Abstract
Purpose
Patients with advanced hepatocellular carcinoma (HCC) have few treatment options. Thymalfasin (thymosin α-1) is an immunomodulator that may increase response to ablative therapy through direct anti-tumor action or enhanced protection against infections. We compared transarterial chemoembolization (TACE) plus thymalfasin with TACE alone for unresectable HCC.
Methods
In this phase II, randomized trial, 25 patients received either TACE plus thymalfasin (1.6 mg SC, 5 times weekly; n = 14) or TACE alone (n = 11) for 24 weeks. Response was defined as transition to transplant eligibility or lack of disease progression through week 72. Survival was assessed through 24 months post-treatment.
Results
Eight of fourteen (57.1%) patients in the TACE + thymalfasin group versus 5 of 11 (45.5%) patients in the TACE-only group became responders (P = 1.0). Four of fourteen TACE + thymalfasin patients versus none of 11 TACE-only patients became eligible for transplant. Median overall survival time was 110.3 weeks for the TACE + thymalfasin group versus 57.0 weeks for the TACE-only group (P = 0.45). Seven patients in each group experienced serious adverse events; there were no bacterial infections in the TACE + thymalfasin group versus 4 in the TACE-only group. There were 3 deaths in the TACE + thymalfasin group and 5 in the TACE-only group.
Conclusions
In patients with unresectable HCC, TACE + thymalfasin resulted in numerically higher rates of survival and tumor response, including transplant candidacy, with fewer bacterial infections, than TACE alone. Treatment regimens for HCC including thymalfasin as an immunomodulator should be evaluated in larger trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor in the world, and the third most common cause of cancer death [1, 2]. Between 500,000 and 1 million new cases of HCC are diagnosed annually [2, 3]. Evidence documents that the incidence of HCC is rising across several countries, including the United States [4–6]. The prognosis for a patient with HCC is generally poor and highly dependent on tumor stage and underlying liver function [7]. For patients with small tumors and well-compensated liver function, curative options include surgical resection, liver transplantation, or local ablation [8]. However, most patients presenting with HCC have advanced tumor-stage disease and the prognosis is usually poor [5, 7]. Detected after the onset of symptoms, HCC has a 5-year survival rate of 0–10% [7–10].
Among patients with advanced disease who do not qualify for surgical or liver transplantation therapies, the only non-chemotherapeutic treatment that has been shown to increase survival is sorafenib [11]. For patients who are not surgical candidates but have tumors small enough for ablative therapy with reasonable liver function, radiofrequency ablation and transarterial chemoembolization (TACE, a combination of regional chemotherapy and some form of hepatic artery occlusion) have been shown to improve survival in randomized clinical trials [9–13]. TACE can induce tumor necrosis in more than 50% of patients, and response to treatment was associated with increased survival in a large, phase III study [10, 14]. Treatment guidelines now recommend TACE for nonsurgical patients with large or multifocal HCC and no vascular invasion or extrahepatic spread [8]. However, fewer than 2% of patients achieve a complete response with TACE; during follow-up, tumor regrowth commonly occurs and TACE procedures must be repeated as needed [8]. Combining TACE with adjuvant therapies such as thymalfasin may increase its efficacy in this difficult-to-treat patient population, although there are no large, randomized, phase III trials to document the benefit of combined therapies with ablative treatments.
Thymalfasin, or thymosin α-1, is an immunomodulatory and antiviral agent that is approved in 35 countries worldwide. It has been primarily used for the treatment of chronic hepatitis B in China and has been studied in numerous patient populations in the United States. Thymalfasin promotes T-cell differentiation, enhances cytokine (IFN-γ, IL-2, IL-3) production, and downregulates T-cell apoptosis [15–23]. It has been shown to decrease tumor cell growth both in vitro and in vivo [24–28] and has demonstrated therapeutic usefulness in several types of cancer, including non-small cell lung cancer and malignant melanoma [29–31]. One phase II study has been completed that examined the efficacy and safety of thymalfasin for the treatment of HCC [32]. This study demonstrated that thymalfasin 0.9 mg/m2 SC biweekly for 6 months plus TACE (using 40–60 mg of doxorubicin) resulted in longer survival than historical controls treated with TACE alone (82% vs. 41% at 7 months after end-of-treatment; P < 0.05). Patients in the TACE + thymalfasin group also showed significant increases in levels of CD8 and NK cells, consistent with other trials in which thymalfasin appeared to prevent chemotherapy- or radiation-induced immune suppression [29, 33]. This mechanism may theoretically protect patients from complications of chemotherapy such as infections.
