Abstract
Purpose
Surveillance of patients of cirrhosis of liver is practiced for early detection of HCC. No data from any developing country on cost-effectiveness of such a program are available.
Methods
Economic evaluation of HCC surveillance was embedded in a prospective study undertaken to estimate the incidence of HCC in 194 cirrhotics. The protocol consisted of 6 monthly abdominal ultrasound (US) and serum alphafetoprotein (AFP) estimation, and yearly triple phase CT. Cost was estimated from the hospital and patient perspectives. Cost-effectiveness ratios for detecting a case of HCC were estimated. Modeling was done to estimate cost effectiveness with different combinations of diagnostic tests.
Results
Cost-effectiveness ratios of HCC surveillance program per HCC case detected were estimated as US$ 280 from the hospital perspective. From patient perspective, these were US$ 9,965 for outstation and US$ 2,808 for local patients. Cost-effectiveness ratio for direct medical cost per case of HCC detected by 6 monthly US and AFP, the EASL protocol, was estimated to be US$ 1,510 in the private sector.
Conclusion
The cost of HCC surveillance program is exorbitant for India (gross national income per capita US$ 620) and possibly other low/middle income countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Screening of patients with cirrhosis of liver is a well-accepted approach for early detection of HCC. However, an effective surveillance program for the detection of cancer among at-risk individuals can be recommended as a standard protocol only if it also meets the criteria of being cost-effective. An effective program which is beyond the reach of most of the patients in a country is a meaningless strategy. The studies by Bolondi et al. [1] from Italy, Mima et al. [2] from Japan, Yuen et al. [3] from Hong Kong have reported the cost of detecting one patient of HCC as US$ 17,934, US$ 25,000, and US$ 1,167, respectively. On the face of it, these are enormous costs for patients from developing countries. But the healthcare costs always need to be viewed in the local socio-economic context. Even though 80% patients of liver cancer belong to Asia and Africa [4], with an overwhelming burden in the low and middle income countries, cost-effectiveness of HCC surveillance in these countries has not been studied. We undertook economic evaluation of HCC surveillance program in this prospective longitudinal study.
Material and methods
The economic evaluation was undertaken as a part of a prospective study for the estimation of incidence of HCC in patients of cirrhosis at the All India Institute of Medical Sciences (AIIMS), New Delhi, a leading tertiary care referral hospital in India. The study was approved by the Institutional Ethics Committee. The primary outcome measure was cost per HCC patient detected from the hospital and the patient perspective.
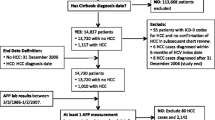
During April 2001 to November 2004, a total of 301 consecutive patients of cirrhosis of liver of all etiologies were enrolled in the study. The various etiologies of cirrhosis were due to hepatitis B (41%), hepatitis C (27%), dual infection of hepatitis B and C (7%), and others (comprising of autoimmune, alcoholic, and cryptogenic, 25%).
These patients were subjected to baseline screening by abdominal ultrasound (US), serum alpha-fetoprotein (AFP) estimation, and triple phase CT (TPCT) of the liver to detect HCC. One hundred and seven patients with cirrhosis were found to have HCC at presentation and were thus excluded from further evaluation. The cohort of the remaining 194 cirrhosis patients free of HCC at the outset were surveilled by US and AFP every 6 months, and TPCT scan every 12 months, for detecting HCC. The recall strategy was the one proposed by the European association for the study of liver (EASL) [5] The nodules detected were staged according to the Barcelona Clinic Liver Cancer (BCLC) Staging [6]. This surveillance program was subjected to economic evaluation proposed by Eisenberg [7].
Diagnostic criteria followed for HCC was the modified EASL criteria [5]. This consisted of (a) positive fine needle aspiration cytology (FNAC) or (b) any two of the following—AFP more than 300 ng/ml and arterialisation of the mass on TPCT/MRI. Contrast-enhanced MRI was used in rare occasions only when diagnosis could not be founded by TPCT, AFP, or FNAC.
The first step in the economic evaluation was a detailed cost identification exercise for the HCC screening program per annum. The second one was the estimation of cost-effectiveness ratios in terms of cost of detecting one HCC case.
Cost identification
Hospital perspective
Cost identification here refers to the estimation of unit costs of the diagnostic tests employed for the HCC surveillance at AIIMS, which is a government hospital with subsidized services. For estimating unit costs of these three tests (US, TPCT, and AFP), the capital and recurrent cost of TPCT and US tests was estimated on an annual basis. The capital cost consisted of the cost of the machines and their installation (including construction, air conditioning, furnishing, etc.). Cost of the land and the original civil construction of the hospital building was not accounted for. Annutized cost at 3% discount rate (using value of annuity as 8.5302) was applied [8].
