Abstract
Background
Variceal bleeding is a major complication of portal hypertension, which is associated with significant mortality. Moreover, patients surviving a variceal bleeding episode have very high risk of rebleeding, which is associated with mortality as high as that of the first bleed. Because of this, prevention of bleeding from gastroesophageal varices has been one of the main therapeutic goals since the advent of the first effective therapies for portal hypertension.
Aim
This review deals with the present day state-of-the-art pharmacological prevention of variceal bleeding in primary and secondary prophylaxis.
Results
Pharmacological therapy aims to decrease portal pressure (PP) by acting on the pathophysiological mechanisms of portal hypertension such as increased hepatic vascular tone and splanchnic vasodilatation. Propranolol and nadolol block the beta-1 in the heart and the peripheral beta-2 adrenergic receptors. Beta-1 blockade of cardiac receptors reduces heart rate and cardiac output and subsequently decreases flow into splanchnic circulation. Beta-2 blockade leads to unopposed alpha-1 adrenergic activity that causes splanchnic vasoconstriction and reduction of portal inflow. Both effects contribute to reduction in PP. Carvedilol is more powerful in reducing hepatic venous pressure gradient (HVPG) than traditional nonselective beta-blockers (NSBBs) and achieves good hemodynamic response in nearly 75 % of cases. Simvastatin and atorvastatin improve endothelial dysfunction mainly by enhancing endothelial nitric oxide synthase (eNOS) expression and phosphorylation and NO production. In addition, statins deactivate hepatic stellate cells and ameliorate hepatic fibrogenesis. These effects cause a decrease in HVPG and improve liver microcirculation and hepatocyte perfusion in patients with cirrhosis. In addition, several promising drugs under development may change the management of portal hypertension in the coming years.
Conclusion
This review provides a background on the most important aspects of the treatment of portal hypertension in patients with compensated and decompensated liver cirrhosis. However, despite the great improvement in the prevention of variceal bleeding over the last years, further therapeutic options are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variceal bleeding is a major complication of portal hypertension, which is associated with significant mortality, greater than that of acute myocardial infarction. Moreover, patients surviving a variceal bleeding episode have very high risk of rebleeding, which is associated with mortality as high as that of the first bleed. Because of this, prevention of bleeding from gastroesophageal varices (GEV) has been one of the main therapeutic goals since the advent of the first effective therapies for portal hypertension [1].
Traditionally, prevention of variceal hemorrhage (VH) has been divided into prevention of the first bleeding episode (or “primary prophylaxis”) and prevention of recurrent VH (“secondary prophylaxis”) [2, 3]. In terms of death risk, however, this classification is not very accurate, as both categories include patients with low and high risk of dying. This is mostly due to the fact that the more important determinant of mortality is the degree of liver failure, with patients without liver disease (with so-called noncirrhotic portal hypertension) or with compensated cirrhosis [Child–Turcotte–Pugh (CTP) class A] having negligible mortality [4], while this is quite high in patients with advanced, decompensated cirrhosis [those who already had complications of cirrhosis, such as ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatic encephalopathy (HE) or jaundice before the bleeding, which are in CTP class B and C]. In addition, there have been efforts at “preprimary prophylaxis” or prevention of development of GEV [5].
Prevention of first hemorrhage from gastroesophageal varices versus prevention of first episode of clinical decompensation
Over recent years, knowledge of the natural history of cirrhosis has been better characterized [6]. On top of the broad classification into “compensated” and “decompensated” stages, it is now recognized that distinct substages can be distinguished [7]. Thus, compensated cirrhosis can be divided according to the degree of portal hypertension (evaluated by measurement of HVPG) into an initial substage where HVPG can be elevated but remains below 10 mmHg (“mild,” subclinical portal hypertension) [4]. At this stage, there is no hyperdynamic circulation, the effect of nonselective beta-blockers (NSBBs) on HVPG is minimal, there are no complications of portal hypertension, and the risk of developing these is almost negligible [8]. This is followed by a second substage in which HVPG increases above 10 mmHg, which defines “clinically significant portal hypertension” (CSPH), which is associated with the risk of developing portosystemic collaterals and GEV, and predicts overt clinical decompensation (ascites, VH, and HE) [9], postsurgical decompensation [10], and hepatocellular carcinoma (HCC) [11].
It is important to note that this substage can be recognized at present with the use of new noninvasive tools for assessing portal hypertension (liver and spleen elastography, methacetin breath test, contrast-enhanced ultrasound) [12–14]. This substage is further subdivided according to absence/presence of GEV, the latter having a higher risk. At this point, patients exhibit hyperdynamic circulation and have greater HVPG response to NSBBs [8]. The more frequent decompensating event is ascites, followed by VH and HE. Development of these complications defines entry into the decompensated stage, which can be further subdivided according to the nature of the complication (bleeding versus nonbleeding) and whether this is the first episode of decompensation or if there have been previous decompensation episodes.
At the most recent Baveno VI conference it was concluded that the aim of future studies in portal hypertension should be modified according to this stage classification [2]. The recent American Association for the Study of Liver Diseases (AASLD) guidance document endorsed this view [15]. Therefore, it is currently recommended that, in compensated cirrhosis with mild portal hypertension, the aim of therapy should be preventing the advent of CSPH, which is probably best achieved by treating the specific cause of cirrhosis, and by supporting healthy lifestyle habits [16]. In compensated patients with CSPH, the goal of therapy should be to prevent decompensation and death/liver transplantation (including competing risk analysis). This is best achieved by drug therapy, or alternatively, by endoscopic therapy. In decompensated cirrhosis, therapy aims to prevent further decompensation and death/liver transplantation, and suggested treatment is the combination of drug and endoscopic therapy (Table 1).