In the current randomized, active-controlled study, we hypothesized that for patients with unresectable HCC, adding thymalfasin to TACE would increase tumor response and survival time compared with treatment with TACE alone. The total daily subcutaneous dose of thymalfasin, 1.6 mg 5 times weekly, was chosen on the basis of the dose shown to be most effective in causing tumor regression in a previous study using thymalfasin as part of a treatment regimen for metastatic melanoma [34]. The control group was treated with TACE alone since TACE was the standard of care for palliative treatment of patients with unresectable HCC at the time of this trial.
Materials and methods
Study design
A phase II, open-label, randomized, active-controlled study was performed in adult patients with unresectable HCC. Unresectable was defined as not treatable by surgical resection due to the presence of portal hypertension or by liver transplant due to the patient’s disease severity being outside UNOS/Milan criteria (http://www.unos.org/) or due to comorbid conditions prohibiting a surgical procedure. Patients were randomized 1:1 to either TACE plus thymalfasin, 1.6 mg SC 5 times weekly, or TACE alone for 24 weeks (Fig. 1). Randomization was carried out centrally using a randomization table and was stratified by clinical staging using the Okuda classification system [35]. After the treatment period, patients were monitored for 48 weeks (through week 72) and received TACE at the discretion of the investigator if tumor growth occurred. Patients were assessed regularly throughout treatment and follow-up by history and physical examination, chest radiographs, and laboratory measurements. Magnetic resonance imaging (MRI) or computed tomographic (CT) scan was used to assess tumors at screening and weeks 8, 24, 48, and 72. The same imaging technique was always used for a given patient. Tumor response was assessed using the response evaluation criteria in solid tumors (RECIST) and included complete response (CR), partial response (PR), stable disease (SD), or progression of disease (PD) [36]. Other variables assessed were overall survival and survival rates, achievement of eligibility for liver transplantation because of tumor downstaging, changes in α-fetoprotein (AFP) levels, and Eastern Cooperative Oncology Group (ECOG) performance status [36]. Patients who completed the study through week 72 were followed for survival via telephone contact every 12 weeks for up to 24 months following the completion of treatment, for a total treatment plus follow-up time of 30 months after the date of randomization. Tumor measurements and interpretation of the scans were performed centrally by radiologists blinded to treatment assignment.
In the TACE plus thymalfasin arm, the first injection of thymalfasin was administered on the same day that the patient had the TACE procedure, immediately prior to the procedure, or within 48 h after the procedure. If the patient underwent subsequent TACE procedures, thymalfasin injections continued according to the ongoing injection schedule (5 times a week, Monday through Friday). TACE procedures could have been performed up to 4 times during the protocol. The number of TACE procedures per patient was determined by the treating physician in accordance with the participating site’s guidelines for performing TACE. TACE procedures were performed under radiographic control following the site’s guidelines and used either doxorubicin or cisplatin. The total dose of doxorubicin given with the total number of TACE events was to remain less than 450 mg/m2, and each patient should have recovered bone marrow function prior to the next cycle. The dose of cisplatin with each TACE procedure was 100 mg/m2 or less. The dose could be modified to comply with the participating site’s TACE guidelines.
Patients
Eligible patients were adults, 18 years or older, with unresectable HCC, a Child-Pugh classification of A or B, and a MELD (Model for End-Stage Liver Disease) score of no more than 20. HCC must have been diagnosed by liver biopsy, or, if biopsy was contraindicated, by either one of the following: (1) a hepatic mass larger than 2 cm on cross-sectional imaging with the AFP level at least 1000 ng/ml or (2) a hepatic mass on ultrasound, MRI, or CT scan, with the AFP level less than 1000 ng/ml when one of the following was present: one additional image by a different technique suggestive of HCC; the hepatic mass had doubled in diameter over time; or the AFP level had progressively risen to more than 200 ng/ml and tripled from the mean baseline level. The HCC had to be documented as unresectable on the basis of tumor size or location, or on the patient’s insufficient liver function to support hepatectomy. In addition, the patient was judged ineligible for liver transplant either on the basis of staging criteria (patients were judged ineligible for transplant if they had a single HCC nodule >5 cm in diameter, or more than 3 nodules present, or up to 3 nodules with 1 or more nodules >3 cm in diameter) or on the basis of an assessment by the transplant team. Patients were also required to have hematocrit of more than 30%, platelet count of more than 50000/μl, white blood cell count of more than 2.0 × 109/l, polymorphonuclear leukocytes of more than 1.0 × 109/l, and serum creatinine level less than 1.5 mg/dl. Female patients were required to use birth control or to be surgically sterile or postmenopausal.
