Abstract
Purpose
The effectiveness of imaging (including apparent diffusion coefficient [ADC] of diffusion-weighted magnetic resonance imaging [DWI]) and laboratory variables for predicting early tumor recurrence and overall survival after surgery in hepatocellular carcinoma (HCC) patients are analyzed.
Methods
The present study included 116 consecutive patients with HCC who underwent partial hepatectomy. Patients were classified into two groups: patients with and without early recurrence (<1 year). Preoperative imaging variables (tumor number, size, shape, capsule, ADC, and venous invasion) and laboratory variables were evaluated to predict early recurrence using univariate and multivariate analyses. Overall survival was calculated using the Kaplan–Meier method.
Results
Twenty patients (17 %) developed early recurrence after surgery. Multivariate logistic regression analysis showed that tumor ADC (p = 0.0002), aspartate aminotransferase (p = 0.0121), and serum prothrombin time activity percentage (p = 0.0082) were statistically significant for predicting early recurrence. The optimal ADC cutoff value for predicting early recurrence obtained from receiver operating characteristic analysis was ≤0.898 × 10−3 mm2/s. In patients with ADC ≤0.898 × 10−3 mm2/s, the 3- and 5-year survival rates (77 and 56 %, respectively) were significantly decreased compared with those in patients with ADC >0.898 × 10−3 mm2/s (97 and 97 %, respectively; p = 0.0015).
Conclusions
Low tumor ADC value by DWI was a risk factor for early postoperative HCC recurrence and was associated with lower patient survival rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgery is the mainstay of treatment for anatomically resectable hepatocellular carcinoma (HCC) [1–5]. Given recent improvements in early detection, surgical techniques, and postoperative management, an increasing number of patients undergo curative resection of HCC. Despite these remarkable improvements, however, the rate of postoperative recurrence of either intrahepatic metastasis or metachronous multicentric HCC remains high [6]. Early tumor recurrence, which is defined as intrahepatic recurrence within 1 year, after potentially curative resection for HCC is one of the most important factors impacting prognosis and outcome of the disease. Recurrent disease is the leading cause of death during the first year [7], and the time from resection to recurrence is an independent prognostic factor for survival after recurrence [8, 9].
The histological differentiation of HCC has been reported as a predictor of early recurrence of HCC after curative resection [10, 11]. Tumor differentiation can only be evaluated invasively, through either biopsy or histological examination of the surgical specimen. Recent reports have indicated that apparent diffusion coefficient (ADC) values of diffusion-weighted magnetic resonance (MR) imaging (DWI) correlate well with the degree of HCC differentiation. Significantly lower ADC values were observed with moderately and poorly differentiated HCCs compared with well-differentiated HCCs [12, 13]. Based on this knowledge, we hypothesized that ADC can be used as a biomarker for predicting early tumor recurrence and patient survival.
The purpose of the present study was to determine the preoperative variables, including ADC, which may help predict early recurrence and subsequent outcomes in patients undergoing potentially curative resections for HCC.
Materials and methods
Patients
The present study followed the principles of the Declaration of Helsinki and was approved by the institutional review board at our institution. Written informed consent was waived. The present study included 151 patients who underwent partial hepatectomy for HCC in the department of surgery at our institution between 1 January 2004 and 31 December 2009. The remnant liver was routinely evaluated for additional tumors with intraoperative ultrasonography. Data from before, during, and after surgery were retrieved from electronic patient charts (database) at the institution.
Patients excluded from the study included those: (1) who received transarterial chemoembolization (TACE) or radiofrequency ablation before surgical treatment (n = 4), (2) whose preoperative radiological evaluation with three-phase dynamic enhanced multi-detector computed tomography (CT) and DWI during the month before surgery was unavailable (n = 27), (3) who had fibrolamellar carcinoma (n = 1), (4) whose postoperative follow-up data was unavailable (n = 2), and (5) who died within 1 month after surgery (operative mortality; n = 1).
This process resulted in a study population of 116 patients (88 men and 28 women) aged 35–85 years (mean [SD]: 66.85 [10.89] years). Patient characteristics are shown in Table 1.
