Abstract
The epidemiology of hepatocellular carcinoma (HCC) in the Asia Pacific will undergo significant change over the next few decades as the prevalence of viral hepatitis declines and the burden of metabolic diseases increases. As the Asia Pacific embraces continued affluence, obesity and diabetes rates are burgeoning, becoming increasingly important to the incidence of HCC. Obesity and diabetes are established risk factors for HCC, either as substrates for non-alcoholic fatty liver disease (NAFLD) or as independent carcinogens themselves. This review summarises the epidemiological data on changing HCC trends in the Asia Pacific, particularly as it pertains to the emerging problem of NAFLD-related HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Asia Pacific region has undergone massive socioeconomic changes in the past 2 decades, and with this, a dramatic shift away from communicable diseases as public health priorities to a health landscape dominated by obesity, diabetes and their co-morbidities. The rates of obesity and diabetes are burgeoning in the Asia Pacific, driving not only a growth in non-alcoholic fatty liver disease (NAFLD), but also in NAFLD-related complications including hepatocellular carcinoma (HCC). NAFLD, the hepatic manifestation of the metabolic syndrome, can no longer be considered a disease of the West, as prevalence figures in the Asia Pacific are comparable to many Western countries, with estimates reaching up to 40 % [1]. There is compelling epidemiological and biological evidence that non-alcoholic steatohepatitis (NASH), the more aggressive entity in the fatty liver disease spectrum, can progress to HCC [2, 3]. This has significant implications for the Asia Pacific, a region that already has the heaviest burden of liver cancer.
HCC is the predominant type of primary liver cancer and is the third leading cause of cancer death worldwide [4]. More than three-quarters of all HCC cases are from Asia, where the dominant risk factors are chronic infection with hepatitis B (HBV) or C (HCV) [5]. With the implementation of HBV immunisation programmes and access to more effective treatments for HBV and HCV, the prevalence of viral hepatitis and thus its role in HCC are expected to decrease over the coming decades. In this review, we outline the changing trends in HCC epidemiology across the Asia Pacific as the role of viral hepatitis declines and metabolic risk factors become increasingly prevalent as the principal risk for liver cancer. We also review data on the incidence, clinical features and outcomes of NASH-related HCC in the region and identify priorities for further research.
HCC in the Asia Pacific: burden of disease and changing epidemiology
There is a striking disparity in the global distribution of HCC, with the Asia Pacific representing the peak axis of HCC incidence and mortality. In the most recent estimate from the International Agency for Research on Cancer (IARC), approximately 750,000 new cases of liver cancer were reported in 2008 [6]. The greatest burden lies in Eastern and Southeastern Asia, Middle and Western Africa, and the Western Pacific, with China alone reporting 55 % of all new cases [7]. Age-standardised incidence rates (ASIRs) for liver cancer in the Asia Pacific surpass rates in most of the developed world including North America and Northern Europe where the incidence is less than 5 per 100,000 persons [5, 8]. Registries in Korea, Japan, Vietnam, China, Shanghai and Hong Kong all report very high incidence rates of greater than 20/100,000 persons [9]. However, robust epidemiological studies on HCC incidence in the Asia Pacific, particularly in less developed countries, are lacking. Figure 1 summarises the most recent ASIR from selected countries in the region, using data from GLOBOCAN 2008, an IARC database compiling information from cancer registries across the world [6].
The geographic variation in HCC incidence corresponds to the distribution of the dominant risk factors, HBV and HCV, a phenomenon termed “geographic parallelism” [10]. HBV is highly endemic across the Asia Pacific with more than 8 % of the population being surface antigen positive compared to <2 % of the population in North America and Northern Europe [8]. The HBV is a class 1 carcinogen and accounts for 80 % of HCC cases across the Asia Pacific [11]. The exception to this is Japan and Australia, where HCV is the dominant risk factor. In the seminal study by Beasley et al. [12] in 1981, the relative risk (RR) of HCC in patients with chronic HBV was 223 compared to uninfected persons. Subsequently, several prospective cohort studies confirmed an RR between 5- and 100-fold for developing HCC in persons with chronic hepatitis B infection [9]. In contrast to the Asia Pacific, HCV is the major risk factor for HCC in regions of low HCC incidence and accounts for 10–20 % of all cases globally [8].