Patients were excluded from the study if they were taking any hepatotoxic or immunosuppressive drug, had portal vein thrombosis or hepatic artery malformation, were infected with human immunodeficiency virus, had a malignancy other than HCC within the prior 10 years (except for curatively treated skin cancer or surgically cured in situ carcinoma of the cervix), used alcohol or intravenous drugs within the prior 3 months, had been previously treated with thymalfasin, or if they were poor medical or psychiatric risks in the opinion of the investigator. Patients were also excluded if they failed to meet the requirements of Child-Pugh category or MELD score or if HCC was amenable to treatment by surgical resection or transplantation.
Efficacy endpoints
Efficacy variables included response rate. A patient became a responder by fulfilling either of two criteria: (1) becoming eligible for transplant by week 72 or (2) demonstrating a lack of progression through week 72. Progression was defined, per RECIST criteria [36], as 20% or more increase from baseline in the sum of the maximum diameters of the target lesions or the appearance of one or more new lesions. Patients could fulfill the criteria for lack of progression at any of the prespecified imaging times (weeks 8, 24, 48, and 72) through week 72. If a patient had a CR or PR that occurred before progression, the patient was classified as a responder. Patients who withdrew prior to week 72 were classified as nonresponders unless they became eligible for transplant. A patient’s tumor response would also be classified as missing equals failure if data on tumor size were not available (modified intent-to-treat [MITT]).
Other efficacy analyses included survival, ECOG performance status, decrease in AFP, no new metastases, best tumor response, and duration of tumor response. ECOG performance status was assessed at screening and weeks 4, 12, 24, 48, and 72. ECOG categories included grades 0, 1, 2, 3, 4, and 5, where 0 is “fully active without restriction,” 4 is “completely disabled” (confined to bed or chair), and 5 is “dead” [37]. Using AFP, a complete responder was defined as having normal AFP and a partial responder was defined as having at least a 50% reduction from baseline in the AFP level. AFP was measured by local laboratories at baseline and weeks 4, 12, 24, 36, 48, and 72. Best tumor response was defined as the best response recorded from the start of treatment until disease progression or recurrence. Duration of response was defined as the time from the best response to the time of progressive disease.
Safety analyses
Safety parameters included adverse events, serious adverse events, laboratory abnormalities, and deaths. Safety analyses were evaluated for all randomized patients who received at least one dose of study medication or a TACE procedure. The WHO Toxicity Grading System was used for all adverse events.
Statistical analyses
A sample size of 18 patients per treatment arm was planned for this pilot study. The sample size estimate was based on the tumor response endpoint in the MITT population, which included all randomized patients who received at least one dose of study medication or a TACE procedure. The response rate was expected to be 50% in the TACE + thymalfasin treatment arm and 20% in the control arm (TACE alone). Assuming α = 0.068 and β = 0.2 (corresponding to a power of 80%), 18 subjects per group (36 subjects in total) were needed to detect a difference of 30% (i.e., 50% vs. 20%) between the two groups. Since this study was designed primarily as a safety study, we estimated the small number of patients who could be recruited in a short time period to help complete the initial clinical research component of the medication development. Therefore, we did not target a statistically significant difference in efficacy (P < 0.05) as the value to power the study. Descriptive statistics were planned to summarize efficacy variables. These included sample size, mean, standard deviation, median, minimum and maximum for continuous variables, and number and percentage of patients for categorical variables.
Efficacy and safety analyses were completed using the MITT method, in which all patients who received one dose of thymalfasin and at least one embolization were included. For the tumor response efficacy endpoint (response) and for biochemical (AFP) response, patients with missing data were considered not to have a response for that endpoint. A two-sided 95% confidence interval for both the median overall survival and the percentage survival was calculated.