Incidence of varices, bleeding, and clinical decompensation, and risk factors
GEV are present in about one-third of patients with compensated cirrhosis, whereas this figure increases to 85 % in patients with decompensated cirrhosis [17, 18]. In patients with compensated cirrhosis, incidence of varices is 7 % per year [5] and progression from small to large varices occurs at a rate of about 10 % per year [19]. The advent of noninvasive tools has limited the number of patients requiring screening endoscopy; previously, it was considered that all patients should have endoscopy at diagnosis of cirrhosis, while since Baveno VI it has been recognized that patients with liver stiffness <20 kPa and platelet count >150,000/mm3 have very low probability (<5 %) of having high-risk varices, and in them endoscopy can be safely avoided. These figures are being modified by new studies, resulting in still greater numbers of patients in whom screening endoscopy can be safely avoided or delayed.
The incidence of variceal hemorrhage is around 10–15 % per year, depending on the severity of liver disease, size of varices, and presence of red wale marks (areas of thinning of the variceal wall) [1, 20]. Factors associated with poor outcomes in patients with VH are presence of bacterial infections and HVPG >20 mmHg, which is mostly observed in patients in CTP class C [21, 22]. If untreated, recurrent variceal bleeding occurs in two-thirds of patients, usually within 1–2 years [23]. However, the more common decompensating event is not development of variceal bleeding, but ascites, which is over twice as frequent as variceal hemorrhage.
Obesity and alcohol abuse are associated conditions that worsen prognosis of patients with cirrhosis of any etiology. Obesity has been shown to exacerbate liver fibrosis and clinical decompensation, and is associated with lack of regression of cirrhosis in patients with viral cirrhosis [24–26], while weight loss and exercise are associated with a fall in HVPG and in inflammatory biomarkers [27]. Alcohol intake can increase HVPG and worsen prognosis of hepatitis C virus (HCV)- and nonalcoholic steatohepatitis (NASH)-related cirrhosis [28, 29]. Therefore, adhering to a healthy lifestyle is an important aspect of therapy in cirrhosis of any etiology.
Available drugs for treatment of portal hypertension
Rational basis of therapy
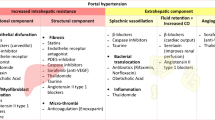
Rational pharmacological therapy aims to decrease portal pressure (PP) by acting on the pathophysiological mechanisms of portal hypertension. As in any vascular territory, according to Ohm’s law, the pressure gradient across the portal system is determined by the product of the blood flow in the portal vein and the vascular resistance that opposes the flow. Therefore, drugs or procedures that reduce the hepatic vascular resistance and/or portal flow will decrease PP.
In chronic liver disease, increased intrahepatic resistance to portal blood flow is the initial mechanism that leads to portal hypertension. Intrahepatic vascular resistance increases due to structural changes inherent to cirrhosis, such as fibrosis, regenerative nodules, vascular occlusion, and sinusoidal remodeling (mechanical component). In addition, hepatic sinusoidal endothelial dysfunction (disequilibrium between an increased production and response to vasoconstrictors, and deficient production and response to vasodilators) results in a dynamic increase of intrahepatic vascular tone (functional component).
During the evolution of the disease, when the PP gradient increases to 10 mmHg or above, portosystemic collaterals (including gastroesophageal varices) start to develop and blood flow increases due to splanchnic arteriolar vasodilatation, which contributes to worsening and perpetuation of portal hypertension despite the formation of extensive portosystemic collaterals through which portal flow is diverted to systemic circulation, bypassing the liver. Formation of collaterals is a consequence not only of dilatation of preexisting (but virtually closed) vascular channels connecting the portal and systemic circulation due to increased PP, but also of vascular endothelial grow factor (VEGF)-derived angiogenesis.
Augmented NO production in the splanchnic vasculature is the major factor leading to splanchnic vasodilatation, exacerbating portal hypertension, which increases flow into the portal venous system and is associated with systemic vasodilatation. This splanchnic vasodilation becomes so marked as to provoke arterial hypotension and decreased “effective” or “central” blood volume, which in turn leads to activation of compensatory endogenous neurohumoral vasoactive systems, with ensuing retention of sodium and water resulting in increased blood volume and cardiac output (hyperdynamic circulatory state) and, in more advanced stages, circulatory dysfunction and formation of ascites. Therefore, drugs for portal hypertension should ideally decrease PP without further enhancing systemic vasodilatation.
Pharmacological agents for portal hypertension
Drugs that decrease intrahepatic vascular resistance
-
1.
Acting on the structural component of increased hepatic resistance
Elimination or suppression of the etiologic agent (hepatitis B and C viruses, alcohol intake, etc.) frequently induces at least partial regression of cirrhosis and lowers PP in patients with compensated cirrhosis and portal hypertension. Drugs targeting fibrous septa degradation and prevention of fibrosis progression would have a beneficial impact and decrease PP. Although no antifibrotic drugs have yet been approved, several drugs have shown beneficial effect in preclinical and clinical studies in patients with cirrhosis [6, 30] and constitute a field of intensive research.
-
2.
Drugs acting on liver endothelial dysfunction and increased hepatic vascular tone
Drugs that improve intrahepatic endothelial dysfunction also have potential antifibrotic effects, since restoring the phenotype of the hepatic endothelium has a beneficial impact in terms of deactivating stellate cells and decreasing extracellular matrix deposition, therefore modifying both the structural and functional components of increased hepatic resistance.
NO donors
Nitrovasodilators (nitroglycerin, isosorbide 5-mononitrate) increase the availability of NO in the hepatic circulation, produce dilatation of the hepatic vasculature, and ameliorate portal hypertension. However, their vasodilatory effect also extends to the systemic circulation, potentially causing arterial hypotension and activating endogenous vasoactive systems. Isosorbide 5-mononitrate in monotherapy is not recommended since, it has been shown to be clinically ineffective [31, 32].
