic review and meta-analysis. JGH Open. 2021;5(11):1236–1249. https://doi.org/10.1002/jgh3.12658
Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. 2019;37(1):65–72. https://doi.org/10.2337/cd18-0026.PMID:30705499;PMCID:PMC6336127
Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. https://doi.org/10.1016/j.jhep.2017.07.027
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. https://doi.org/10.1002/hep.29085
Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. https://doi.org/10.1002/hep.27368
Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta analysis. Gastroenterology. 2020;158(6):1611-1625.e12. https://doi.org/10.1053/j.gastro.2020.01.043
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–1291. https://doi.org/10.1016/j.jhep.2021.07.035
Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912–918. https://doi.org/10.1053/j.gastro.2020.11.051
Paik JM, Mir S, Alqahtani SA, Younossi Y, Ong JP, Younossi ZM. Dietary risks for liver mortality in NAFLD: global burden of disease data. Hepatol Commun. 2021. https://doi.org/10.1002/hep4.1707
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: MHN. Specific author contributions: Study design: ADD, VHN, MHN. Data collection: ADD, VHN. Data analysis: ADD, VHN, MHN. Drafting of the article: ADD, VHN, MHN. Data interpretation, review and/or revision of the manuscript: All authors. Study concept and study supervision: MHN.
Corresponding author
Ethics declarations
Conflict of interest
Mindie H. Nguyen: Research support: Pfizer, Enanta, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, B. K. Kee Foundation; Consulting and/or Advisory Board: Intercept, Exact Science, Gilead, GSK, Eli Lilly, Laboratory of Advanced Medicine, Janssen. Ramsey Cheung: Research support: Gilead. Allen D. Dao, Vy H. Nguyen and Takanori Ito have no relevant financial or non-financial interests to disclose.
Informed consent
All participants gave written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dao, A.D., Nguyen, V.H., Ito, T. et al. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol Int 17, 225–236 (2023). https://doi.org/10.1007/s12072-022-10436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10436-2