Recurrent cost consisted of the ongoing expenditure such as maintenance of the machine, electricity consumption, number of films used, cost of processing the films (developer and fixer), etc. Cost of consumables for CT and US facilities like linen, miscellaneous necessities like syringes, needles, drugs, spirit, cotton soap, etc., were also estimated on the basis of market price. Staff salaries for the twin facility of CT and US were also ascertained from records and key informants.
After estimating the overall cost (combining capital and recurrent cost), the cost per unit test for US and CT was deduced. Thereafter, user fees earned per test were subtracted to finally obtain the net cost per test of CT and US.
The cost of AFP test was based on the AFP kit cost, consumables and the technician salary estimated on a monthly basis. After adjusting for the user charges, the net cost per test of AFP was also calculated. This estimate did not include the capital costs invested in land, construction, and infrastructure of the laboratory. The recurrent costs and the time devoted by faculty and other staff, etc., were negligible and therefore not included.
Based on the net costs of the tests, the cost of surveillance program (2 US, 2 AFP, and 1 TPCT each year), was calculated per patient per year.
Patient perspective
Three domains of cost were taken into account:
-
(i)
Direct medical cost: cost of test, disposables and contrast material.
-
(ii)
Direct non-medical cost: cost of travel, food, lodging for patient and attendant(s).
-
(iii)
Indirect cost: loss of wages of the patient and attendant(s) during health care.
For direct medical costs, charges paid for the tests and typical expense on disposables and contrast medium were taken into account. For direct non-medical and indirect costs, the information was based on a cross-sectional survey among 56 non-randomly selected patients (27 outstation, 29 local). The sample size for this exercise was estimated for outstation and local patients separately. A sample size of 25 each was estimated for each category. With this sample size, a two-sided 95% confidence interval (CI) for the average total cost would extend Rs. 2,000 from the observed mean, assuming the standard deviation (SD) of the total cost as Rs. 5,000 for outstation patients. Similarly, a two-sided 95% CI would extend Rs. 400 from the observed mean assuming SD of the total cost as Rs. 1,000 for local patients.
The cost in rupees was also converted into US dollars (US$) for comparison taking the prevailing rate of one US$ equal to Rs. 45.
Cost-effectiveness of the surveillance program
For cost-effectiveness analysis, the cost per HCC detected was calculated from the hospital and patient perspectives, separately. The incidence of HCC among the newly diagnosed patients of cirrhosis was obtained from the cohort study, which was 3.53 per 100 person years [9].
Modeling for direct medical cost with different screening systems including EASL protocol
With the aim of looking at relative costs of screening protocols using different mix and frequency of diagnostic tests from the patient perspective, modeling for direct medical costs, at AIIMS and in private sector in Delhi, was undertaken. These costs were estimated for different protocols, that is, (i) 6 monthly US alone, (ii) 6 monthly US plus AFP (EASL Protocol) [5], and (iii) 6 monthly US plus AFP along with annual TPCT. The information for private sector was based on enquiries on charges from three major laboratories in Delhi.
Results
On surveillance of 194 cirrhosis patients, nine of them developed HCC on a cumulative follow up of 563.4 person-years. The incidence of HCC among patients of cirrhosis was estimated to be 1.60 per 100 person-years in the cohort study [9].
Of these nine HCC patients, four were found to be at BCLC-A stage and survived for 9, 11, 43, and 52 months, respectively. Two patients were at BCLC-B stage and survived for 58 months each (with treatment), while the other died at 2 months without treatment. The remaining 3 patients were at BCLC-C stage and only one could be offered palliative therapy and survived for 7 months while the other two died at 4 and 5 months each as no definite therapy could be offered to them.
Cost identification
Hospital perspective
The cost of triple phase CT (TPCT) and US tests were estimated by analyzing the capital and recurrent costs at AIIMS (Table 1). Likewise, cost of AFP test was also estimated. The capital cost for AFP was Rs. 4,725, recurrent cost Rs. 1,160, and these two contributed to the total cost of AFP per annum as Rs. 5,585. The cost per unit test of AFP was deduced as Rs. 75. After taking into account the user charges, the net cost per AFP test was a gain of Rs. 95 to the hospital.
The overall cost of HCC screening program followed in this study (2 US + 2 AFP and 1 CT annually) to the hospital was estimated to be Rs. 445 (US$ 10).
Patient perspective
For this estimate direct (medical and non-medical) and indirect costs were computed. Table 2 depicts the overall cost from the patient perspective for undertaking HCC screening.
Cost-effectiveness of the surveillance program
Using the estimate of the incidence of HCC and the cost identified in the preceding sections, the cost-effectiveness of the program expressed as cost per HCC case detected was calculated (Table 3). For local patients, the cost per HCC detected was Rs. 126,345 (US$ 2,808). For outstation patients, the cost per HCC detected was more than three times, Rs. 448,440 (US$ 9,965). From the hospital perspective, the cost was Rs. 12,606 (US$ 280) per HCC detected.