Simvastatin and atorvastatin improve endothelial dysfunction mainly by overexpressing transcription factor KLF-2, which enhances eNOS expression and phosphorylation and NO production, and reduces propensity for thrombosis and angiogenesis. In addition, statins deactivate hepatic stellate cells and ameliorate hepatic fibrogenesis [33, 34]. These effects cause a decrease in HVPG, and improve liver microcirculation and hepatocyte perfusion [35] in patients with cirrhosis. Specifically, recent data have shown that statin users with compensated HCV cirrhosis have lower incidence of decompensation (ascites and VH) and lower mortality than nonusers [36], and a randomized placebo-controlled clinical trial showed a significant improvement in mortality by associating simvastatin with NSBB and endoscopic band ligation (EBL) in patients treated after an episode of variceal bleeding [37]. These results need further validation.
Anti-alpha-adrenergic agents
Prazosin is an alpha-1 adrenergic antagonist that reduces HVPG by decreasing hepatic vascular tone and hepatic resistance, which increases liver blood flow. Its vasodilatory effect in the systemic circulation decreases arterial pressure and leads to activation of endogenous vasoactive systems [38], therefore it should not be used in monotherapy. However, in combination with propranolol, the deleterious systemic effect is attenuated, while the decrease in PP is more intense than with the association of propranolol plus isosorbide 5-mononitrate [39].
Carvedilol is a NSBB with intrinsic anti-alpha-adrenergic activity and mild vasodilating effect, resembling the effects of the combination of propranolol and prazosin. Carvedilol has a greater effect in decreasing HVPG than traditional NSBBs (propranolol and nadolol) and is becoming widely used in treatment of portal hypertension (see below) [40, 41].
Drugs that decrease portal and collateral blood flow by counteracting splanchnic vasodilatation
Increased splanchnic blood flow does not occur in cirrhosis until the patient develops clinically significant portal hypertension and hyperdynamic circulation. This is why the drugs discussed below are not indicated in patients with mild portal hypertension (HVPG <10 mmHg) or in those with no varices.
Nonselective beta-adrenergic blockers (NSBBs)
Propranolol and nadolol block the beta-1 adrenergic receptors in the heart and the peripheral beta-2 adrenergic receptors. Beta-1 blockade of cardiac receptors reduces heart rate and cardiac output and subsequently decreases flow into the splanchnic circulation. Beta-2 blockade leads to unopposed alpha-1 adrenergic activity that causes splanchnic vasoconstriction and a further reduction of portal inflow. Both effects contribute to the reduction in PP. Beyond reduction in PP, NSBBs also have other beneficial effects in cirrhosis such as reducing bacterial translocation and spontaneous bacterial peritonitis (SBP) due to shortening of intestinal transit time and decreased bacterial overgrowth. Treatment with NSBBs has to be stepped up gradually until a maximal tolerated dose (<240 mg for propranolol, <160 mg for nadolol) or when the heart rate is below 55 bpm or systolic blood pressure <100 mmHg. The goal is to reduce HVPG below 12 mmHg or at least 20 % from baseline value, which is associated with a reduction in the risk of bleeding, of rebleeding and ascites development, SBP, and hepatorenal syndrome, and improved survival. However, only 40–50 % of the patients achieve such hemodynamic response. Combination therapy with vasodilators (isosorbide 5-mononitrate or prazosin) enhances the reduction of HVPG in up to a third of nonresponders [32]. Unfortunately, noninvasive tests do not allow accurate assessment of the PP response to NSBBs.
Carvedilol is a NSBB that is more powerful in reducing HVPG than propranolol or nadolol, because in addition to beta-blockade it relaxes the increased hepatic vascular tone due to anti-alpha-adrenergic activity. It achieves good hemodynamic response in nearly 75 % of cases [40–42]. Carvedilol has its maximal effects on PP already at low dosages (12.5 mg per day) that are better tolerated than effective doses of traditional NSBBs. Because of these advantages, it is becoming the most widely used beta-blocker for management of portal hypertension in compensated cirrhosis. This was added to the most recent Baveno VI consensus guidelines. However, its safety in decompensated patients with ascites has been questioned due to the risk of systemic hypotension, although it appears that this is only associated with high dosages of carvedilol (25 mg per day or greater).
Once initiated, treatment with NSBBs should be maintained lifelong. NSBB administration should be monitored with caution in patients with refractory ascites, and the dosage decreased or withdrawn if patients develop systolic blood pressure <90 mmHg, severe hyponatremia with serum sodium <130 meq/L, or acute kidney injury [15].
New drugs under development
Several promising drugs under development may change the management of portal hypertension in the coming years. Most of them are focused on targeting hepatic vascular resistance via NO modulation (obeticholic acid, udenafil, serelaxin, antioxidants) and hepatic fibrosis (simtuzumab), but there are also other agents with promising preclinical data (anticoagulants, angiogenesis inhibitors, emricasan, taurine, etc.), whose mechanisms of actions lie beyond the scope of this review [43] (Figs. 1, 2, 3).
Treatment of portal hypertension in patients with compensated chronic liver disease
Treatment of patients with compensated cirrhosis and mild portal hypertension
This substage of compensated cirrhosis is defined by increased HVPG (above 5 mmHg) but <10 mmHg. Patients in this stage do not have GEV or other complications of portal hypertension (PH) and are known to have very low risk of clinical decompensation. As mentioned above, the goal of therapy is to prevent development of CSPH (and thereby GEV and decompensation). Patients at this stage of cirrhosis have not yet developed hyperdynamic circulation [8], and increased intrahepatic resistance is the main mechanism leading to PH. Accordingly, therapy has to be directed toward correcting the etiology of cirrhosis. Patients in this stage are more likely to show partial (or eventually total) regression of cirrhosis after successful etiological treatment, as shown for patients with cirrhosis due to hepatitis B virus (HBV) [26]. Drugs that decrease portal inflow, such as NSBBs, are not adequate in this substage, since hyperdynamic syndrome has not yet developed [8].