Results
Study population
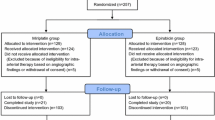
Of 28 patients who were randomized, 3 withdrew before receiving treatment and 25 received at least one dose of study medication or a TACE procedure (Fig. 2). Fourteen patients received TACE plus thymalfasin and 11 received TACE only. There were 20 successful TACE procedures for the 14 TACE plus thymalfasin patients and 21 successful procedures for the 11 TACE-only patients.
Baseline demographic and disease characteristics of the study population are shown in Table 1. Among randomized patients, 79% were male, mean age was 60 years, and the racial and ethnic makeup was mixed (43% of patients were White). Among treated (MITT) patients at baseline, the median number of target lesions was 2 and the median sum of their diameters was 10.01 cm (Table 1). Fifty-five percent (6/11) of patients in the TACE-only group and 57% (8/14) of patients in the TACE plus thymalfasin group were anti-HCV positive. One of eleven (9%) patients in the TACE-only group and 2 of 14 (14%) patients in the TACE plus thymalfasin group were HBsAg positive.
Five patients (TACE plus thymalfasin: n = 1; TACE only: n = 4) completed 24 weeks of treatment and 48 weeks of follow-up (through week 72 of the study) (Fig. 2). Reasons for study discontinuation included transplant (TACE plus thymalfasin: n = 4), patient decision (TACE plus thymalfasin: n = 2; TACE only: n = 1), loss to follow-up (one in each treatment group), protocol violation (TACE plus thymalfasin: n = 2; TACE only: n = 1), and death (TACE plus thymalfasin: n = 2; TACE only: n = 4).
Response rate
Through 72 weeks, 57.1% (8/14) of patients in the group receiving TACE + thymalfasin became responders versus 45.5% (5/11) in the group receiving TACE only (P = 1.0). Among the 8 responders in the group receiving TACE + thymalfasin, 4 patients became eligible for liver transplant whereas none of the 5 responders in the TACE-only group became eligible for transplant. Among the 4 patients who became eligible for transplant, 2 were classified as Okuda stage II and 2 as Okuda stage I at baseline.
Survival
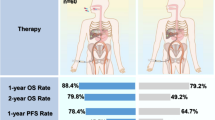
Median overall survival time was 110.3 weeks for the group receiving TACE + thymalfasin versus 57.0 weeks for group receiving TACE only (P = NS; Fig. 3). When transplant patients’ data were censored at the time of transplant, survival time for the group receiving TACE + thymalfasin was 83.3 weeks. At 24 weeks, 48 weeks, and 2 years, survival rates were consistently higher for the group that received TACE + thymalfasin (Table 2). Results for mean and median survival by baseline Okuda stage are shown in Table 3.
ECOG performance status
At 24 weeks (end of treatment), 3 patients in each treatment group had ECOG scores of 2 or less. At 48 weeks, 5 patients who received TACE only and no patient who received TACE + thymalfasin had ECOG scores of 2 or less. It is unknown how many patients had other ECOG scores at these 2 time points.
AFP response
At 24 weeks, 14.3% (2/14) of patients receiving TACE + thymalfasin versus 18.2% (2/11) of patients receiving TACE only demonstrated a complete (normal AFP) or partial response (≥ 50% reduction from baseline; P = NS). Ten of fourteen patients in the group receiving TACE + thymalfasin and 6 or 11 patients in the group receiving TACE only had missing AFP levels at 24 weeks. At 48 weeks, 7.1% (1/14) of patients in the TACE + thymalfasin group versus 9.1% (1/11) of patients in the TACE-only group were complete or partial responders. Twelve of fourteen and 8 of 11 patients in the 2 groups had missing AFP values at 48 weeks.
New metastases
At week 24, 1 patient in the TACE-only group demonstrated new lesions. At week 72, 1 patient in the TACE + thymalfasin group demonstrated new lesions.
Best tumor response
The best tumor responses, by RECIST criteria, are shown in Table 4. No patient in either treatment group demonstrated a complete response. Partial response was achieved by 14.3% (2/14) of patients who received TACE + thymalfasin and 18.2% (2/11) of patients who received TACE only. Stable disease was the best tumor response for 35.7% (5/14) of patients who received TACE + thymalfasin and 18.2% (2/11) of patients who received TACE only. Mean duration of response was 64.9 weeks for TACE only and 78.8 weeks for TACE + thymalfasin.