Follow-up after surgery and standard of reference for early recurrence
Mean and median follow-up periods for surviving patients after hepatic resection were 28 and 24.5 months (range 3–70 months), respectively. After surgery, patients were regularly followed up every 3 months using abdominal ultrasound, determination of the levels of serum tumor markers, such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence-II (PIVKA-II; if the level was elevated before surgery). Dynamic CT or MRI was also performed every 3 months for the first year and every 6 months thereafter. Follow-up dynamic CT was performed using iodine contrast agent, while two types of contrast agents were used in the follow-up dynamic MRI. Conventional or extracellular gadolinium-based contrast agent was used until January 2008. Gadoxetic acid was used since January 2008. Additional contrast-enhanced ultrasound, CT during arterial portography, CT during hepatic angiography, and angiography were performed when necessary. Early tumor recurrence was defined as intrahepatic recurrence identified within 1 year after surgery.
The presence of postoperative recurrence was determined by a radiologist with 11 years of experience in abdominal radiology, who served as a coordinator in the present study. Postoperative HCC recurrence was considered to be present if a newly appearing focal nodule showed hypervascularization demonstrated on arterial-phase images of dynamic CT or MRI and contrast washout on delayed-phase images [3, 14, 15].
Contrast-enhanced CT technique
CT examinations were performed in all patients using a 16-detector row scanner (Aquilion 16; Toshiba Medical Systems, Japan) at the following settings: tube voltage, 120 kV; tube current, 280–400 mA (automatically adjusted to the body size of each patient); rotation period, 0.5 s; detector collimation, 1 × 16 mm (16 rows with 1.0 mm section thickness); and beam pitch, 0.94. Images with an effective 5 mm section thickness were reconstructed every 5 mm to provide contiguous sections.
Precontrast CT images were obtained, and then non-ionic contrast medium (Omnipaque 300; Daiichi Pharmaceutical, Tokyo, Japan) containing iodine (300 mg/mL) was administered intravenously using a power injector (Auto Enhance A-50; NemotoKyorindo, Tokyo, Japan) within 30 s as a bolus of 2.0 mL/kg body weight up to a maximum of 150 mL. The injection rate varied depending on patient body weight (2.6–5.0 mL/s). Image acquisition in the arterial, portal venous, and delayed phases began 40, 60, and 180 s, respectively, after the initiation of contrast medium injection [16].
MRI technique
MRI was performed on all patients using a 1.5-T superconducting magnet (Signa Lx, GE Medical Systems, Milwaukee, WI, USA) with an 8-channel body phased-array coil. DWI was performed using the respiratory-triggered technique during free breathing under the following conditions: sequence, single-shot spin-echo echo-planar with a parallel imaging technique (factor = 2); fat-suppression technique, spatially selective radio frequency; scan direction, axial; b value, 500 and 1,000 s/mm2; directions of diffusion gradients, three orthogonal directions; repetition time/echo time, 8,000–10,000/73.2–73.4 ms; matrix, 128 × 128; slice number, 60; slice thickness, 4–6 mm; field of view, 40 cm; excitation number, 4; and acquisition time, ~5–6 min.
CT and DWI variables analysis
The preoperative CT scan of each patient was reviewed by a gastrointestinal radiologist with 5 years of experience in hepatobiliary imaging. No clinical, laboratory, or pathology information other than the presence of HCC was provided during image analysis.
The tumor number, size, shape, and capsule were evaluated. Tumor size was evaluated by measuring the longest diameter on the axial plane. For a patient with multiple tumors, the tumor with the longest diameter was used for statistical analysis. Tumor shape was classified into two categories: simple nodular (round with well-defined margin and no indentation/bulging) or not [17]. Tumor capsule integrity was classified into present or absent/uncertain [18]. The number of tumor lesions that appeared to be hyperdense on arterial-phase images and washed out on delayed-phase images was counted. Invasion into the portal or hepatic veins was considered present, if a tumor thrombus was observed in the lumen of major hepatic or portal vein branches.