The dominant role of HBV in shaping HCC incidence is declining. Since 1992 when the World Health Assembly recommended universal vaccination against HBV for all newborns, more than 90 % of countries have adopted the policy and more than 70 % are delivering three immunisation doses [13]. It is projected that notable decreases in hepatitis B-related HCC will be seen over the next 2 to 3 decades, allowing for the latency between infection and cancer development. Taiwan was one of the first countries to vaccinate and give HBV immunoglobulin to newborns in 1984 [14]. The impact of this programme is already evident in children and adolescents, where liver cancer incidence has significantly declined in vaccinated children aged 6–19 compared to those unvaccinated at birth (RR 0.31, p < 0.001 age and sex adjusted) [15].
The epidemiology of HCV-related HCC is also changing across the globe. In the US, the incidence of HCC has tripled in the last 30 years, largely due to chronic HCV infection [16]. This trend is expected to plateau by 2020 [17] as the contribution from the cohort infected in the 1960–1970s declines, and more effective antiviral therapies become available. In Japan, where 79 % of HCCs are associated with HCV [11], principally from an infection wave beginning in the 1920s and increasing after World War II, the incidence has already declined in the last decade [18]. The ASIR of HCC in men in Osaka, Japan, rose from 29.2 to 41.9 cases per 100,000 persons between 1981 and 1987 and thereafter progressively declined to 24 cases per 100,000 in 2003 [18].
Decreases in virus-related HCC are likely to be countered by the emergence of NAFLD as a major cause of liver disease. Based on epidemiological data, and the trend to continued affluence, NAFLD is likely to become the dominant cause of HCC in the US and other developed countries [17]. Likewise, as the Asia Pacific verges on the brink of its own NAFLD and metabolic syndrome epidemic, NASH will become an important determinant of HCC epidemiology in the region.
Obesity and diabetes in the Asia Pacific: increasing substrates for NAFLD and NAFLD-related complications
Obesity
According to the most recent World Health Organisation estimate, more than 1.8 billion people are overweight and this is expected to increase to 2.16 billion by 2030 [19]. Although in absolute terms the prevalence of overweight individuals is low in Southeast Asia (14 % in both sexes) compared to the Americas (62 % in both sexes) [20], the most rapid increases in obesity prevalence have occurred in Asia Pacific countries. In China, the prevalence of overweight and obesity increased by an alarming 414 % between 1982 and 2002 from 3.7 to 19 % [21]. In Japan, the prevalence increased by 46 % between 1980 and 2000 from 16.7 to 24 % [21].
Importantly, several studies have established that visceral adiposity is higher in Asians compared to Caucasians with a similar BMI [22–24]. Abdominal obesity is highly prevalent across the Asia Pacific, particularly in South Asia (India and Pakistan), where 58 % of men and 78 % of women are abdominally obese according to International Diabetes Federation criteria [25]. This has important implications as visceral adipose tissue (VAT) is immunologically and metabolically active and associated with multiple end-organ effects including NAFLD and hepatic carcinogenesis [26].
Diabetes
In concert with the epidemic of obesity, the Asia Pacific will undergo exponential increases in diabetes prevalence over the next 2 decades. In a recent study by the China National Diabetes and Metabolic Disorders Study Group, the prevalence of diabetes in China was estimated at 9.7 %, and pre-diabetes 15.5 % [27]. This translates to 92.4 million people with diabetes and 148.2 million with pre-diabetes. By 2030, the worldwide prevalence of diabetes will increase by a further 114 %, with India and China sharing the highest burden [28]. Furthermore, among the top 10 countries to have the highest prevalence of diabetes in 2030, an additional four—Indonesia, Pakistan, Bangladesh and the Philippines—are in Asia [28]. Of concern, in a study by the Asia Pacific Cohort Studies Collaboration, the current prevalence of diabetes in South Korea, Tonga and Thailand already exceeds their projections for 2030 [29].