Modeling for direct medical cost with different screening systems including EASL protocol
Cost per HCC case detected based on direct medical costs at AIIMS and private sector in Delhi was estimated in different models (Table 4). It is evident that costs in the private sector are nearly three times at AIIMS. Cost-effectiveness for direct medical cost per case of HCC detected by 6 monthly US and AFP (EASL protocol) [5] was estimated to be Rs. 24,080 (US$ 535) at AIIMS and Rs. 67,988 (US$ 1510) in the private sector.
Discussion
Cost is a fundamental consideration in healthcare. An excellent treatment is largely meaningless until and unless it becomes affordable for most people who need it. If the cost of detecting or treating a disease is exorbitant, it does not reach the patients with limited resources. Further, at a more general level, choices must be made between alternative uses of resources, and these decisions must consider both cost and outcome, since there are not enough resources to provide all the medical care technically possible or that patients might prefer to receive [7].
In the context of this study, we asked the following questions. How much does it cost to detect HCC in patients of cirrhosis in our setting? How does it compare with such estimates in other countries?
The net costs of different tests at AIIMS hospital were as follows: US, Rs. 85 or US$ 2 (loss), TPCT Rs. 465 US$ 10 (loss), and AFP Rs. 95 US$ 2(gain), respectively. On the whole, AIIMS hospital is providing services at a subsidized cost to enable low income patients to access quality tertiary care services. It is notable that the hospital exempts user charges for the poorest, a discount that has been factored into the above estimates. It is also important to note that the cost estimates are to be seen in the context of existing services at this hospital embedded in a platform of a large institution operational for several decades. It was not possible to apportion certain costs (such as land, building, initial cost of establishing laboratories, indirect benefits to staff, etc.), and, therefore, the true hospital costs would be somewhat higher.
From the patient perspective, the cost to those belonging to Delhi and those from outside were very different, because the latter incur much higher expenses on transportation, boarding, and loss of wages, etc. The outstation patients typically stayed in Delhi for 2 days at each visit for tests. The annual cost of the screening program as implemented in this study (2 US, 2 AFP, 1 TPCT each year) for local patients was Rs. 4,460 (US$ 100), whereas for the outstation patients the costs were over three times, i. e. Rs. 15,830 (US$ 350).
Over three times differential between the costs to the outstation and local patients brings out the enormity of direct non-medical (transport, boarding) and indirect costs (loss of wages of patient and attendant) for patients who, because of lack of access to requisite services nearby, seek care at distant tertiary care institutions in big cities. Thus, these additional costs are a function of the distance where services of acceptable quality are available.
The point estimate of the cost-effectiveness ratios for surveillance program used in this study from the patient perspective was Rs. 126,345 (US$ 2,808) for each HCC detected for local patients and Rs. 448,440 (US$ 9,965) for the outstation patients.
European Association for the Study of the Liver (EASL) protocol [5] envisages 6 monthly US and AFP. Cost-effectiveness ratio for direct medical cost per case of HCC detected to the patient by this protocol was estimated to be Rs. 67,988 (US$ 1510) in the private sector. This roughly amounts to 3 to 4 months’ salary of a middle-rung physician in government sector in India.
When placed in the societal context, the above costs are too exorbitant for a vast majority of patients in India to afford. Firstly, the per capita gross national income per annum in India is just around US$ 620 in the country [10, 11]. Secondly, public spending on health in India continues to be low, at around 1.0% of the gross domestic product (GDP) [9, 11]. Such spending puts India at the bottom 20% countries [12]. This malady is further compounded by the fact that health insurance is rudimentary in the country. Except for a small minority of people employed in the government and private sectors, citizens have no choice but to incur personal expenditure to purchase healthcare. Not surprisingly, almost 80% of all health spending is out-of-pocket at the point of service [12]. While the better off may be able to absorb this financial burden to a varying extent, the middle income and the poor people cannot meet such expenses. This problem is not trivial. According to one estimate, one-fourth of patients who were not poor when they entered the hospital fell into poverty because of hospital expenses [12].
Hospitals where optimum diagnostic and therapeutic services are available in the public sector at subsidized cost or with no profit are a rarity in India and other developing countries. Overall, 82% outpatient care services and 56% hospitalizations occur in the private sector [12]. For services requiring sophisticated diagnostic work-up such as in the present case, the availability of care in the non-profit or subsidized system is negligible, and patients have to seek care from the for-profit private sector. Therefore, accepting these realities, cost considerations for a recommended surveillance program for HCC in cirrhosis patients should be driven primarily by the patient perspective and taken from the standpoint of the private sector costs.
Thus, in the setting of India, as possibly also other low/middle income countries, the cost of HCC surveillance appears to be intimidating enough to avoid seeking care for most people. For others, such expenditures could be back-breaking, plunging them into debt and deeper poverty.
