Abstract
Aim
Wisteria floribunda agglutinin positive (WFA+) Mac-2-binding protein (M2BPGi) is a noninvasive glyco-marker for liver fibrosis. This study evaluated the utility of serial measurement of serum M2BPGi and total M2BP as a predictor of fibrosis and the development of hepatocellular carcinoma (HCC).
Methods
This study included 119 patients with chronic hepatitis C (CHC). Of these patients, 97 were treated with IFN-based therapy and 22 were treated with daclatasvir and asunaprevir. Serum M2BPGi values were measured prior to, at the end of, and at 24 weeks after the completion of treatment. As subanalysis, serum total M2BP levels were measured in patients treated with pegylated-interferon and ribavirin.
Results
In patients treated with IFN-based therapy, M2BPGi levels were elevated at the end of treatment but decreased afterwards. In contrast, M2BPGi levels in patients treated with IFN-free therapy decreased immediately after starting the treatment without transient elevation. Though pre-treatment M2BPGi levels significantly correlated with fibrosis in both patients with a sustained virological response (SVR) and non-SVR, post-treatment M2BPGi levels decreased regardless of the degree of fibrosis in patients with SVR. In multivariate analysis, non-SVR and HCC development were independent factors associated with M2BPGi level ≥2.2. In patients treated with pegylated-interferon and ribavirin, total M2BP levels were positively correlated with fibrosis and HCC development.
Conclusion
Real-time monitoring of the serum M2BPGi level after antiviral therapy for CHC patients could be a helpful screening tool for assessing the risk of HCC. M2BP and its glycan structure could be associated together with hepatocarcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related death in the world [1]. Hepatitis C virus (HCV) is a major cause of HCC, with 170 million people presently infected [2]. Although the efficacy of anti-viral therapy has dramatically improved with direct-acting antiviral agents (DAAs), HCC continues to develop in patients, even in those with a sustained virological response (SVR), particularly in those with advanced liver fibrosis or severe steatosis or those from an older age group [3, 4]. Therefore, an assessment of liver fibrosis and/or the risk of HCC development are essential for the management of patients with chronic hepatitis C (CHC), not only before treatment but also after achieving SVR.
Recently, Wisteria floribunda agglutinin positive (WFA+) Mac-2-binding protein (M2BP), so called M2BP glycosylation isomer (M2BPGi) was originally discovered to be a novel serum glyco-marker for liver fibrosis in glycoproteomic biomarker screening studies [5, 6]. Recent cross-sectional studies have reported that the serum level of M2BPGi at one time point is associated not only with a progression in liver fibrosis during CHC infection, but also with hepatocarcinogenesis [7–10]. However, the glycan structure may change during the clinical course of CHC including during anti-HCV therapy, and serial changes in M2BPGi levels during the course of antiviral treatment, especially after achieving SVR are unclear. Moreover it is also unknown which of the mechanisms are more important for liver fibrosis and HCC development, increase of M2BP itself or changes of its glycan structures.
In this report, we analyzed serial changes in serum M2BPGi and total M2BP levels during an anti-HCV treatment period in order to evaluate their clinical significance and usefulness for the assessment of liver fibrosis and HCC risk.
Patients and methods
Patients
One hundred nineteen patients with HCV who were treated at Tokyo Medical and Dental University Hospital or affiliated hospitals participating in the Ochanomizu-Liver Conference Study group were included in the present study. All patients had no history of HCC and other chronic liver diseases. Before starting antiviral treatment, patient characteristics, biochemical data, hematological data, virological data and treatment details were collected. A fibrosis 4 (Fib-4) index and aspartate aminotransferase (AST)-to-platelet ratio index (APRI) were also calculated as reported before [11, 12].
Histological evaluation
Liver biopsy was done before the initiation of therapy for 108 (90.8 %) of the 119 analyzed subjects. To obtain liver specimens, ultrasound-guided liver biopsies were performed using 18 gauge needles. The stage of fibrosis and the grade of inflammatory activity were scored by two pathologists according to the classification of Desmet et al. [13].