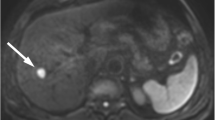
Tumor ADC measurements were obtained and analyzed. The standards for measuring the ADCs of the lesions were as follows. Regions of interest (ROIs) were drawn over a lesion at the level of the maximum diameter of the lesion, as seen on DWI at b values of 500 and 1,000 s/mm2. We ensured that the ROIs for each lesion were carefully placed within the confines of the lesion. ADC values were calculated by the same radiologist. Cysts and necrotic areas were not included in the ROIs. Signal intensities were measured three times, and the average intensity was calculated for each lesion. ADCs were calculated with the following formula: ADC = ln (S1/S2)/(b2 − b1), where ln is the natural logarithm, and S1 and S2 are the signal intensities of the lesion obtained using DWI at b1 (500 s/mm2) and b2 (1,000 s/mm2), respectively. All ADC values were expressed as mean (SD) in square millimeters per second.
Preoperative laboratory variable analysis
The following variables were recorded and analyzed: AFP, PIVKA-II, hepatitis serology, alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, prothrombin time activity percentage (PTp), albumin, total bilirubin, and AST platelet ratio index (APRI), which is a non-invasive liver fibrosis test [19, 20].
Statistical analysis
ADC values of the tumors were correlated with histological differentiation of the HCCs (tumor grade), which were reported in the pathological reports of the surgical specimen. Mean with standard deviation of ADC values for each tumor grade was calculated. Wilcoxon test was used for the comparisons of ADC values.
Univariate analysis was performed with χ2 or Fisher’s exact tests for categorical or binary variables and the Wilcoxon test for continuous variables. The logistic regression test was used for multivariate analysis. To study the effect of a continuous variable on survival time, we used the receiver operative characteristic (ROC) curve to determine the optimal cutoff value (discrete variable) for significant variables identified with multivariate analysis. Overall survival was calculated using the Kaplan–Meier method, and the log-rank test was used to assess differences in survival. Differences with a p value <0.05 were considered to be significant. Statistical analysis was performed using MedCalc v. 11.3.8.0 (MedCalc software, Mariakerke, Belgium).
Results
Correlation between tumor grade and ADC values
The mean (standard deviation) of the ADC values of poorly differentiated HCCs was 0.71 × 10−3 mm2/s, which was significantly smaller than those of moderately differentiated (0.86 × 10−3 mm2/s, p = 0.0307) and well-differentiated (0.93 × 10−3 mm2/s, p = 0.0064) HCCs (Fig. 1).
Early recurrence
Among the 116 patients included in the study, 20 (17 %) showed early tumor recurrence within 1 year. Of the 20 early recurrences, 19 (95 %) were remote from the resection line.
Of the 20 patients with early recurrence, 19 (95 %) underwent significant additional therapy after the diagnosis of recurrence (re-resection [n = 2], radiofrequency ablation [n = 1], and TACE [n = 16]). The patient who received no treatment had widespread intrahepatic recurrence and died from the disease. There were six deaths among patients with early recurrence and seven deaths among patients without early recurrence.
Variables predictive of early recurrence
By univariate analysis, six variables were more frequent in patients with early recurrence after liver resection. These were serum levels of AFP (p = 0.0013), PTp (p = 0.0026), AST (p = 0.0166), albumin (p = 0.0225), APRI (p = 0.0042), and tumor ADC (p < 0.0001) (Table 1). By multivariate analysis, three variables were statistically significant for predicting early tumor recurrence: tumor ADC (p = 0.0002), serum AST (p = 0.0121), and PTp (p = 0.0082) (Table 2).
The optimal cutoff value of ADC determined with ROC analysis for predicting early recurrence was ≤0.898 × 10−3 mm2/s. ADCs of ≤0.898 × 10−3 mm2/s were observed in 19 (95 %) of 20 patients with early recurrence. The optimal cutoff value of AST determined with ROC analysis for predicting early recurrence was >40 IU/L. ASTs of >40 IU/L were observed in 12 (60 %) of the 20 patients with early recurrence. The optimal cutoff value of PTp determined with ROC analysis for predicting early recurrence was ≤72.3 %. Of the 20 patients, 14 (70 %) with early recurrence had a PTp of ≤72.3 %.
