Abstract
Background
Antiviral treatments for hepatitis B virus (HBV) are not established in patients with HBV-related hepatocellular carcinoma (HCC).
Aim
To investigate the safety and efficacy of lamivudine (LAM) in patients with HBV-related HCC who were treated with radiofrequency ablation (RFA).
Methods
RFA-treated patients with HBV-related HCC were retrospectively divided into those who received LAM (LAM group) and those who did not (nontreatment group). The first-year changes in serum alanine aminotransferase (ALT), total bilirubin (TBIL), and albumin (ALB) levels were compared in corresponding subsets based on Child-Pugh classification (Mann–Whitney U test) and between one-to-one matched pairs (Wilcoxon signed rank test), who were selected on the basis of their propensity scores for receiving LAM. Overall and recurrence-free survival was also compared.
Results
Complete ablation of HCC was achieved in 104 patients with HBV-related HCC between January 2000 and December 2005. LAM was administered to 33 patients after RFA. Serum HBV-DNA became negative by TMA method in 24 (73%) patients. Four patients showed redetection of HBV-DNA with ALT elevation. Subset analysis based on initial Child-Pugh class and paired analysis with matching revealed significant decreases in ALT and bilirubin levels and increases in ALB levels in the first year in the LAM group (ΔALT = −17, ΔALB = +0.3, and ΔTBIL = −0.2) compared with controls (ΔALT = +5, ΔALB = ±0.0, and ΔTBIL = +0.3). Overall survival and recurrence-free survival did not differ between the two groups. No specific adverse effect was observed in the LAM group.
Conclusion
LAM after RFA for HBV-related HCC was safe and improved liver function. Further studies are needed to evaluate its effect on survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the major consequences of chronic hepatitis B virus (HBV) infection [1–3]. The prevalence of HBV infection widely differs geographically, and is very high in many countries in East Asia and sub-Saharan Africa. Those countries also show a high incidence of HBV-related HCC. Surgical resection can be performed for HCC at the early stages, but is frequently contraindicated by background cirrhosis common in HCC patients. Although liver transplantation is a potential treatment for HCC with liver dysfunction, its feasibility is limited by the scarcity of donor organs. Radiofrequency ablation (RFA) is an alternative treatment for HCC, applicable to patients with moderately impaired liver function [4–10]. The prognosis after RFA is reported to be comparable to that after surgical resection.
Patients with HBV-related HCC treated with RFA are to be confronted with two serious problems: recurrence of HCC and exacerbation of liver dysfunction. Although recurrent HCC may be treated with repeated RFA or other therapeutic modalities, additional treatments may be prohibited by further deterioration in liver function. Moreover, liver failure can be the direct cause of death even in patients without HCC recurrence. Thus, preservation of liver function is essential for improving prognosis of HBV-related HCC patients.
Lamivudine (LAM), 2′3′-dideoxy-3′-thiacytidine, is a potent reverse transcriptase inhibitor and has been used for the treatment of chronic hepatitis B as well as HIV infection [11]. LAM has been shown to be safe and well tolerated in patients with HBV infection, including those with severely decompensated cirrhosis [12–14]. With the inhibition of HBV replication, inflammatory reaction in the liver subsides and the liver can be protected from further deterioration in function. The antiviral therapy with LAM appears to be suitable also for patients with HBV-related HCC after cancer treatment but there have been few reports. The objective of this study is to elucidate the safety and efficacy of LAM treatment in HBV-related HCC patients treated with RFA.
Patients and methods
Patients
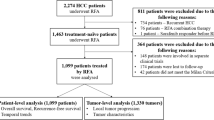
This is a retrospective study on clinical experience in a single center. Between January 2000 and December 2005, a total of 1050 patients were admitted to our hospital for RFA treatment of HCC. HBs antigen was positive in 110 patients and 104 of them received curative RFA therapy, as judged by subsequent imaging studies (Fig. 1). In this study, the medical records of these 104 patients were reviewed.
RFA was performed percutaneously, using monopolar radiofrequency generator (CC-1 Cosman Coagulator, Radionics, Burlington, MA, USA) and internally cooled-tip radiofrequency electrode (Radionics) as described elsewhere [4, 10]. The effectiveness of ablation was evaluated with contrast-enhanced computed tomography in each patient. At the time of RFA, baseline characters of enrolled patients, including age, sex, and biochemical tests, were recorded. Serum level of HBV-DNA was determined by the transcription-mediated-amplification (TMA) method.