Anti-HCV therapy and definitions of responses to therapy
Fifty-five patients were treated by combination therapy with PEG-IFN-α2b or PEG-IFN-α2a and RBV. The standard duration of combination therapy was 24 weeks for genotype 2 and 48 weeks for genotype 1. Forty-two patients received 12 weeks of triple therapy that included SMV or TVR, PEG-IFN and RBV followed by 12 weeks of dual therapy that included PEG-IFN and RBV. Twenty-two patients received 24 weeks of IFN-free therapy with ASV and DCV. SVR was defined as serum HCV RNA undetectable 24 weeks after the cessation of treatment.
Measurement of M2BPGi
Serum M2BPGi levels were measured prior to, at the end of, and at 24 weeks after the completion of antiviral treatment. M2BPGi quantification was performed based on a lectin-antibody sandwich immunoassay using a fully automatic HISCL-2000i immunoanalyzer (Sysmex Co., Hyogo, Japan) [5]. Measured values of M2BP conjugated to WFA were indexed, with values calculated using the following equation:
where the [WFA+M2BP] sample represents the WFA+M2BP count of the serum sample (PC is the positive control, NC is the negative control). The positive control was supplied as a calibration solution standardized to yield a COI value of 1.0 [6].
Measurement of serum total M2BP level
Serum total M2BP levels were measured prior to, at the end of, and at 24 weeks after the completion of treatment in 55 patients treated with PEG-IFN and RBV. M2BP concentration was determined by using an ELISA kit (Immuno-Biological Laboratory, Gunma, Japan, code# 27362) according to manufacturer’s instruction.
Follow-up and diagnosis of HCC
All patients were followed up for between 3 and 12 months by the measurement of blood counts and liver biochemistry. Diagnostic imaging, either by US, CT, or MRI was performed at an interval of every 3–12 months. A diagnosis of HCC was made based on positive results of typical vascular patterns as revealed by either contrast-enhanced CT, contrast-enhanced MRI or angiography. The mean follow-up period was 7.1 years (range 0.83–11.4 years).
Statistical analysis
An SPSS software package (version 20; SPSS, Chicago, IL, USA) and graph pad prism (version 5.0; Graph Pad Software Inc., San Diego, CA, USA) were used for statistical analysis. Discrete variables were evaluated by Fisher’s exact probability test, and distributions of continuous variables were analyzed by a Mann–Whitney U test.
All p values were calculated by two-tailed tests, and those less than 0.05 were considered statistically significant.
Results
Chronological changes in M2BPGi according to antiviral therapy
The clinical characteristics of the 119 patients prior to treatment are summarized in Table 1.
M2BPGi values were significantly elevated at the end of treatment and decreased after the completion of treatment in patients treated with IFN-based therapy (Fig. 1, p < 0.0001). On the other hand, M2BPGi levels uniformly decreased after starting IFN-free treatment (Fig. 1, p = 0.003). There were no differences between values of those infected with HCV genotype 1 and 2 in serum M2BPGi measured at pretreatment (p = 0.366), end of treatment (p = 0.831), and 24 weeks after treatment completion (p = 0.093), regardless of the standard duration of combination therapy.
Chronological changes in M2BPGi according to virological response
Among 64 patients with SVR, M2BPGi values were significantly decreased after the completion of treatment compared with those at start of treatment (Fig. 2, p < 0.0001), even with progression of liver fibrosis (Supplementary Fig. 1a, p = 0.045). In 12 patients without SVR, there were no significant differences of serum M2BPGi values between before and after treatment (Fig. 2, p = 0.24), regardless of the stage of fibrosis (Supplementary Fig. 1b).
Serum M2BPGi values according to HCC development
During follow-up, HCC developed in 8 of 119 patients [median period after completion of treatment 7.1 years (range 0.83–11.4 years)]. The clinical details of them are summarized in Supplementary Table 1. Serum M2BPGi levels before treatment in patients with HCC were significantly higher than those in patients without HCC (Fig. 3a, p = 0.033); this difference was more marked after treatment (Fig. 3a, p = 0.0004). And even in patients with SVR, serum M2BPGi levels after treatment in patients with HCC were also significantly higher than those in patients without HCC (Supplementary Fig. 2, p = 0.0007). In patients without HCC, M2BPGi values were significantly decreased after the completion of treatment (Supplementary Fig. 3a, p < 0.0001), whereas in patients with HCC there were no differences between before and after treatment (Supplementary Fig. 3a, p = 0.70). Though post-treatment AFP levels were also positively correlated with development of HCC (supplementary Fig. 4, p = 0.0023), there were no correlations with HCC analyzed only in patients with SVR (p = 0.27).