In addition to suppressing the etiologic agent (e.g., HBV, HCV, alcohol, iron overload), some drugs have been shown to have “antifibrotic” properties in preclinical studies and to decrease HVPG in patients with cirrhosis (simvastatin, obeticholic acid, emricasan, enoxaparin) and are currently being investigated in randomized controlled trials (RCTs), mostly in cirrhosis due to NASH [30, 44–49]. Statins decrease hepatic fibrogenesis, improve intrahepatic endothelial dysfunction, reduce PP, and improve liver perfusion and liver function [35]. The stage of cirrhosis that will benefit most from statins remains to be determined. This also applies to other antifibrotic agents.
In addition, these patients benefit from lifestyle interventions, with diet to correct obesity/overweight or sarcopenia, moderate physical exercise, and abstinence from alcohol. These recommendations are valid for patients with cirrhosis of any etiology.
Patients with compensated cirrhosis and CSPH, but without gastroesophageal varices requiring treatment
CSPH is defined as HVPG ≥10 mmHg, the threshold value for development of varices and clinical decompensation, among other outcomes. Varices requiring treatment are those of moderate/large size or that exhibit abundant red color signs on their walls (which is rarely the case in compensated patients). Therefore, most patients with small varices should be managed as specified in this section.
A large, multicenter, randomized, placebo-controlled trial in patients with compensated cirrhosis without GEV showed no differences between placebo and NSBB (timolol) in prevention of varices (so-called preprimary prophylaxis) [5]. Therefore, no specific portal-pressure-reducing treatment to prevent formation of varices is recommended. However, this study included patients with and without CSPH. Since the response to NSBB differs between these groups [8], the negative results of this timolol study might have been due to roughly half the patients having no CSPH.
It is now considered that, in patients with cirrhosis and CSPH but without varices needing treatment, the objective of treatment should no longer be to prevent varices, but to prevent clinical decompensation. Drugs that decrease intrahepatic resistance and/or decrease splanchnic blood flow are appropriate at this stage. The preliminary results of a large RCT using NSBB to prevent decompensation of cirrhosis (the PREDESCI study) are very encouraging [50].
Patients with compensated cirrhosis and gastroesophageal varices requiring treatment
Patients at this stage have, by definition, CSPH, because the lowest HVPG in these patients is 10–12 mmHg [51, 52]. This clinical setting was previously described as “primary prophylaxis of variceal hemorrhage,” and the main objective was to prevent the first episode of VH. In these patients, the risk of bleeding depends mainly on the endoscopic assessment of the size of varices (small versus medium/large-sized GEV) and presence of red wale signs. Because of this different risk, therapeutic guidelines distinguish between these different situations. As already mentioned, prevention of clinical decompensation is probably the most appropriate endpoint at this stage, because ascites, not variceal bleeding, is the most common decompensating event [9].
Primary prophylaxis of VH is indicated in patients at high risk of bleeding. These are (a) patients with medium/large varices, (b) patients with small varices with red wale signs, and (c) decompensated patients with small varices [20].
The drugs that have been more widely used are traditional NSBBs (propranolol, nadolol). More recently, carvedilol, because of its greater portal-pressure-reducing effect, easier dosage, and better tolerance, is becoming the most widely used NSBB for portal hypertension. Other drugs that decrease PP (isosorbide mononitrate, angiotensin II antagonists, prazosin) have not been used in phase III RCTs or shown ineffective.
In this setting, reduction in HVPG to ≤12 mmHg or ≥20 % from baseline was shown to protect from VH and represent an “optimal response” to NSBB [53, 54], as well as with a decreased incidence of decompensation [42]. Reductions in HVPG >10 % with NSBB have also been associated with lower incidence of first VH, of ascites, and of death in some [55, 56] but not all studies [57].
Prevention of first variceal hemorrhage in patients with medium/large esophageal varices
Eight RCTs have compared NSBBs with no therapy/placebo. Metaanalysis of these studies [1] showed a clear benefit of NSBBs in preventing first variceal bleeding episode. A meta-analysis of 19 RCTs (including unpublished abstracts) comparing NSBBs with EVL [58] showed that EVL was associated with lower rates of upper gastrointestinal (GI) bleeding and VH, without differences in mortality. The beneficial effect of EVL on bleeding was not confirmed in subgroup analyses limited to 7 trials with adequate bias control [59] or to 12 fully published studies [60]. Two trials comparing EVL with carvedilol showed either greater efficacy of carvedilol [61] or comparable efficacy [62]. Accordingly, the current recommendation is to use either NSBBs (propranolol, nadolol, or carvedilol) or EVL to prevent first VH in patients with medium/large varices, and that choice of treatment should be based on local resources and expertise, patient preference and characteristics, contraindications, and adverse events [3, 2].
Advantages of NSBBs are low cost, ease of administration, and not requiring specific expertise. In addition, hemodynamic responders to NSBBs have lower incidence of decompensation and death. All these aims are easier to achieve with carvedilol than with traditional NSBBs. Importantly, once a patient is on NSBB, there is no need for repeat esophagogastroduodenoscopy (EGD) during therapy. Disadvantages of NSBBs are that approximately 15 % of patients may have absolute or relative contraindications, and another 15 % may require dose reduction or discontinuation due to common side-effects (e.g., fatigue, weakness, and shortness of breath). These resolve upon dose reduction or discontinuation, but that may be discouraging for patients and their physicians [63]. The rare patient who cannot tolerate even a low dose of carvedilol (3.25 mg) should be switched to serial EVL.