Lamivudine treatment
The decision to prescribe LAM, which became available in Japan from November 2000, after RFA treatment was at the discretion of each patient and the physician in charge on discussing merits and demerits of the therapy. In particular, the possibility of emergence of LAM-resistant HBV mutants remained a major concern since no other anti-HBV agents were available at that time. This situation practically precluded studies with randomized assignment.
When indicated, LAM was given at a dose of 100 mg per day orally after obtaining written informed consent. The serum level of HBV-DNA was monitored with TMA method every month, together with biochemical tests for liver function such as alanine aminotransferase (ALT), albumin (ALB), and total bilirubin (TBIL). Recurrence of HCC was monitored with ultrasonography and computed tomography every 3–4 months, and RFA was repeated when necessary. Blood tests and imagings were also applied to those patients who did not choose to receive LAM.
Effects of lamivudine
Although the indication of LAM was not based on rigid criteria, certain factors concerning liver function and HCC status were likely to have affected the decision. Retrospectively, we calculated the propensity score, or “probability,” of receiving LAM for each patient by using unconditional logistic regression. Then, a matched control was selected from the patients who did not receive LAM for each patient who did, by using the propensity score thus derived as the matching factor.
Changes in liver function indices were compared in three distinct manners. First, sequential changes in the average values were compared among overall patients. Second, subset analysis was performed on the basis of the initial liver function classified by Child-Pugh score. Then, pair-wise comparison was performed between each patient in the LAM group and a matched control selected as described in the following section.
Statistic procedures
The propensity score for LAM administration was calculated by using a logistic regression model with LAM administration as the dependent variable and sex, age, liver function (represented by Child-Pugh score), and HCC stage as the independent variables. Since all patients who subsequently received LAM were positive for serum HBV-DNA before administration, controls were included only when they were also positive. A control was selected for each patient who received LAM by using the propensity score as the matching variable, the maximum distance of which was set at 0.1, with an SAS macro, gmatch (http://www.mayoresearch.mayo.edu). The changes in serum ALT, ALB, and TBIL levels in the first year were compared between the cases and controls with Wilcoxon signed rank test. Survival rates were calculated with the Kaplan–Meier method and assessed with the log-rank test. P values less than 0.05 were regarded as statistically significant. All analyses were performed with SAS software for Windows version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of the 104 patients enrolled, 33 patients received LAM after ablation therapy. Serum HBV-DNA was positive in all these patients before LAM administration. In the remaining 71 patients, serum HBV-DNA was positive in 45 and was below the detection threshold in the remaining 26 patients. The propensity score for LAM administration was calculated among 78 patients positive for HBV-DNA: 33 who received LAM and 45 who did not. In logistic regression, patients in Child-Pugh classes B and C were more likely to have received LAM than those in class A, although the difference was not statistically significant (odds ratio: 2.04, P = 0.2030). Other factors, namely, sex, age, and tumor stage, were not significant either (Table 1). However, by using the estimated coefficients for those four independent variables, the probability, or propensity score, of each patient receiving LAM could be calculated. The propensity score showed a concordance index of 0.652 for the prediction of LAM administration. On the basis of the propensity score, one matched control was selected for each patient who received LAM, allowing the maximum difference of 0.1 in the score. Thus, 28 one-to-one matched pairs were created.
Antiviral efficacy of lamivudine
Serum HBV-DNA became negative by TMA assay in 1–9 months of administration in 24 patients (73%) who received LAM. Among those patients, serum HBV-DNA became detectable again in one (10%), six (35%), and seven (58%) patients at 1, 2, and 3 years, respectively. Serum HBV-DNA remained positive but decreased within the detection range in the remaining 9 patients. ALT level was decreased into the normal range in six patients and remained above the normal range in three patients. Reactivated hepatitis, defined as redetection of HBV-DNA and elevation of ALT level higher than 2× the upper normal limit, was seen in four. Two of them received adefovir treatment, which was effective in achieving negative HBV-DNA and normal ALT. The other two died of recurrent HCC before administration of adefovir. Serum ALT levels were normalized in a total of 25 patients (76%): serum HBV-DNA was negative in 19 and positive in 6. Serum ALT remained abnormal in the remaining 8 patients, and 5 of them were negative for serum HBV-DNA. No adverse effects attributable to LAM were recorded.
Changes in liver function
Changes in overall liver function indices among the 104 patients are shown in Fig. 2A–C, where serum ALT, ALB, and TBIL levels during 4 years were compared between the LAM group and the nontreatment group. Although the baseline levels of ALT and bilirubin were significantly higher, and that of ALB significantly lower, in the LAM group the difference lost significance after 1 year.