Table 5 compares cost-effectiveness ratios of screening programs from different studies with the estimates from this study. The cost of detecting a single HCC in the present study is much lower than that reported from Japan [2] and Italy [1]. It is, however, higher than that reported from Hong Kong [13]. It should be noted that cost-effectiveness depends on a number of factors, not just the costs of services, but also the incidence of the disease in the setting concerned. The lower the occurrence of HCC, the higher would be the cost of detecting a single positive patient.
In this study the cost-effectiveness was framed against detecting a clinical outcome using different diagnostic system. Another dimension of cost-effectiveness is to estimate the cost of saving a life year or of saving disability adjusted life years (DALYs) or gaining quality adjusted life years (QALY). For these estimates, information on costs beyond detection of disease (such as the costs of therapeutic procedures, follow up, etc.), and on outcomes (death, disability, etc.) would need to be computed.
This can be done either by large-scale follow-up studies or by a decision analysis model (the Markov model) [14, 15]. The latter takes into account health outcomes based on assumptions from the existing secondary data. Using Markov approach, three studies employing US and AFP have estimated the cost of per life year saved varying from $ 48,00 to 284,000 in Switzerland [16], $74,000 in the US [17], and $ 26,698 in the US [18]. The present study was not designed to address these aspects of economic evaluation.
To conclude, the present study estimates, for the first time, the cost-effectiveness of HCC surveillance in patients of cirrhosis in a developing country, both from the hospital and the patient perspective. Since this cost seems unaffordable, alternative strategies need to be devised to reduce the cost of surveillance program to make it accessible to the low income patients who are the usual victims of this devastating disease. Some cost containment approaches worth exploring are (i) screening only high-risk patients (such as those harboring hepatitis B or C replicating viruses), (ii) judiciously employing diagnostic tests and doing away with the tests which have low diagnostic yield, and (iii) employing telemedicine to cut down the non-medical and indirect costs. In addition, the hunt for low cost tests/markers of HCC must go on to make early diagnosis a reality for the most needy patients in developing countries.
References
Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost-effectiveness analysis. Gut. 2001;48:251–9.
Mima S, Sekiya C, Kanagawa H, Kohyama H, Gotoh K, Mizuo H, et al. Mass screening for hepatocellular carcinoma: experience in Hokkaido, Japan. J Gastroenterol Hepatol. 1994;9:361–5.
Yuen MF, Lai CL. Screening for hepatocellular carcinoma: survival benefit and cost-effectiveness. Ann Oncol. 2003;14:1463–7.
Pisani P, Parkin DM, Ferlay J. Estimates of the world wide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891–3.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. EASL Panel of Experts on HCC. EASL panel of experts on HCC: clinical management of hepatocellular carcinoma. Conclusions of the Barcelona EASL conference. European Association for the study of liver. J Hepatol. 2001;35:421–30.
Llovet JM, Burroughs A, Bruix J. Hepatocelluar carcinoma. Lancet. 2003;362:1907–17.
Eisenberg JM. Clinical economics: a guide to the economic analysis of clinical practices. J Am Med Assoc. 1989;262:2879–86.
Drummond MF, Brien O’, Stoddart GL, Torrence GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
Paul SB, Acharya SK, Sreenivas V, Gulati MS, Madan K, Gupta AK et al. Incidence of hepatocellular carcinoma among Indian patients of cirrhosis of liver. J Gastroenterol Hepatol. 2006;21:A 466.
World Bank. The World Development Report. Washington, DC; 2004.
UNICEF. State of the world’s children 2006. New York; 2005.
Peters DH, Yazbeck AS, Sharma RR, Raman GNV, Pritchett LH, Wagstaff A. Better health systems for India’s poor: findings, analysis and options. Washington, DC: The World Bank; 2002.
Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–5.
Kassirer JP, Mosokowitz AJ, Lau J, Pauker SG. Decision analysis: a progress report. Ann Intern Med. 1987;106:275–91.
Beck JR, Pauker SG. The Markov model in medical prognosis. Med Decis Making. 1993;12:419–58.
Sarasin FP, Giostra E, Hadengne A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422–34.
Saab S, Ly D, Nieto J, Kanwal F, Lu D, Raman S, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytic model. Liver Transplant. 2003;7:672–81.
Arguedas MR, Chen VK, Elboubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost utility analysis. Am J Gastroenterol. 2003;98:679–90.
Acknowledgements
The study was partly funded by Indian Council of Medical Research, New Delhi. India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, S.B., Sreenivas, V., Gulati, M.S. et al. Economic evaluation of a surveillance program of hepatocellular carcinoma (HCC) in India. Hepatol Int 2, 231–236 (2008). https://doi.org/10.1007/s12072-008-9054-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-008-9054-5