Advantages of EVL are that it can, theoretically, be done in the same session as screening endoscopy and has few contraindications. Disadvantages are cost, the risks associated with sedation, plus the risk of causing dysphagia, esophageal ulcerations, strictures, and bleeding. Although the number of side-effects is greater with NSBBs, the severity of side-effects is greater with EVL, with reports of deaths resulting from EVL-induced bleeding. In addition, because EVL is a local therapy that does not act on the pathophysiology of PH, it cannot prevent other complications from portal hypertension. Also, after variceal eradication, surveillance endoscopies are necessary to detect variceal recurrence, which approaches 90 %.
RCTs comparing the combination of NSBBs plus EVL versus EVL alone for prevention of first VH have failed to reveal a benefit from combination therapy, with a (predicted) higher number of adverse events in the combination therapy group [64].
Prevention of first variceal bleeding in patients with small esophageal varices
Patients with small varices require treatment to prevent the first VH only when the varices are at high risk of bleeding (with red wale marks and/or occurring in a CTP C patient) [20]. This represents only a fraction of patients, and no specific studies have been conducted in this specific population. However, there is consensus in recommending that these patients be treated with propranolol or carvedilol, because performing EVL in these cases and defining eradication may be difficult. Regarding low-risk small varices, some studies show that NSBBs or carvedilol may delay growth of small varices [65, 66], but this was not found in other studies [67, 68] (Table 2).
Management of patients after recovering from an episode of acute esophageal variceal hemorrhage
This clinical scenario was traditionally referred to as “secondary prophylaxis of variceal hemorrhage.” However, it is now recognized that therapy needs to take into account the presence or absence of other complications of cirrhosis. In patients with variceal hemorrhage as the sole complication of cirrhosis, risk of death is relatively low; therefore, therapy should aim at prevention of additional complications (including variceal rebleeding). On the contrary, patients who experience variceal bleeding in the context of other complications defining clinical decompensation (ascites, HE) are at high risk of death, so the aim of therapy should be to improve survival (with liver transplantation as a competing event) [2] (Table 2).
Current recommendations face the difficulty that these specific aims have not been explored as main endpoints in clinical trials so far. Therefore, present recommendations are only pertinent with regards to prevention of recurrent variceal hemorrhage. Patients who recover from the first episode of VH have high rebleeding risk (60 % in the first year), with mortality of about 33 %. Therapy to prevent rebleeding is therefore mandatory in these patients and should be instituted before the patient is discharged from hospital, except in those having had transjugular intrahepatic portosystemic shunt (TIPS) as part of the treatment of the acute bleeding episode.
First-line therapy is the combination of NSBBs (propranolol or nadolol) plus EVL. Metaanalysis comparing combination therapy with monotherapy using either EVL or drug therapy has demonstrated that combination therapy is significantly more effective than EVL alone in preventing recurrent GI hemorrhage. However, combination therapy is only marginally more effective than drug therapy (NSBB + nitrates) alone, with a tendency for better survival with NSBBs alone [69], suggesting that pharmacological therapy is more important part of combination therapy. Therefore, in patients who cannot tolerate NSBBs, rather than relying only on EVL, it is wise to consider TIPS, particularly if the patient has another complication (e.g., ascites) that could benefit from TIPS.
The combination of NSBBs plus low-dose isosorbide mononitrate (ISMN) has a greater portal-pressure-reducing effect than NSBBs alone. In metaanalysis, the combination of NSBBs and ISMN was not significantly better than NSBBs alone, but had a higher rate of side-effects [70].
In secondary prophylaxis of VH, carvedilol has been compared with EVL alone [71] or with NSBB + ISMN [72], but not with the combination of NSBB + EVL. Therefore, there are insufficient data to recommend carvedilol alone for prevention of rebleeding, and there are insufficient data on its safety in this population (although it is presumably safe at low doses). Carvedilol, particularly at dosages >25 mg/day, may decrease arterial pressure [41] and should not be used in patients with refractory ascites (even in the setting of primary prophylaxis).
A recent multicenter, placebo-controlled RCT showed that addition of simvastatin (40 mg/day) was not associated with a significant reduction in rebleeding (compared with placebo), but was associated with a significant improvement in survival, mainly related to a decrease in deaths from bleeding or infection [37]. There were two instances of rhabdomyolysis in two patients with bilirubin over 5 mg/dL; pharmacokinetic considerations suggest that, in CTP class C patients, the dosage should not exceed 20 mg/day. Moreover, the study suggested no benefit of adding simvastatin in the subset of Child C patients.
TIPS is the treatment of choice in patients who fail standard therapy for prevention of variceal rebleeding (NSBB + EVL). Until recently, trials comparing TIPS and endoscopic therapy had used bare TIPS stents [73]. However, in a recent RCT using the recommended covered stents for TIPS, which was compared with the combination of EVL or glue injection plus NSBBs, TIPS patients had less rebleeding (0 versus 29 %), but there were no differences in survival and with higher incidence of early encephalopathy in the TIPS group [74].
The lowest rebleeding rates are observed in patients on secondary prophylaxis who are HVPG responders (defined as reduction in HVPG below 12 mmHg or >20 % from baseline) [23]. Therefore, HVPG-guided therapy performed in centers where HVPG measurements are readily available would be a reasonable strategy.