NAFLD
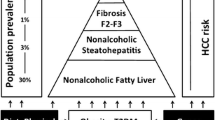
Obesity and diabetes are key substrates for the development of NAFLD. Accordingly, along with the explosion of obesity and diabetes in the Asia Pacific, a parallel rise in NAFLD has been observed. Although large regional variations exist, at least 10 % of the population in Asia has fatty liver detected on ultrasonography, while rates of over 25 % have been reported in several countries including India, Japan, Sri Lanka and Korea (Table 1) [30–35]. The prevalence of NASH is more difficult to estimate as studies where histology is available are biased toward select (usually tertiary hospital) populations. In the limited number of cross-sectional studies where liver histology is reported, over 50 % of Asian NAFLD patients are noted to have NASH [36].
The metabolic risk factors associated with NAFLD in the West are closely linked to the epidemiology of NAFLD in Asia Pacific countries. In a study from India, 88 % of patients with diabetes had evidence of fatty liver on ultrasound [37]. Another from Shanghai indicated that the presence of diabetes increased the risk of NAFLD by 31-fold [95 % confidence interval (CI) 14.18–70.35] [38]. NAFLD is also highly prevalent in the Asian obese. For example, in Japan, using annual health check data, the prevalence of NAFLD was 10–20 % in the non-obese, 50 % for those with a BMI between 25 and 30 kg/m2, and 80 % for those with a BMI over 30 [39].
In addition to affluence driving metabolic risk in the Asia Pacific, Asians may also have a genetic predisposition to developing fatty liver. Apolipoprotein C3 (APOC3) is a protein inhibitor of lipoprotein lipase that hydrolyses triglycerides in chylomicrons, VLDL, LDL and diacylglycerols [40]. In a US study, polymorphisms in APOC3 were associated with NAFLD in 38 % of lean young Asian Indian men compared to 9 % of non-Asian Indian men [41]. The prevalence of NAFLD in wild-type homozygotes (Asian or non-Asian) was 0 %. Furthermore, carriers of the variant alleles (C-482T and T-455C) were found to have a 60 % increase in plasma triglyceride concentration and a two-fold increase in post-prandial plasma triglyceride and retinyl fatty ester concentrations after an oral fat-tolerance test. The authors proposed that APOC3 variants result in increased APOC3, which inhibits lipoprotein lipase. This causes hypertriglyceridemia due to increased chylomicron remnants, which are preferentially taken up by the liver, resulting in NAFLD. This report has not been replicated and thus larger cohort studies, inclusive of other ethnic groups, are needed.
Longitudinal studies on the natural history of NAFLD in the Asia Pacific region are lacking. However, studies from Western cohorts firmly establish the risk of steatohepatitis progressing to cirrhosis and from there to HCC [42, 43]. Thus, it is reasonable to assume that the burden of NASH-associated complications in the Asia Pacific will also increase over the coming decades. If NASH supersedes viral hepatitis as the dominant cause of liver cancer in the region, it will represent one of the most dramatic shifts in cancer epidemiology, warranting new paradigms in public health policy and prevention strategies across the region.
Epidemiology of NASH HCC in the Asia Pacific
The true incidence of NASH HCC is difficult to establish as there is a paucity of prospective longitudinal studies worldwide, particularly so in the Asia Pacific. Studies on this evolution are difficult to perform, largely owing to the long, slowly progressive course of NAFLD [43]. From the longitudinal studies available, it is clear that NASH can progress to HCC and this risk is comparable to that from hepatitis C-related cirrhosis. In a recent prospective cohort study from the US, 25/195 (12.8 %) of NASH cirrhotic patients developed HCC during a median follow-up of 3.2 years [44]. The yearly cumulative incidence of HCC was 2.6 % in NASH cirrhosis compared to 4.0 % in HCV cirrhosis, a statistically insignificant difference (p = 0.09). The study concluded that patients with NASH cirrhosis have a greatly increased risk of HCC, rivalling the risk in HCV cirrhosis. In another recent prospective cohort study from centres in Australia, the US and Europe, 6/247 (2.4 %) patients with NASH cirrhosis/advanced fibrosis developed HCC during a mean follow-up of 85.6 months [42], suggesting a lower annual incidence of HCC.