M2BPGi and M2BP levels according to HCC development. M2BPGi (a) and M2BP (b) levels before and 24 weeks after treatment in patients without (non-HCC) and with HCC. The top and bottom of each box represent the first and third quartiles. The length of each box represents interquartile ranges within which are located 50 % of values. The line through the middle of each box represents the median. The error bars are 5–95 percentile. “COI” denotes cutoff index
Predictive potential of M2BPGi levels for advanced fibrosis and HCC development
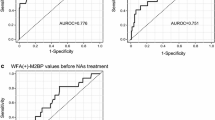
To investigate the predictive potential of serum M2BPGi levels for advanced liver fibrosis and HCC development, receiver operating characteristic (ROC) analyses were performed both before and after treatment. For predicting advanced fibrosis (F3–4), the area under the ROC curve (AUROC) for pre-treatment M2BPGi levels was greater compared with that in post-treatment M2BPGi levels (Fig. 4a, AUROC = 0.848 vs. 0.784). The predictive nature of pre-treatment levels of M2BPGi compared with post-treatment levels [positive predictive value (PPV) = 0.688 vs. 0.462] for advanced fibrosis was superior. For predicting advanced fibrosis, pre-treatment M2BPGi levels yielded the highest AUROC with a level of 0.848 followed by hyaluronic acid (0.789), type 4 collagen (0.805), APRI (0.757), and Fib4 index (0.810) (Supplementary Fig. 5a). The predictive ability of M2BPGi levels for the future development of HCC was superior for post-treatment levels compared with pre-treatment levels (Fig. 4b, AUROC = 0.973 vs. 0.742, PPV = 0.500 vs. 0.224). Both cut-off values for pre-treatment M2BPGi levels predicting advanced fibrosis and post-treatment M2BPGi levels predicting HCC development were 2.2 COI. Post-treatment M2BPGi value was also a useful predictor limited in patients with SVR (Supplementary Fig. 6). For predicting development of HCC, post-treatment M2BPGi levels yielded the highest AUROC with a level of 0.973 followed by APRI (0.743), Fib4 index (0.777) and AFP (0.752) (Supplementary Fig. 7). In multivariate analyses, the progression of liver fibrosis, pre-treatment APRI and HCC development were independent factors that were positively correlated with pre-treatment M2BPGi ≥ 2.2 COI (Table 2). On the other hand, failure of viral eradication and HCC development were identified as factors positively correlated with post-treatment M2BPGi ≥ 2.2 COI (Table 3).
Predictive potential of M2BPGi for advanced fibrosis (F3 or F4) and HCC development (ROC analysis). Receiver operating characteristic (ROC) curves and areas under the ROC curves (AUROC) for serum M2BPGi levels in the diagnosis of F3 or F4 stage of liver fibrosis (a) and for the prediction of HCC development (b) before treatment and 24 weeks after treatment.“AUC” denotes area under the ROC curve. “PPV” denotes positive predictive value. “NPV” denotes negative predictive value
Subgroup analysis of total M2BP level in patients treated with PEG-IFN and RBV
M2BP level was significantly positively correlated with M2BPGi values prior to (r 2 = 0.229, p = 0.0004), at the end of (r 2 = 0.389, p < 0.0001), and at 24 weeks after treatment completion (r 2 = 0.361, p < 0.0001). Serum M2BP level had significant correlation with fibrosis (p = 0.01, r 2 = 0.20), whereas they had no significant correlation with activity index (p = 0.72, r 2 = 0.01). Chronological changes in M2BP levels had no identical tendency after PEG-IFN and RBV therapy (p = 0.09). Moreover, there were no differences of M2BP levels between pre- and post-treatment regardless of the SVR status (Supplementary Fig. 8, p = 0.41 and p = 0.080). Both pre- and post-treatment M2BP levels in patients with HCC were significantly higher than those in patients without HCC (Fig. 3b, p = 0.043 and p = 0.0002).