Safety
Twenty-five patients received at least one dose of study medication or a TACE procedure and were included in the safety analyses (TACE + thymalfasin: n = 14; TACE only: n = 11). Fourteen TACE + thymalfasin patients had a total of 20 successful TACE procedures and 11 TACE-only patients had a total of 21 successful TACE procedures. The median number of thymalfasin doses for the 14 patients in the TACE + thymalfasin group was 72 (range = 1–131).
On-study safety is summarized in Table 5. Through 72 weeks, most patients in both treatment groups experienced at least one adverse event. The most common adverse events were nausea (TACE + thymalfasin 57%; TACE only 64%) and fatigue (TACE + thymalfasin 43%; TACE only 82%). Seven TACE plus thymalfasin patients experienced 20 serious adverse events and 7 TACE-only patients experienced 26 serious adverse events. Among these, bacterial infections were reported for 4 TACE-only patients (sepsis 1, bacterial peritonitis 1, cholecystitis 1, and catheter-related infection 1) versus no for TACE plus thymalfasin patient.
No patient discontinued study treatment because of an adverse event; 1 patient in the TACE + thymalfasin group discontinued during the follow-up period (prior to the week 72 visit) because of terminal hepatorenal syndrome. There were 35 severe, life-threatening, or fatal adverse events in the TACE + thymalfasin group and 33 in the TACE only groupFootnote 1; 3 of these were fatal for TACE + thymalfasin patients and 1 was fatal for a TACE-only patient. From the start of the study through 30 months postrandomization, there were three deaths in the TACE + thymalfasin group and five in the TACE-only group. No death was judged to be related to thymalfasin.
Of the 23 adverse events judged possibly or probably related to thymalfasin, most were mild and resolved without sequelae. Only three of these events occurred in more than one patient: nausea (n = 2), fatigue (n = 2), and nipple pain (n = 2). Among laboratory assessments of patients receiving TACE + thymalfasin, the variables with the greatest percentage of patients that shifted from normal at baseline to above normal at any time during the study were monocytes (28.6%) and chloride (21.4%); the variables with the greatest percentage of patients that shifted from normal at baseline to below normal at any time during the study were lymphocytes (21.4%) and calcium (21.4%). Overall, thymalfasin was well tolerated.
Discussion
The results of this pilot study show that for patients with unresectable HCC, adding thymalfasin to a regimen of TACE is generally well tolerated and may improve outcomes. Response as defined in the protocol was higher among patients who received thymalfasin and overall survival time for that group was nearly twice that of the group treated with TACE alone, although the differences for these endpoints did not reach statistical significance in this small study. Similarly, the percentage of patients surviving after 2 years favored the TACE plus thymalfasin arm by 6%. The longer survival time may reflect better tumor response or fewer TACE-associated adverse events, or a combination of these factors. As the current trial was primarily a safety study, the small sample size and lack of statistical significance for differences in response rates are secondary to our finding that adding thymalfasin to TACE is generally well tolerated and merits further investigation as a treatment regimen.
TACE has been shown to improve short-term survival among patients with unresectable HCC but has associated adverse events similar to those caused by systemic chemotherapy, including immune suppression. Some patients receiving TACE develop severe infectious complications including hepatic abscess, sepsis, or cholecystitis [8, 10]. In addition, patients with advanced HCC who are candidates for TACE have impaired immune function due to effects of the cancer, to previous treatment regimens, or to underlying cirrhosis [38–41]. In this context, thymalfasin may be beneficial, since it has been shown to stimulate T-cell proliferation and differentiation and to induce TH1-type immune responses [15–23]. Previous clinical trials evaluating thymalfasin for different types of cancer have shown it to be potentially effective in restoring immune function that may have been depressed by the cancer or by prior treatments [29].
Consistent with the results of previous studies [29–33], our results suggest an association between thymalfasin therapy and restored immune function. In the current trial, patients who received thymalfasin in addition to TACE experienced no bacterial infections versus those who received TACE alone, among whom there were four infections including sepsis, cholecystitis, and bacterial peritonitis. In a previous study of thymalfasin in combination with TACE for HCC, Stefanini et al. [32] showed that TACE + thymalfasin improved survival compared with historical controls treated with TACE only and was associated with increases in cytotoxic T cells (CD8) and NK cells (CD16 and CD56) after completion of treatment. The present, active-controlled study also demonstrated increased survival compared with treatment with TACE alone and an absolute difference in survival rate (in favor of the TACE + thymalfasin arm) of 23% at end of treatment (6 months) and 6% at 2 years. The lack of bacterial infections observed among thymalfasin-treated patients suggests restored immune function that may have contributed to longer survival time.