To date, the only longitudinal study from Asia to examine HCC in NAFLD is by Yatsuji et al. [45]. In this Japanese study of 68 NASH patients with cirrhosis followed for a mean of 41 months, the 5-year HCC rate was 11.3 %. The 5-year occurrence rate of HCC in controls with HCV-related cirrhosis was higher at 30.5 %; however, this difference was not significant (p = 0.185). In another, large retrospective cohort study of 6,508 Japanese patients with NAFLD, 184 patients with significant fibrosis were identified using the AST-to-platelet ratio index [46]. In this group, 6/184 (3.26 %) of patients developed HCC in the 6-year follow-up period. A limitation of this study was that although patients with HCV antibody or hepatitis B surface antigen were excluded, hepatitis B core antibodies were not measured and thus occult HBV infection cannot be ruled out. This has important implications, as demonstrated in a recent meta-analysis of eight prospective studies (six from Japan), which gave a pooled 2.44-fold increased risk of HCC in patients with occult HBV infection [47]. The prevalence of NASH HCC in Japan can also be estimated from nationwide surveys. In a nationwide survey of 33,379 patients with cirrhosis, 2.1 % (n = 647) were attributed to NASH and 31.5 % (n = 199) of those with NASH cirrhosis were found to have HCC [48].
Incidence and prevalence data on NASH HCC from other Asia Pacific countries are lacking. India has one of the highest rates of NAFLD in Asia. From what is known about the prevalence of NAFLD in India, it is possible to provide rough estimates of the prevalence of NASH-associated HCC (Fig. 2). On current estimates, there may be ~120 million people in India with NAFLD [49], and in a recent prospective epidemiological study, 31 % of those with NAFLD have NASH on histology [50]. This would equate to a possible 37 million people with NASH. Overall, NASH progresses to cirrhosis in 10 % of cases [2, 51], equating to 3.7 million people. Retrospective studies suggest that up to 25 % of NASH-cirrhosis cases are complicated by HCC [2, 52], suggesting a potential staggering 930,000 people in India with NASH HCC. In the recent landmark Million Death Study, cancer mortality rates in India were estimated from verbal autopsy findings of 122,429 deaths from 1.1 million homes in India [53]. Liver cancer was the fourth leading cause of cancer mortality with an age-standardised cancer mortality rate of 6.8 per 100,000 (99 % CI 5.4–8.1).
Prevalence figures for NASH HCC are an underestimate if the contribution of NASH to cryptogenic cirrhosis (CC) is taken into account. There is compelling evidence that a majority of patients with CC have undiagnosed NASH [54]. In a US study of 105 patients with HCC, 29 % were found to have CC [55]. Of those with CC, half had clinical or histological features consistent with NAFLD. Similar evidence has emerged from the Asia Pacific. In a study from India of living-donor liver transplant recipients, two-thirds of patients with a pre-transplant diagnosis of CC were ultimately diagnosed with NAFLD on their explant liver [56]. Likewise, in a case-control study from Japan, clinical features of NAFLD—obesity, visceral adiposity, diabetes and hypertriglyceridemia—were more frequent in CC patients than in controls with virus-associated cirrhosis [57].
Clinical characteristics of NASH HCC in the Asia Pacific
Interestingly, despite the scarcity of incidence and prevalence data on NASH HCC in Asia, there is some literature on the characteristics of the tumors, particularly from Japan [1]. In a cross-sectional Japanese study, the clinical features of 87 patients with histology-proven NASH who developed HCC were assessed [58]. The median age was 72 years. The male-to-female ratio was 1.6:1, which is consistent with the gender disparity seen in HCC of other etiologies. Over half of the patients had obesity, diabetes and hypertension. Importantly, although cirrhosis and advanced fibrosis were the key risk factors for HCC (present in 51 and 72 % of cases, respectively), 28 % (n = 25) of tumors developed in those with stage 1 or 2 fibrosis. This finding in the largest cohort of non-cirrhotic NASH-HCC patients to date supports multiple worldwide case reports and series of HCC in NASH occurring in the absence of cirrhosis [17]. Further prospective studies are needed to elucidate the true frequency of this entity.