Management in less common clinical scenarios
Gastric varices
Gastric varices (GV) are present in 10–20 % of patients with cirrhosis. Sarin’s classification is the most commonly used for risk stratification and management of GV [75]. The most common are GOV1: EV extending below the cardia into the lesser curvature (75 % of GV). These are usually managed as esophageal varices. GOV type 2 (GOV2) are EV extending into the fundus. Isolated GV type 1 (IGV1) are located in the fundus, while isolated GV type 2 (IGV2) are located elsewhere in the stomach. These are extremely infrequent in patients with cirrhosis. GV are much more frequent in patients with extrahepatic portal vein and/or splenic vein occlusion.
For management of GV, few RCTs are available, most with small sample size and, in many occasions, without adequate stratification according to type of GV or presence/severity of liver disease. These limitations make the following recommendations less robust than those for esophageal varices.
Prevention of first hemorrhage from gastric varices
There is only one RCT on primary prevention of gastric VH, including patients with large GOV2 and IGV1 who were randomized to endoscopic obturation by cyanoacrylate (glue) injection, NSBBs, and observation [76]. Only 15 % of patients had IGV1. Glue injection was associated with lower bleeding rate (10 %) than NSBBs (38 %) or observation (53 %). Survival was better in the glue-treated group (93 %) compared with observation (74 %), but not different from NSBBs (83 %). Firm recommendations cannot be derived from this trial. The least invasive treatment is NSBBs, having the associated advantage that it may prevent other complications of cirrhosis.
Prevention of rebleeding from gastric varices
Endoscopic variceal obturation (cyanoacrylate glue injection): In a RCT, repeated cyanoacrylate injection was superior to NSBB in preventing rebleeding and mortality in patients with cardiofundal varices [77]. In another trial, addition of NSBBs to cyanoacrylate injection did not improve rebleeding or mortality compared with cyanoacrylate injection alone [78].
TIPS: A RCT including patients with GOV1 and GOV2 varices showed TIPS to be more effective than glue injection in preventing rebleeding [79], but with higher incidence of encephalopathy and no differences in survival.
Balloon-occluded retrograde transvenous obliteration (BRTO): This procedure for treatment of fundal varices associated with a large gastro- or splenorenal communication involves retrograde catheterization of the left renal vein, followed by balloon occlusion and slow infusion of sclerosant to obliterate the gastro/splenorenal collateral and fundal varices [80–82]. Several variations of the technique exist. BRTO has the theoretical advantage over TIPS that it does not shunt portal blood flow away from the liver. No randomized trials have compared BRTO with other therapies.
Patients with refractory ascites or after spontaneous bacterial peritonitis
Observational studies have raised concerns regarding use of NSBBs in patients with refractory ascites, due to the finding of increased mortality [59] or greater incidence of postparacentesis circulatory dysfunction in patients under NSBBs [83]. A retrospective study showed that NSBBs improved survival in patients with ascites, but in a subanalysis limited to those surviving an episode of SBP, NSBBs worsened survival and had higher risk of hepatorenal syndrome (HRS) [84].
These concepts have been challenged by three subsequent studies assessing large cohorts of patients with ascites [85–87] that have shown either no differences [86] or even improved survival [85, 87] in patients treated with NSBBs, including patients with refractory ascites. An additional study showed that ongoing treatment with NSBBs was associated with improved survival in patients with acute-on-chronic liver failure [88].
The discrepancies between these studies might have been influenced by the use of unusually high doses of NSBBs in the studies that initially suggested a harmful effect from NSBBs, as shown by two recent publications reporting that, in patients with decompensated cirrhosis, dosages of propranolol ≥160 mg/day were associated with worse survival, whereas dosages up to 160 mg/day were associated with improved survival [87]. The second study showed almost identical findings in patients with SBP [89]. It is important to note that dosages of propranolol of 160 mg/day or above (or >80 mg/day if using nadolol) are very rarely (if ever) required in decompensated cirrhosis if the recommended titration steps for adjusting propranolol dosage are adhered to.
Therefore, current evidence does not support a harmful effect of NSBBs in decompensated cirrhosis. In these patients, especially in those with true refractory ascites or SBP, the dosage of NSBBs should be carefully titrated and high dosages avoided. Also, these patients need careful monitoring and the NSBB dose reduced (or discontinued) with development of severe hypotension (systolic blood pressure < 90 mmHg), hyponatremia (serum sodium <130 mEq/L), or unexplained deterioration of renal function [2]. NSBBs might be reintroduced after correction of renal function/circulatory state.
Patients with hepatocellular carcinoma
Most RCTs for prevention of VH have excluded patients with HCC, and the few including HCC patients excluded those with advanced disease. Therefore, the best treatment for these patients remains unknown. Observational data suggest increased risk of bleeding and worse prognosis in these patients [90–92]. However, there are no data to suggest decreased efficacy of treatments to prevent bleeding (NSBBs, EVL, or TIPS if technically feasible) as compared with no intervention. A recent multicenter study in Spain reported that patients with HCC frequently do not receive secondary prophylaxis after recovering from acute VH, which was independently associated with mortality after adjusting for HCC stage and degree of liver dysfunction [92]. This suggests that HCC patients should receive the same secondary prophylaxis as patients without HCC, including those who have portal vein thrombosis. Patients with advanced HCC should be treated within the context of the end-of-life care plan of the patient.
Patients experiencing first variceal hemorrhage while on prophylactic therapy with NSBBs or EVL
An increasing number of patients with cirrhosis experience the first variceal bleeding episode under primary prophylaxis with NSBBs or EVL. These patients have been excluded from most trials on prevention of rebleeding, so the best therapeutic approach for them is unclear. A recent study showed that rebleeding and mortality were significantly higher in patients who bled while on prophylactic NSBBs (clinically “nonresponders”), as compared with those who bled without having been on NSBB [93]. These findings suggest that patients who bleed while on primary prophylaxis with NSBBs may need a more effective therapy, such as TIPS.