Restored immune function may have also resulted in better tumor response. Response as defined in the protocol was higher in the TACE + thymalfasin arm, and 4 of 14 patients in that group, versus none in the TACE-only group, became eligible for liver transplant. In those patients, the tumor response allowed patients to be downstaged, thus enabling them to fulfill UNOS/Milan criteria for transplantation. The addition of thymalfasin likely boosted cellular immunity, which, in turn, may have helped control tumor growth and spread [42, 43]. These results are consistent with those of Shuqun et al. [44], who found that for HCC patients who underwent hepatectomy, adding thymalfasin to TACE postoperatively delayed HCC recurrence and increased survival time.
In this study, the safety and tolerability profile of thymalfasin was comparable with that of TACE alone with respect to the frequency of adverse events and the frequencies of severe, life-threatening, and fatal adverse events. Among adverse events possibly or probably related to thymalfasin, most were mild and resolved without sequelae, and only one (nipple pain) occurred in 10% or more of thymalfasin-treated patients. These results are consistent with those of previous clinical studies, in which thymalfasin has been shown to have an excellent safety profile [32, 45–48]. When evaluating new agents for HCC, it is crucial to determine that they do not worsen outcomes and survival due to associated toxicity, compared with placebo or existing treatments [49, 50]. The current trial incorporated TACE as an active control, since it is a proven effective therapy for patients with unresectable HCC. Our results suggest the potential benefits and limited risks of adding thymalfasin to TACE for this patient population and justify a larger, phase III trial to evaluate this regimen. Furthermore, in light of the results obtained with sorafenib and its recent regulatory approval for HCC, combination trials of TACE plus thymalfasin with a kinase inhibitor or thymalfasin with a kinase inhibitor should also be considered.
Notes
Of the severe, fatal, or life-threatening events, a subset met the Food and Drug Administration (FDA) criteria for serious adverse events. Some severe events did not meet the FDA seriousness criteria.
References
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–2576. doi:10.1053/j.gastro.2007.04.061
Gish RG. Hepatocellular carcinoma: overcoming challenges in disease management. Clin Gastroenterol Hepatol 2006;4:252–261. doi:10.1016/j.cgh.2006.01.001
Thomas MB. Hepatocellular carcinoma: the need for progress. J Clin Oncol 2005;23:2892–2899. doi:10.1200/JCO.2005.03.196
Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127(5 Suppl 1):S5–S16. doi:10.1053/j.gastro.2004.09.011
Kim WR, Gores GJ, Benson JT, Therneau TM, Melton LJ III. Mortality and hospital utilization for hepatocellular carcinoma in the United States. Gastroenterology 2005;129:486–93
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA J Clin 2007;57:43–66. doi:10.3322/canjclin.57.1.43
Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: a population-based study. Clin Gastroenterol Hepatol 2006;4:104–110. doi:10.1016/S1542-3565(05)00745-7
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–1236. doi:10.1002/hep.20933
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–1917. doi:10.1016/S0140-6736(03)14964-1
Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–1738. doi:10.1016/S0140-6736(02)08649-X
Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, et al., SHARP Investigators Study Group. Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): results of a phase III randomized, placebo-controlled trial (SHARP trial). J Clin Oncol (Meeting Abstracts) 2007;25(Suppl):Abstract LBA1
Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1):S179–S188. doi:10.1053/j.gastro.2004.09.032
Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA 2008;299(14):1669–1677. doi:10.1001/jama.299.14.1669
Llovet JM, Bruix J; Barcelona-Clinic Liver Cancer Group. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429–442. doi:10.1053/jhep.2003.50047