Studies on the prognosis and outcomes of NASH HCC are limited. To date, the only long-term prospective cohort study on outcomes is from Japan [59]. In that study, 34 NASH-HCC patients were age- and sex-matched to 56 patients with HCV HCC. The 5-year recurrence rate after curative treatment was 69.8 % in NASH HCC and 83.1 % in HCV-related HCC, a difference that was not statistically significant. Risk factors for recurrence in NASH-HCC patients were fibrosis stage and tumor size, and in the HCV-HCC group, obesity, diabetes and tumor burden. The 5-year survival rate between the two groups was also comparable at 55.2 % in NASH-HCC and 50.6 % in HCV-HCC patients. These findings differ from a recent retrospective study from the US where patients with NASH HCC were found to have better overall survival after curative therapy than patients with HCV or alcohol-related HCC (at 3 years, 61 vs. 36 %, respectively, p = 0.029) [60]. In this study, however, the groups differed significantly in their clinical and tumor characteristics and were not case-control matched.
Thus, although the literature on NASH HCC in Asia is limited, of variable quality and arises predominantly from Japan, clinicians need to be aware that HCC can develop in NASH cirrhosis at a rate rivalling HCV cirrhosis. NASH-HCC patients tend to be older, male, and often have multiple metabolic co-morbidities that will contribute not only to their liver-related mortality but also overall mortality. Importantly, even though current HCC surveillance guidelines do not address the risk of non-cirrhotic NASH HCC, clinicians need to be aware of this entity.
Obesity and diabetes as risk factors for HCC, independent of NAFLD
Obesity and diabetes increase the burden of HCC in the Asia Pacific not only via the development of NAFLD, but perhaps also as an independent co-carcinogen. Extensive epidemiological studies have demonstrated a strong association between obesity and HCC. In a recent systematic review of ten cohort studies, one nested case-control study and two case-control studies, obese individuals were found to have a 1.4–4.1 RR of HCC [61]. Four out of the 13 studies included were from the Asia Pacific—two from Japan, one from Taiwan and one from Korea—representing a total Asian cohort of more than 1.5 million. In a systematic review of obesity and liver cancer in the Japanese, five out of nine cohort studies reported a weak to strong positive association between BMI and liver cancer risk, while three case-control studies reported a strong positive association [62]. Overall, overweight/obese individuals had an RR of 1.74 (95 % CI 1.33–2.28) for developing liver cancer compared with individuals with normal weight. However, an important caveat is that the data do not exclude the possibility that patients with obesity and diabetes developed NAFLD that then progressed to HCC.
There is emerging literature that the association between obesity and various cancers, including HCC, is mediated by visceral rather than generalised body fat [26]. VAT is directly related to the degree of inflammation and fibrosis in NASH [63] and is thus linked to the progression to NASH HCC. VAT also promotes a pro-tumorigenic environment of systemic chronic inflammation that can have effects on the liver and other tissues [26]. Of interest, in a Japanese cross-sectional study, individuals with a higher visceral fat area on CT (>130 cm2 in males and >90 cm2 in females) had higher rates of HCC recurrence after curative treatment than those with lower visceral fat areas (75.1 vs. 43.1 %, respectively at 3 years, p = 0.018) [64]. This has important ramifications for the Asia Pacific given that Asians (particularly South Asians) have a tendency to greater VAT accumulation than their Western counterparts.