Discussion
Previous cross-sectional studies have shown M2BPGi to be a useful marker for liver fibrosis [5, 6, 14–16] and treatment outcome for triple therapy including SMV or TVR [17]. In the present study, we analyzed serial M2BPGi levels prior to, at the end of, and 24 weeks after treatment among patients treated with IFN-based and IFN-free therapy. M2BPGi levels were transiently elevated at the end of treatment, but decreased afterwards among patients treated with IFN-based therapy, while M2BPGi levels uniformly decreased after starting IFN-free treatment. In the subgroup analysis, M2BP levels in patients with HCC were significantly higher than those in patients without HCC, which suggested that M2BP and its glycan structure could be associated with hepatocarcinogenesis. On this basis, our study is the first to demonstrate a strict association between serial M2BPGi levels and HCC development after antiviral therapy both among IFN-based and IFN-free treatment.
Although pre-treatment M2BPGi values could distinguish the progression of fibrosis (Supplementary Fig. 9a), post-treatment levels in SVR patients no longer differentiated fibrosis stages as shown in Fig. 2. Though APRI and Fib-4 index also significantly decreased in patients with SVR (Supplementary Fig. 10a, b), such an improvement in liver fibrosis after 24-week-post-treatment is unlikely even in SVR patients. It would be reflected in the reduction of inflammation (Supplementary Fig. 10c, d). Therefore the predictive potential of M2BPGi levels for fibrosis stage at a higher precision may require the presence of HCV. Previous cross-sectional studies for evaluation of M2BPGi levels in patients with chronic hepatitis B or non-alcoholic steatohepatitis, which demonstrated less predictive characteristics with lower cut-off levels for advanced liver fibrosis [18], support this hypothesis.
We found that M2BPGi levels at the end of treatment were uniformly elevated by IFN-based therapy, and decreased after completion of treatment (Fig. 1). These dynamic changes were similar to those of IFN-stimulated genes. On the other hand, M2BPGi levels uniformly decreased after starting IFN-free treatment with asunaprevir and daclatasvir (Fig. 1). Therefore, it is suggested that glycan structures of M2BP reflect not only the stage of liver fibrosis, but also the immune response induced by IFN or other cytokines in the presence of HCV. In contrast, M2BPGi levels after treatment more efficiently distinguished between patients showing HCC development and those without.
In multivariate analyses, pre-treatment M2BPGi levels were affected by APRI, stage of fibrosis, and HCC development, whereas post-treatment M2BPGi levels were affected by SVR and HCC development. Therefore, it is suggested that M2BPGi levels after treatment more directly reflect a potential for hepatocarcinogenesis, probably due to a decrease in the influence of factors, such as the immune response to HCV infection.
M2BP has been shown to be enhanced by IFN stimulation in an HCV replicon system [19] and to increase in breast, prostate, and lung cancers in humans [20–22] and HCC in a rat model [23]. More recently, it was reported that M2BP increased with the progression of liver fibrosis in CHC patients [24]. However, the biological mechanisms responsible for glycan alteration of M2BP in all points for immune response to HCV, liver fibrosis and hepatocarcinogenesis are still unknown. In our subgroup analysis treated with PEG-IFN and RBV, serum M2BP levels were significantly positively correlated with progression of fibrosis and HCC development. It was suggested that both the increase of M2BP production and the alteration of glycan structure of M2BP occurred by progression of fibrosis or development of HCC.
This study has a few limitations, the first being the retrospective nature and small number of patients with HCC studied. Because the incidence of HCC development after achieving SVR was low, longer-term longitudinal studies in larger cohorts are required to verify the generality of our results. A second limitation is the absence of post-treatment liver biopsies. However, a post-treatment liver biopsy from an ethical viewpoint is impossible, especially in patients showing SVR, while a remarkable improvement in liver fibrosis immediately after achieving SVR is unlikely. Our results, which demonstrate the usefulness of post-treatment M2BPGi levels for predicting the future development of HCC in patients with SVR, were not affected by the absence of post-treatment liver biopsies.
In conclusion, post-treatment monitoring of serum M2BPGi has the potential to predict future HCC development in patients undergoing anti-HCV therapy; thus, constructing a post-treatment-surveillance system for HCC using this glyco-marker would be helpful in this regard.