Knutsen AP, Freeman JJ, Mueller KR, Roodman ST, Bouhasin JD. Thymosin-alpha1 stimulates maturation of CD34+ stem cells into CD3+4+ cells in an in vitro thymic epithelia organ coculture model. Int J Immunopharmacol 1999;21:15–26. doi:10.1016/S0192-0561(98)00060-5
Ohta Y, Sueki K, Yoneyama Y, Tezuka E, Yagi Y. Immunomodulating activity of thymosin fraction 5 and thymosin alpha 1 in immunosuppressed mice. Cancer Immunol Immunother 1983;15:108–113. doi:10.1007/BF00199700
Ohta Y, Tezuka E, Tamura S, Yagi Y. Thymosin alpha 1 exerts protective effect against the 5-FU induced bone marrow toxicity. Int J Immunopharmacol 1985;7:761–768. doi:10.1016/0192-0561(85)90163-8
Serrate SA, Schulof RS, Leondaridis L, Goldstein AL, Sztein MB. Modulation of human natural killer cell cytotoxic activity, lymphokine production, and interleukin-2 receptor expression by thymic hormones. J Immunol 1987;139:2338–2343
Sztein MB, Serrate SA, Goldstein AL. Modulation of interleukin-2 receptor expression on normal human lymphocytes by thymic hormones. Proc Natl Acad Sci USA 1986;83:6107–6111. doi:10.1073/pnas.83.16.6107
Hsia J, Sarin N, Oliver JH, Goldstein AL. Aspirin and thymosin increase interleukin-2 and interferon-gamma production by human peripheral blood lymphocytes. Immunopharmacology 1989;17:167–173. doi:10.1016/0162-3109(89)90045-3
Leichtling KD, Serrate SA, Sztein MB. Thymosin alpha 1 modulates the expression of high affinity interleukin-2 receptors on normal human lymphocytes. Int J Immunopharmacol 1990;12:19–29. doi:10.1016/0192-0561(90)90064-T
Osheroff PL. The effect of thymosin on glucocorticoid receptors in lymphoid cells. Cell Immunol 1981;60:376–385. doi:10.1016/0008-8749(81)90279-3
Baumann CA, Badamchian M, Goldstein AL. Thymosin alpha 1 antagonizes dexamethasone and CD3-induced apoptosis of CD4+CD8+ thymocytes through the activation of cAMP and protein kinase C dependent second messenger pathways. Mech Ageing Dev 1997;94:85–101. doi:10.1016/S0047-6374(96)01860-X
Rasi G, Silecchia G, Sinibaldi-Vallebona P, Spaziani E, Pierimarchi P, Sivilia M, et al. Anti-tumor effect of combined treatment with thymosin alpha 1 and interleukin-2 after 5-fluorouracil in liver metastases from colorectal cancer in rats. Int J Cancer 1994;57:701–705. doi:10.1002/ijc.2910570516
Pica F, Fraschetti M, Matteucci C, Tuthill C, Rasi G. High doses of thymosin alpha 1 enhance the anti-tumor efficacy of combination chemo-immunotherapy for murine B16 melanoma. Anticancer Res 1998;18:3571–3578
Mastino A, Favalli C, Grelli S, Rasi G, Pica F, Goldstein AL, et al. Combination therapy with thymosin alpha 1 potentiates the antitumor activity of interleukin-2 with cyclophosphamide in the treatment of the Lewis lung carcinoma in mice. Int J Cancer 1992;50:493–499. doi:10.1002/ijc.2910500327
Garaci E, Pica F, Mastino A, Palamara AT, Belardelli F, Favalli C. Antitumor effect of thymosin alpha-1 interleukin-2 or thymosin alpha-1/interferon alpha, beta following cyclophosphamide in mice injected with highly metastatic Friend erythroleukemia cells. J Immunother Emphasis Tumor Immunol 1993;13:7–17
Umeda Y, Sakamoto A, Nakamura J, Ishitsuka H, Yagi Y. Thymosin alpha 1 restores NK-cell activity and prevents tumor progression in mice immunosuppressed by cytostatics or X-rays. Cancer Immunol Immunother 1983;15:78–83. doi:10.1007/BF00199694
Schulof RS, Lloyd MJ, Cleary PA, Palaszynski SR, Mai DA, Cox JW Jr, et al. A randomized trial to evaluate the immunorestorative properties of synthetic thymosin alpha 1 in patients with lung cancer. J Biol Response Mod 1985;4:147–158
Favalli C. Combination therapy in malignant melanoma. Presentation at the third international symposium on combination therapies. Houston, TX: Institute for Advanced Studies in Immunology & Aging; 1993
Lopez M, Carpano S, Cavaliere R, Di Lauro L, Ameglio F, Vitelli G, et al. Biochemotherapy with thymosin alpha 1, interleukin-2 and dacarbazine in patients with metastatic melanoma: clinical and immunological effects. Ann Oncol 1994;5:741–6