Diabetes has also been established as an independent risk factor for HCC. Several large-scale population-based cohort studies from the US and Europe demonstrated a 1.86- to 4-fold increase in HCC risk with diabetes [2]. Asian people with diabetes also have a higher risk of HCC. For example, in a systematic review and meta-analysis of four cohort studies and one case-control study from Japan, diabetes was associated with a significantly increased risk of HCC (OR 3.64, 95 % CI 2.61–5.07) [65]. In a recent large population-based cohort study from Taiwan by Lai et al. [66], the incidence of HCC was twice as high in diabetic patients than in non-diabetics.
Importantly for the Asia Pacific, there appears to be a synergistic effect of obesity and diabetes with viral hepatitis on HCC risk. In the Lai et al. study [66], the hazard ratio for HCC associated with diabetes was 1.73 (95 % CI = 1.47–2.03), and this increased to 72.4 (95 % CI = 42.9–122) among patients with diabetes, hepatitis C and cirrhosis. In a study of 23,820 participants in Taiwan with 14 years of follow-up, a BMI over 30 in individuals without viral hepatitis was associated with a two-fold increased risk of HCC [67]. In individuals with HCV, obesity increased the risk of HCC four-fold. Diabetes increased the risk of HCC three-fold in HCV and two-fold in HBV. When both obesity and DM were present with either virus, the risk of HCC increased by a staggering 100-fold. NAFLD itself, independent of obesity and diabetes, also appears to act synergistically with viral hepatitis to increase HCC risk. In a prospective study of 161 Japanese patients with chronic hepatitis C, steatosis independently increased the risk of HCC by 2.89-fold (95 % CI = 1.24–6.37; p < 0.0135) compared to hepatitis C patients without steatosis [68]. This association was not confirmed in an Australian study that, unlike the Japanese study, matched patients for fibrosis and therefore excluded the potential confounding effect of fibrosis on HCC development [69]. Further prospective studies with larger cohorts are needed to define this relationship among NAFLD, viral hepatitis and HCC risk.
Mechanisms of hepatocarcinogenesis in NASH
The precise pathogenesis of HCC in NASH is not known, but three key pathways postulated include: inflammation and adipocytokines; hyperinsulinaemia and the insulin-like growth factor (IGF)-1 axis; and the generation of reactive oxygen species (ROS). Chronic inflammation is intimately related to tumorigenesis, influencing the proliferation of cancer cells, angiogenesis, the risk of metastases and response to cancer treatment [70]. NASH and its principal risk factors, obesity and diabetes, are associated with the release of multiple proinflammatory cytokines and adipocytokines including TNF-α and IL-6. In murine models, NASH induced by dietary manipulation promoted diethyl nitrosamine-initiated HCC development, and this process was associated with increased TNF-α signalling [71]. Adiponectin, the most abundant adipokine, is secreted at reduced levels in obesity and NAFLD, and this may also contribute to hepatocarcinogenesis [17]. In mice, adiponectin has been shown to substantially reduce HCC development, and in tissue microarray of human HCC patients, adiponectin was inversely correlated with HCC size [72].
NASH is associated with insulin resistance and hyperinsulinaemia. Chronic hyperinsulinaemia reduces the expression of IGF binding protein (IGFBP)-1 and -2, resulting in increased levels of free IGF-1 [73, 74]. It is widely postulated, based on in vitro studies, that increased IGF-1 promotes hepatocarcinogenesis through its mitogenic and anti-apoptotic effects [73, 75]. However, recent clinical studies have suggested that circulating IGF-1 levels are in fact decreased in HCC and low IGF-1 has been correlated with poor overall survival [76]. A possible explanation is that as IGF-1 is synthesised mainly in the liver, levels decline in HCC and cirrhosis [77]. The relationship between the IGF-axis and HCC development remains incompletely understood, and further in vitro and epidemiological studies are needed to define this.