Overall survival
Overall, 3- and 5-year survival rates of all patients were 86 and 73 %, respectively (Fig. 2). In patients with tumor ADCs of ≤0.898 × 10−3 mm2/s, the 3- and 5-year survival rates were 77 and 56 %, respectively, which were significantly lower than those in patients with tumor ADCs of >0.898 × 10−3 mm2/s (p = 0.0015; Fig. 3). Only one death occurred among patients with ADCs of >0.898 × 10−3 mm2/s during the same period, and the 5-year survival was 97 %.
Significant difference also occurred between the survival rates of patients with serum ASTs of >40 and ≤40 IU/L (p = 0.0276) and a PTp of ≤72.3 and >72.3 % (p = 0.0276). In patients with serum ASTs of >40 IU/L, the 3- and 5-year survival rates were 75 and 64 %, respectively. Four deaths occurred in the same period among patients with ASTs of <40 IU/L, and the 5-year survival rate was 78 %. The 3- and 5-year survival rates in patients with a serum PTp of ≤72.3 % were 72 and 72 %, respectively. Six deaths occurred in the same period among patients with a PTp of >72.3 %, and the 5-year survival rate was 85 %.
Discussion
Intrahepatic tumor recurrence after therapy is due to either intrahepatic metastasis or multicentric primary HCC [9, 21, 22]. Chronic viral hepatitis or cirrhosis are usually present in HCC patients and are risk factors for the development of HCC. Therefore, HCC recurrence owing to intrahepatic metastasis or multicentric primary HCC is still inevitable, even if HCC is surgically resected. However, differentiating intrahepatic metastasis that originated from the primary tumor from newly developed multicentric HCC is difficult. Imamura et al. [22] and Poon et al. [23] have suggested that early and late recurrence of HCC after resection could roughly represent intrahepatic metastasis and multicentric HCC, respectively. Early recurrence has a greater clinical significance than late recurrence considering the poor survival of patients with early recurrence.
In the present study, univariate analysis showed that serum levels of AFP, PTp, AST, albumin, APRI, and tumor ADC are statistically significant for predicting early recurrence of HCC. However, only tumor ADC, serum AST, and PTp showed statistical significance in multivariate analysis.
The histological differentiation of HCC has been reported as a predictor of early recurrence after curative resection [10, 11]. A lower mean ADC was reported in moderately to poorly differentiated HCC compared to well-differentiated HCC [12]. In the present study, a tumor ADC of ≤0.898 × 10−3 mm2/s was observed in 19 of 20 patients (95 %) with early recurrence. The early recurrence in patients with low tumor ADC may be due to poor histological tumor differentiation.
In the present study, high-serum AST of >40 IU/L was observed in 60 % of patients with early recurrence and was significantly associated with poor survival compared to that of patients with low-serum AST. This result is consistent with that of a previous study [24]. Serum PTp of ≤72.3 % was observed in 70 % of patients with early recurrence and was also significantly associated with poor survival compared to that of patients with high-serum PTp. The reason for the high rate of early recurrence in patients with high-serum AST and low PTp is unclear.
Although venous invasion has been reported to be significant for predicting early recurrence of HCC after surgical resection [11, 25], the presence of venous invasion identified with CT had no statistically significant impact on the incidence of early HCC recurrence (p = 0.973) in the present study. This unexpected result could be due to the small number of patients with positive portal vein invasion in the present study (n = 4) and the more aggressive treatment for patients whose cancers displayed this characteristic. However, the observation that venous invasion showed no statistical significance in early HCC recurrence is consistent with those of previous studies [26].
Large tumor size, absence of tumor capsule, and elevated serum AFP levels have been reported as predictors or risk factors for early tumor recurrence [11, 25, 27]. In the present study, these factors were not statistically significant for predicting early recurrence using multivariate analysis. The Barcelona Clinic Liver Cancer staging system was not significant for predicting early recurrence in the present study, which may be due to selection bias because only patients who underwent surgery were included in the study, and a small number of patients with stages C and D cancers were included.
The present study had several limitations, including retrospective design and lack of histological proof of recurrent HCC. Tumor node metastasis staging, a standard staging system for tumors of other organs, was not analyzed as a variable owing to criticisms of this staging system. Lack of evaluation of Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) is another limitation, since AFP-L3 is believed to be an indicator of HCC with poor prognosis [28]. Further study comprehensively including tumor markers would be expected to confirm our results.