Stefanini GF, Foschi FG, Castelli E, Marsigli L, Biselli M, Mucci F, et al. Alpha-1-thymosin and transcatheter arterial chemoembolization in hepatocellular carcinoma patients: a preliminary experience. Hepatogastroenterology 1998;45:209–215
Salvati F, Rasi G, Portalone L, Antilli A, Garaci E. Combined treatment with thymosin alpha 1 and low-dose interferon-alpha after ifosfamide in non-small cell lung cancer: a phase II controlled trial. Anticancer Res 1996;16:1001–1004
Rasi G, Terzoli E, Izzo F, Pierimarchi P, Ranuzzi M, Sinibaldi-Vallebona P, et al. Combined treatment with thymosin-alpha 1 and low-dose interferon-alpha after dacarbazine in advanced melanoma. Melanoma Res 2000;10:189–192. doi:10.1097/00008390-200010020-00012
Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918–928. doi:10.1002/1097-0142(19850815)56:4<918::AID-CNCR2820560437>3.0.CO;2-E
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. doi:10.1093/jnci/92.3.205
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. doi:10.1097/00000421-198212000-00014
Bahr MJ, Manns MP. Function of the immune system in liver cirrhosis. Z Gastroenterol 2001;39:601–607. doi:10.1055/s-2001-16696
Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol 2003;12:297–302
Baumann M, Witzke O, Canbay A, Patschan S, Treichel U, Gerken G, et al. Serum C3 complement concentrations correlate with liver function in patients with liver cirrhosis. Hepatogastroenterology 2004;51:1451–3
Garcia-Tsao G. Bacterial infections in cirrhosis. Can J Gastroenterol 2004;18:405–406
O’Beirne JP, Harrison PM. The role of the immune system in the control of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2004;16:1257–1260. doi:10.1097/00042737-200412000-00003
Campoli M, Ferrone S, Zea AH, Rodriguez PC, Ochoa AC. Mechanisms of tumor evasion. Cancer Treat Res 2005;123:61–88. doi:10.1007/0-387-27545-2_3
Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, et al. Combination transcatheter hepatic arterial chemoembolization with thymosin α1 on recurrence prevention of hepatocellular carcinoma. Hepatogastroenterology 2004;51:1445–1447
Sherman KE, Gordon SC, Iftikar R, Rodriguez-Torres M, Rustgi VK, Di Bisceglie A. Retreatment of treatment experienced HCV patients with pegylated interferon alfa-2a and thymosin alpha-1: pooled analysis of two randomized controlled trials. Hepatology 2007;46:830A. Abstract 1331. doi:10.1002/hep.21985
Camerini R, Mackiewicz A, Testori A, Trefzer U, Jassem J, Ferraresi V, et al. A large first-line, randomized, dose-finding, phase II study on thymosin-alpha 1 (T-alpha 1) plus dacarbazine (DTIC) with or without interferon-alpha (IFN-alpha) compared to DTIC plus IFN-alpha in stage IV melanoma. Tumor response and survival results [meeting abstracts]. J Clin Oncol 2007;25(Suppl). Abstract 8535
Iino S, Toyota J, Kumada H, Kiyosawa K, Kakumu S, Sata M, et al. The efficacy and safety of thymosin alpha-1 in Japanese patients with chronic hepatitis B: results from a randomized clinical trial. J Viral Hepat 2005;12:300–306. doi:10.1111/j.1365-2893.2005.00633.x
You J, Zhuang L, Cheng HY, Yan SM, Yu L, Huang JH, et al. Efficacy of thymosin alpha-1 and interferon alpha in treatment of chronic viral hepatitis B: a randomized controlled study. World J Gastroenterol 2006;12:6715–6721
Gish RG, Hisatake G. Improving clinical trial design for hepatocellular carcinoma treatments. Oncol Rev 2007;1:45–52. doi:10.1007/s12156-007-0006-4
Porta C, Ruff P, Feld R, Feun L, Jeziorski K, Leighton J, et al. Results of a phase III randomized controlled study, the largest ever completed in hepatocellular carcinoma (HCC), comparing the survival of patients with unresectable HCC treated with nolatrexed (NOL) or doxorubicin (DOX). J Clin Oncol 2006;24(Suppl 18). Abstract 96
Acknowledgment
This study was supported by SciClone Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gish, R.G., Gordon, S.C., Nelson, D. et al. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int 3, 480–489 (2009). https://doi.org/10.1007/s12072-009-9132-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-009-9132-3