NASH generates ROS in the liver and this has also been implicated in HCC development [2] through its proinflammatory and -oncogenic signals [78]. ROS may directly induce oncogenic gene mutations including those of the p53 tumor suppressor gene and of the nuclear respiratory factor (Nrf 1) [2]. Importantly, ROSs promote sustained activation of c-Jun amino-terminal kinase (JNK1) and this has been strongly associated with HCC development. In one study, JNK1 activation was present in over 50 % of human HCC samples compared to adjacent non-cancerous tissue [79]. Sustained JNK1 activation increases several genes essential for hepatocyte proliferation [80]. Further research is needed to identify potential JNK1-associated HCC signature genes as these may be potential future therapeutic targets [80].
Future directions
The future burden of NASH associated HCC in the Asia Pacific is becoming increasingly apparent, but there is a clear lack of high-quality epidemiological data across the region. Prospective longitudinal studies on the evolution of HCC in NAFLD are urgently needed to estimate the true incidence of this cancer. Liver biopsies to diagnose NASH are clearly not feasible in large cohorts; however, the use of non-invasive modalities including transient elastography and validated NAFLD fibrosis scoring systems need to be utilised in clinical studies. Although there are a number of studies from Japan that report the clinical characteristics of NASH HCC, the Asia Pacific is such a diverse region that more representative studies, for example from areas of South Asia where the burden of NAFLD is heaviest, are also needed. Our understanding of the frequency and natural history of HCC in non-cirrhotic NASH is in its infancy, and more studies are needed as this has implications for HCC surveillance.
The potential for a genetic predisposition to NASH-associated liver cancer needs further investigation. Emerging evidence suggests polymorphisms in APOC3 may increase the risk of NAFLD in South Asians [41], but whether this and other mutations increase the propensity to HCC development is not known.
Conclusion
With the burgeoning rates of obesity and diabetes, NAFLD and NAFLD-related HCC are public health priorities for the region. Over the next few decades, the epidemiology of liver cancer in the Asia Pacific will significantly alter as the prevalence of viral hepatitis declines and that of NASH increases. There is currently an unmet and urgent need for large-scale, cross-country, prospective studies on the incidence, clinical characteristics and outcomes of NASH HCC in the region. Based on the literature to date, clinicians need to recognise that NASH-related liver cancer occurs more frequently in older men, that metabolic co-morbidities including obesity, diabetes and hypertension often coexist, and that although cirrhosis greatly increases the risk of HCC, tumors may also develop in its absence.
References
Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res 2012;42:1–14
Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–1832
Page JM, Harrison SA. NASH and HCC. Clin Liver Dis 2009;13:631–647
Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol 2012;13:750–751
Sherman M. Epidemiology of hepatocellular carcinoma. Oncology 2010;78(Suppl 1):7–10
Ferlay J, Shin H, Bray F, Forman D, Mathers CD, Parkin D. GLOBOCAN 2008 v2.0, cancer incidence and mortality worldwide: IARC Cancer Base No. 10 [Internet]. Lyon: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr. Accessed 20 Feb 2013
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90
Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15(Suppl 4):5–13
McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011;15:223–243, vii–x
Alam N, Robotin M, Baker D. Epidemiology of primary liver cancer. Cancer Forum 2009;33:88–92
Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol 2009;24:346–353
Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet 1981;2:1129–1133
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–73
Chen DS, Hsu NH, Sung JL, Hsu TC, Hsu ST, Kuo YT, et al. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA 1987;257:2597–2603
Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009;101:1348–1355
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–1491
Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384–1391
Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med 2008;148:820–826
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21
World Health Organization. World Health Statistics 2012. Geneva: World Health Organization; 2012
Asia Pacific Cohort Studies Collaboration. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev 2007;8:191–196
Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res 2001;9:381–387
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–146
He Q, Horlick M, Thornton J, Wang J, Pierson RN Jr, Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab 2002;87:2164–2170
Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obes 2010;2010. doi:10.1155/2010/868573
Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett 2013;330:1–10
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101
Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 2010;375:408–418
Lee CM, Huxley RR, Lam TH, Martiniuk AL, Ueshema H, Pan WH, et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr 2007;16:187–192
Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–415
Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med 2005;22:1141–1145
Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009;50:1029–1034
Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract 2009;84:84–91
Dassanayake AS, Kasturiratne A, Rajindrajith S, Kalubowila U, Chakrawarthi S, De Silva AP, et al. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol 2009;24:1284–1288
Lee K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes Metab 2009;35:447–451
Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol 2011;26(Suppl 1):163–172
Duseja A. Nonalcoholic fatty liver disease in India—a lot done, yet more required! Indian J Gastroenterol 2010;29:217–225
Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol 2005;20:1825–1832
Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol 2011;46(Suppl 1):63–69
Romero-Gomez M. APOC3 polymorphisms and non-alcoholic fatty liver disease: resolving some doubts and raising others. J Hepatol 2011;55:1184–1186
Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med 2010;362:1082–1089
Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208–1216
Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978
Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24:248–254
Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol 2012;107:253–261
Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012;32:231–240
Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2010;45:86–94
Mahady SE, George J. The future liver of the Asia Pacific: fatter and firmer from more fructose and fortune? J Clin Exp Hepatol 2013. doi:10.1016/j.jceh.2012.10.011
Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, Dhibar T, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 2010;51:1593–1602
Agrawal S, Duseja A. Non-alcoholic fatty liver disease: east versus west. J Clin Exp Hepatol 2012;2:122–134
Rosmorduc O, Fartoux L. HCC and NASH: How strong is the clinical demonstration? Clin Res Hepatol Gastroenterol 2012;36:202–208
Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: a nationally representative survey. Lancet 2012;379:1807–1816.
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134–140
Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002;36:1349–1354
Nayak NC, Vasdev N, Saigal S, Soin AS. End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol 2010;41:425–430
Kojima H, Sakurai S, Matsumura M, Umemoto N, Uemura M, Morimoto H, et al. Cryptogenic cirrhosis in the region where obesity is not prevalent. World J Gastroenterol 2006;12:2080–2085
Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428–433; quiz e450
Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, et al. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol 2010;45:960–967
Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012;55:1809–1819
Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma—epidemiological evidence. Aliment Pharmacol Ther 2010;31:1051–1063
Tanaka K, Tsuji I, Tamakoshi A, Matsuo K, Ito H, Wakai K, et al. Obesity and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2012;42:212–221
van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–457
Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009;58:839–844
Noto H, Osame K, Sasazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complicat 2010;24:345–353
Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol 2012;107:46–52
Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 2008;135:111–121
Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer 2003;97:3036–3043
Kumar D, Farrell GC, Kench J, George J. Hepatic steatosis and the risk of hepatocellular carcinoma in chronic hepatitis C. J Gastroenterol Hepatol 2005;20:1395–1400
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–1081
Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer 2009;124:540–546
Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, et al. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology 2010;139:1762–73
Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 2010;61:301–316
Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 2012;71:181–189
Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem 2008;114:71–83
Kaseb AO, Morris JS, Hassan MM, Siddiqui AM, Lin E, Xiao L, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol 2011;29:3892–3899
Bonefeld K, Moller S. Insulin-like growth factor-I and the liver. Liver Int 2011;31:911–919
Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut 2010;59:1303–1307
Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, et al. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol 2009;50:323–333
Chen F, Beezhold K, Castranova V. JNK1, a potential therapeutic target for hepatocellular carcinoma. Biochim Biophys Acta 2009;1796:242–251
Acknowledgements
This work is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, a National Health and Medical Research Council Postgraduate Scholarship, grants from the National Health and Medical Research Council (1006200, 632630, 1047417, 1049857), a Strategic Research Partnership Grant and an Innovator Grant from the Cancer Council NSW, and Translational Cancer Grants from the Cancer Institute NSW and Cancer Australia.
Conflict of interest
Roslyn Vongsuvanh, David van der Poorten, and Jacob George declare no conflicts of interest, financial or otherwise, to disclose.
Compliance with Ethical Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vongsuvanh, R., van der Poorten, D. & George, J. Non-alcoholic fatty liver disease-related hepatocellular carcinoma: a sleeping tiger in the Asia Pacific. Hepatol Int 7 (Suppl 2), 823–832 (2013). https://doi.org/10.1007/s12072-013-9453-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-013-9453-0