In conclusion, HCC patients with low tumor ADC have a statistically significant higher risk of early postoperative recurrence and lower survival. Close postoperative surveillance is recommended for the early detection of recurrence in patients with these factors.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AFP:
-
Alpha-fetoprotein
- APRI:
-
Aspartate aminotransferase platelet ratio index
- AST:
-
Aspartate aminotransferase
- DWI:
-
Diffusion-weighted magnetic resonance imaging
- HCC:
-
Hepatocellular carcinoma
- MR:
-
Magnetic resonance
- PIVKA-II:
-
Protein induced by vitamin K absence-II
- PTp:
-
Prothrombin time activity percentage
- ROI:
-
Region of interest
- TACE:
-
Transarterial chemoembolization
References
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 2005;25:181–200
Yamamoto J, Kosuge T, Saiura A, et al. Effectiveness of hepatic resection for early-stage hepatocellular carcinoma in cirrhotic patients: subgroup analysis according to Milan criteria. Jpn J Clin Oncol 2007;37:287–295
Kudo M. Real practice of hepatocellular carcinoma in Japan: conclusions of the Japan Society of Hepatology 2009 Kobe Congress. Oncology 2010;78(Suppl 1):180–188
Rampone B, Schiavone B, Confuorto G. Current management of hepatocellular cancer. Curr Oncol Rep 2010;12:186–192
Mendizabal M, Reddy KR. Current management of hepatocellular carcinoma. Med Clin North Am 2009;93:885–900, viii
Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10–24
Regimbeau JM, Abdalla EK, Vauthey JN, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol 2004;85:36–41
Choi GH, Kim DH, Kang CM, et al. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol 2008;15:618–629
Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216–222
Kim H, Park MS, Park YN, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J 2009;50:789–795
Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330–339
Muhi A, Ichikawa T, Motosugi U, et al. High-b-value diffusion-weighted MR imaging of hepatocellular lesions: estimation of grade of malignancy of hepatocellular carcinoma. J Magn Reson Imaging 2009;30:1005–1011
Heo SH, Jeong YY, Shin SS, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol 2010;11:295–303
Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005;42:27–34
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–1022
Ichikawa T, Erturk SM, Araki T. Multiphasic contrast-enhanced multidetector-row CT of liver: contrast-enhancement theory and practical scan protocol with a combination of fixed injection duration and patients’ body-weight-tailored dose of contrast material. Eur J Radiol 2006;58:165–176
Liver-Cancer-Study-Group-of-Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Tokyo: KANEHARA & CO., LTD.; 2008
Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 2009;250:435–443
Tural C, Tor J, Sanvisens A, et al. Accuracy of simple biochemical tests in identifying liver fibrosis in patients co-infected with human immunodeficiency virus and hepatitis C virus. Clin Gastroenterol Hepatol 2009;7:339–345
Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol 2010;105:1346–1353
Yamamoto M, Matsuda M, Iimuro Y, et al. Intrahepatic distant metastasis and metachronous multicentric occurrence in solitary hepatocellular carcinoma of less than five centimeters in diameter. Surg Today 1993;23:969–978
Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–207
Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500–507
Park UJ, Kim YH, Kang KJ, et al. Risk factors for early recurrence after surgical resection for hepatocellular carcinoma. Korean J Hepatol 2008;14:371–380
Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003;197:753–758
Lim JH, Jang HJ, Kim EY, et al. Early recurring hepatocellular carcinoma after partial hepatic resection: preoperative CT findings. Korean J Radiol 2000;1:38–42
Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275–283
Yamashita F, Tanaka M, Satomura S, et al. Prognostic significance of Lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinomas. Gastroenterology 1996;111:996–1001
Acknowledgements
No grant support was provided for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muhi, A., Ichikawa, T., Motosugi, U. et al. Diffusion-weighted imaging of hepatocellular carcinoma for predicting early recurrence and survival after hepatectomy. Hepatol Int 7, 662–668 (2013). https://doi.org/10.1007/s12072-012-9383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-012-9383-2