References
D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19:475–505
De Franchis R, Faculty BV. Expanding consensus in portal hypertension. Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol Eur Assoc Study Liver 2015;63(3):743–752
De Franchis R, Faculty BV. Revising consensus in portal hypertension: report of the Baveno V Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol Eur Assoc Study Liver 2011;54(5):1082–1083
Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–832
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005;353(21):2254–2261
Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol Eur Assoc Study Liver 2015;62(S1):S121–S130
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44(1):217–231
Villanueva C, Albillos A, Genescà J, Abraldes JG, Calleja JL, Aracil C, et al. Development of hyperdynamic circulation and response to beta-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63(1):197–206
Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–488
Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients. Prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1122
Ripoll C, Groszmann R, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009;50:923–928
Eisenbrey JR, Dave JK, Halldorsdottir VG, Merton DA, Miller C, Gonzalez JM, et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;268(2):581–588
Stravitz RT, Reuben A, Mizrahi M, Lalazar G, Brown K, Gordon SC, et al. Use of the methacetin breath test to classify the risk of cirrhotic complications and mortality in patients evaluated/listed for liver transplantation. J Hepatol Eur Assoc Study Liver 2015;63(6):1345–1351
Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013; 144(1):102.e1–111.e1
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65(1):310–335
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014; 383(9930): 1749–1761
Pagliaro L, D’Amico G, Pasta L, Politi F, Vizzini G, Traina M, et al. Portal hypertension in cirrhosis: natural history. In: Bosch J, Groszmann RJ, editors. Portal Hypertension. Pathophysiology and Treatment. Oxford, UK: Blackwell Scientific;1994. pp. 72–92.
Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc 2007;65:82–88.
Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 2003;38:266–272
North Italian Endoscopic Club for the Study and Treatment of esophageal varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med 1988;319:983–989
Tandon P, Abraldes J, Keough A, Bastiampillai R, Jakayumar S, Carbonneau M, et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on Child–Pugh class and effects of antibiotics. Clin Gastroenterol Hepatol 2015;13(6):1189–1196
Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, García-Pagán JC, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48(2):229–236
Bosch J, García-Pagán J. Prevention of variceal rebleeding. Lancet 2003;361:952–954
Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology 2009;137(2):549–557
Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54(2):555–561
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381(9865):468–475
Berzigotti A, Villanueva C, Calleja JL, Carlos J, Mesonero F, Bosch J, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 2017;1:1–53
Monto A, Patel K, Bostrom A, Pianko S, Pockros P, McHutchison JG, et al. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology 2004;39(3):826–834
Hutchinson S, Bird S, Goldberg D. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol 2005;3:1150–1159
Yoon YJ, Friedman SL, Lee YA. Anti fibrotic therapies: where are we now? Semin Liver Dis 2016;36(1):87–98. https://doi.org/10.1055/s-0036-1571295.
García-Pagan J, Villanueva C, Vila M, Albillos A, Genesca J, Ruiz-del-Arbol L, et al. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001;121(4):908–914
García-Pagán JC, Morillas R, Bañares R, Albillos A, Villanueva C, Vila C, et al. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003;37(6):1260–1266
Marrone G, Maeso-Díaz R, García-Cardena G, Abraldes J, García-Pagán J, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut 2015;64(9):1434–1443
Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol 2010;53(4):702–712
Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 2009;136(5):1651–1658
Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology 2016; 150(2): 430e1–440e1
Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150(5):1160e3–1170e3
Albillos A, Lledó JL, Rossi I, Pérez-Páramo M, Tabuenca MJ, Bañares R, et al. Continuous prazosin administration in cirrhotic patients: effects on portal hemodynamics and on liver and renal function. Gastroenterology 1995;109(4):1257–1265
Albillos A, García-Pagán J, Iborra J, Bandi J, Cacho G, Pérez-Paramo M, et al. Propranolol plus prazosin compared with propranolol plus isosorbide-5-mononitrate in the treatment of portal hypertension. Gastroenterology 1998;115(1):116–123
Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology 2010;51(6):2214–2218
Sinagra E, Perricone G, D’Amico M, Tinè F, D’Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther 2014;39(6):557–568
Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut 2013;62(11):1634–1641
Nair H, Berzigotti A, Bosch J. Emerging therapies for portal hypertension in cirrhosis. Expert Opin Emerg Drugs 2016;21(2):167–181
Laleman W, Van Landeghem L, Van der Elst I, Zeegers M, Fevery J, Nevens F. Nitroflurbiprofen, a nitric oxide releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats. Gastroenterology 2007;132:709–719
Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol 2015;185:409–419
Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66(1):180–190
Tsochatzis EA, Bosch J, Burroughs AK. New therapeutic paradigm for patients with cirrhosis. Hepatology 2012;56(5):1983–1992
Zubiete-Franco I, Fernández-Tussy P, Barbier-Torres L, Simon J, Fernández-Ramos D, Lopitz-Otsoa F, et al. Deregulated neddylation in liver fibrosis. Hepatology 2016;65(2):694–709
Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012; 143(5):1253.e4–1260.e4
Villanueva C, Albillos A, Genescà J, Garcia-Pagan J, Calleja J, Aracil C, et al. Preventing the decompensation of cirrhosis with beta-blockers in patients with clinically significant portal hypertension. A multicenter double-blind placebo-controlled randomized clinical trial. J Hepatol. 2017;66(1):S97–S98
Lebrec D, De Fleury P, Rueff B, Nahum H, Benhamou J. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980;79:1139–1144
Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5(3):419–424
Feu F, García-Pagán JC, Bosch J, Luca A, Escorsell A, Rodés J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995;346(8982):1056–1059