Abbreviations
- HCV:
-
Hepatitis C virus
- WFA:
-
Wisteria floribunda agglutinin
- M2BP:
-
Mac-2-binding protein
- M2BPGi:
-
Mac-2-binding protein glycosylation isomer
- HCC:
-
Hepatocellular carcinoma
- CHC:
-
Chronic hepatitis C
- PEG-IFN:
-
Pegylated-interferon
- RBV:
-
Ribavirin
- SVR:
-
Sustained virological response
- COI:
-
Cut off index
- DAA:
-
Direct-acting antiviral agent
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AFP:
-
α-Fetoprotein
- US:
-
Ultrasonography
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- PC:
-
Positive control
- NC:
-
Negative control
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- SMV:
-
Simeprevir
- TVR:
-
Telaprevir
- ASV:
-
Asunaprevir
- DCV:
-
Daclatasvir
References
Forner A, et al. Hepatocellular carcinoma. Lancet 2012;379:1245–1255
Mohd Hanafiah K, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–1342
Asahina Y, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010;52:518–527
Asahina Y, et al. α-Fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology 2013;58:1253–1262
Kuno A, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065
Kuno A, et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteom Clin Appl 2013;7:642–647
Fujiyoshi M, et al. Hepatitis Glyco-biomarker study group. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J Gastroenterol 2015;50:1134–1144
Tamaki N, et al. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol Res 2015;45:E82–88
Hanai T, et al. Impact of serum glycosylated Wisteria floribunda agglutinin-positive Mac-2-binding protein levels on liver functional reserves and mortality in patients with liver cirrhosis. Hepatol Res 2015;45:1083–1090
Sasaki R, et al. Serum Wisteria Floribunda agglutinin-positive Mac-2-binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis C after sustained virological response. PLoS One 2015;10:e0129053
Wai CT, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26
Sterling RK, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325
Desmet VJ, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513–1520
Artini M, et al. Elevated serum levels of 90 K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J Hepatol 1996;25:212–217
Cheung KJ, et al. The HCV serum proteome: a search for fibrosis protein markers. J Viral Hepat 2009;16:418–429
Yamasaki K, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2-binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563–1570
Ura k, et al. Serum WFA(+)-M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment Pharmacol Ther 2016;43:114–124
Abe M, et al. Association between Wisteria floribunda agglutinin-positive Mac-2-binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol 2015;50:776–784
Marcello T, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006;13:1887–1898
Shirure VS, et al. Mac-2-binding protein is a novel E-selectin ligand expressed by breast cancer cells. PLoS One 2012;7:e44529
Hu J, et al. Expression and significance of 90 K/Mac-2BP in prostate cancer. Exp Ther Med 2013;5:181–184
Sun L, et al. Functional screen for secreted proteins by monoclonal antibody library and identification of Mac-2-binding protein (Mac-2BP) as a potential therapeutic target and biomarker for lung cancer. Mol Cell Proteom 2013;12:395–406
Vásquez-Garzón VR, et al. Analysis of gene expression profiles as a tool to uncover tumor markers of liver cancer progression in a rat model. Biomed Rep 2015;3:167–172
Tawara S, et al. Evaluation of fucosylated haptoglobin and Mac-2-binding protein as serum biomarkers to estimate liver fibrosis in patients with chronic hepatitis C. PLoS One 2016;11:e0151828
Acknowledgements
This study is based on the participation of six multicenter hospitals (Tokyo Medical and Dental University Hospital, Kashiwa City Hospital, Showa General Hospital, Toride Kyodo General Hospital, Mishima General Hospital).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Asahina and Dr. Kakinuma belong to donation-funded department funded by Chugai Pharmaceutical Co. Ltd., Toray Industries Inc., Bristol-Myers Squibb, Dainippon Sumitomo Pharma Co. Ltd., and Merck Sharp & Dohme.
Financial support
This study was supported by grants for research received from Sysmex Corporation, grants from the Ministry of Education, Culture, Sports, Science and Technology-Japan, the Japan Society for the Promotion of Science, Japan Agency for Medical Research and Development, the Japan Health Sciences Foundation, the Miyakawa Memorial Research Foundation, and the National Institute of Biomedical Innovation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients. The Ethical Committee of Tokyo Medical and Dental University approved this study, which was conducted in accordance with the Declaration of Helsinki (Confirmation No. 1863).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagata, H., Nakagawa, M., Nishimura-Sakurai, Y. et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol Int 10, 956–964 (2016). https://doi.org/10.1007/s12072-016-9754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9754-1