D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131(5):1611–1624
Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology 2009; 137(1): 119–128
Hernández-Gea V, Aracil C, Colomo A, Garupera I, Poca M, Torras X, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with beta-blockers. Am J Gastroenterol 2012;107(3):418–427
Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol 2006;101(3):506–512
Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev 2012;8(8):CD004544
Sersté T, Melot C, Francoz C, Durand F, Rautou P-E, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52(3):1017–1022
Bosch J, Garcia-Tsao G. Pharmacological versus endoscopic therapy in the prevention of variceal hemorrhage: and the winner is. Hepatology 2009;50(3):674–677
Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, Mcavoy NC, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology 2009;50(3):825–833
Shah HA, Azam Z, Rauf J, Abid S, Hamid S, Jafri W, et al. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol Eur Assoc Study Liver 2014;60(4):757–764
Longacre AV, Imaeda A, Garcia-Tsao G, Fraenkel L. A pilot project examining the predicted preferences of patients and physicians in the primary prophylaxis of variceal hemorrhage. Hepatology 2008;47(1):169–176
Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005;100(4):797–804
Merkel C, Marin R, Angeli P, Zanella P, Felder M, Bernardinello E, et al. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004;127(2):476–484
Bhardwaj A, Kedarisetty CK, Vashishtha C, Bhadoria AS, Jindal A, Kumar G, et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2016; gutjnl-2016-311735
Sarin SK, Mishra SR, Sharma P, Sharma BC, Kumar A. Early primary prophylaxis with beta-blockers does not prevent the growth of small esophageal varices in cirrhosis: a randomized controlled trial. Hepatol Int 2013;7(1):248–256
Calés P, Oberti F, Payen J, Naveau S, Guyader D, Blanc P, et al. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial. French-Speaking Club for the Study of Portal Hypertension. Eur J Gastroenterol Hepatol 1999;11(7):741–745
Puente A, Hernández-Gea V, Graupera I, Roque M, Colomo A, Poca M, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int 2014;34(6):823–833
Gluud LL, Langholz E, Krag A. Meta-analysis: isosorbide-mononitrate alone or with either beta-blockers or endoscopic therapy for the management of oesophageal varices. Aliment Pharmacol Ther 2010;32(7):859–871
Stanley AJ, Dickson S, Hayes PC, Forrest EH, Mills PR, Tripathi D, et al. Multicentre randomised controlled study comparing carvedilol with variceal band ligation in the prevention of variceal rebleeding. J Hepatol Eur Assoc Study Liver 2014;61(5):1014–1019
Lo GH, Chen WC, Wang HM, Yu HC. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. J Gastroenterol Hepatol 2012;27(11):1681–1687
Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010;51(1):306
Holster IL, Tjwa ETTL, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + beta-blocker for prevention of variceal rebleeding. Hepatology 2016;63(2):581–589
Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16(6):1343–1349
Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol Eur Assoc Study Liver 2011;54(6):1161–1167
Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut 2010;59(6):729–735
Hung HH, Chang CJ, Hou MC, Liao WC, Chan CC, Huang HC, et al. Efficacy of non-selective β-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: a randomized controlled trial. J Hepatol Eur Assoc Study Liver 2012;56(5):1025–1032
Lo G, Liang H, Chen W, Lai K, Hsu P, Lin C, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007;39:679–685
Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol 2001;12(3):327–336
Saad W. Endovascular management of gastric varices. Clin Liver Dis 2014;18:829–851
Saad W. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol 2012;29(2):118–128
Sersté T, Francoz C, Durand F, Rautou PE, Melot C, Valla D, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol 2011;55(4):794–799
Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective beta-blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146(7):1680–1690
Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015;64(7):1111–1119
Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective beta-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology 2016;63(6):1968–1976
Bang UC, Benfield T, Hyldstrup L, Jensen J-EB, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: a nationwide study based Danish patient registers. Liver Int 2016;36:1304–1312
Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, MacNaughtan J, Bendtsen F, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol Eur Assoc Study Liver 2016;64(3):574–582
Madsen B, Nielsen K, Fialla A, Krag A. Keep the sick from harm in spontaneous bacterial peritonitis: dose of beta blockers matters. J Hepatol 2016;64:1455–1456
Poynard T, Lebrec D, Hillon P, Sayegh R, Bernuau J, Naveau S, et al. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a prospective study of factors associated with rebleeding. Hepatology 1987;7:447–451
Singal A, Jampana S, Singal V, Kuo Y. Hepatocellular carcinoma predicts in-hospital mortality from acute variceal hemorrhage among patients with cirrhosis. J Clin Gastroenterol 2012;46:613–619
Ripoll C, Genescà J, Araujo I, Graupera I, Augustin S, Tejedor M, et al. Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case-control study. Hepatology 2013;58:2079–2088
De Souza A, La Mura V, Reverter E, Seijo S, Berzigotti A, Ashkenazi E, et al. Patients whose first episode of bleeding occurs while taking a betablocker have high long-term risks of rebleeding and death. Clin Gastroenterol Hepatol 2012;10:670–676
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Supported in part by grants from the Instituto de Salud Carlos III (PI 13/00341, and PI14/00182) in a program co-funded by FEDER, EU, and by EU (CLEVER- IAPP-GA-2013-612273). The CIBERehd is funded by the Instituto de Salud Carlos III.
Conflict of interest
J.B. is a consultant for Gilead, Conatus, Exallenz, Actelion, BLB, and Novo, and has received research grants from Conatus. A.B. and V.H.-G. have no disclosures with regards to this study.
Ethical approval
This article does not contain original studies with human participants.
Rights and permissions
About this article
Cite this article
Baiges, A., Hernández-Gea, V. & Bosch, J. Pharmacologic prevention of variceal bleeding and rebleeding. Hepatol Int 12 (Suppl 1), 68–80 (2018). https://doi.org/10.1007/s12072-017-9833-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-017-9833-y