Because of the different baseline characteristics between the two groups (Table 1), we also conducted subset analysis including only those patients positive for baseline HBV-DNA and stratified them on the basis of baseline liver function (Child-Pugh A vs. Child-Pugh B). The changes in the levels of ALT, ALB, and TBIL in the first year were compared between the LAM group and the nontreatment group in an unpaired manner with Mann–Whitney U test. The Child-Pugh A subset included 20 patients in the LAM group and 32 patients in the nontreatment group. The differences between the two groups in the first-year change in ALT, ALB, and TBIL were significant (P = 0.0014, 0.0036, and 0.0092, respectively) (Table 2). In the Child-Pugh B subset (12 in the LAM group and 11 in the nontreatment group), similar trends were observed but none were statistically significant (Table 2).
In the above subset analysis based on liver function, similarity of factors other than liver function was not guaranteed in each subset, and there was a possibility of systemic bias. Thus, we sought to confirm the results by paired statistics, selecting a matched control for each patient in the LAM group on the basis of the propensity score. A total of 28 pairs were provided, and the differences in the first-year changes in ALT, ALB, and TBIL levels were compared with Wilcoxon signed rank test. The difference in changes in each index was statistically significant between the two groups, showing a tendency toward improvement in the LAM group (Fig. 3). Five cases in the LAM group were not matched and excluded from this subanalysis. Changes in the first year in ALT, ALB, and TBIL among those cases were −18.2 + 60.7 IU/l, −0.10 + 0.71 g/dl, and 0.0 + 1.07 mg/dl, respectively.
Changes in liver function indices in paired comparison. The first-year changes in ALT level, ALB concentration, and TBIL concentration (shown as ΔALT, ΔALB, and ΔTBIL, respectively) were compared between each patient in LAM group and corresponding paired control matched by propensity score (total 28 pairs). P values were determined by Wilcoxon signed rank test
Survival
During the observation period, 8 (24.2%) of 33 patients in the LAM group and 32 (45.1%) of 71 patients in the nontreatment group died. Direct consequences of HCC were the cause of death in 7 in the former and 19 in the latter group, whereas liver failure caused death in none in the former and 7 in the latter group. Overall survival did not differ statistically between the two groups (Fig. 4). There was no difference in recurrence-free survival between the two groups. Five patients on LAM developed recurrent HCC during sustained HBV-DNA negativity (median: 9 months, range: 6–22 months).
Discussion
Today, not only LAM but also other new drugs, such as adefovir and entecavir, are vigorously used for chronic hepatitis B [12–16]. LAM, the first agent of this category, was reported to decrease the incidence of HBV-related HCC [14, 17, 18]. However, the effect of LAM on the prognosis of HCC patients has not been well documented. Preservation of liver function, which may be already deteriorated, is a prerequisite for the improvement of prognosis of HCC patients. Interferon therapy to HCV-related HCC patients after cancer treatment seems effective in improving survival [19, 20]. Similar strategies may be applied to HBV-related HCC. We have shown that LAM in HCC patients treated with RFA resulted in significant decreases in serum ALT and bilirubin levels and increases in ALB level. No particular adverse effects were noted, and the emergence of resistant virus was well controlled. The improvement in liver function may be beneficial not only for preventing liver failure but also for broadening treatment options for recurrent HCC.
In the current retrospective study, the indication for LAM administration was not decided systematically except that all patients given LAM were initially HBV-DNA positive. No single factor was a significant determinant of LAM administration, as revealed by logistic regression. Nevertheless, the decision was not completely arbitrary since the determinant used in propensity score adequately predicted LAM administration. In this setting, we performed pair-wise analysis and found significant improvement in liver function with LAM treatment.
We observed no significant differences in the overall and recurrence-free survival between LAM and nontreatment groups. The baseline liver function was poorer and AFP, a strong predictor of recurrence [21], was higher in the LAM group. Noninferior survival in the LAM group may suggest some beneficial effects of LAM administration. However, we did not find significant effects of LAM in multivariate analysis, possibly due to the small number of events (40 deaths). To conclude the efficacy of LAM treatment on recurrence-free or overall survival, either much larger cohort studies or well-designed randomized control trials will be required. The current study demonstrated the safety of LAM administration in HCC patients after HCC treatment, including the data on LAM resistance similar to a previous report [22].
In conclusion, LAM treatment after ablation therapy for HBV-related HCC improved liver function without any particular untoward effects. Its effects on survival remain to be studied in future studies.
References
Shiratori Y, Shiina S, Imamura M, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology 1995;22:1027–33
Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B, C: a prospective study of 251 patients. Hepatology 1995;21:650–5
Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol 1998;28:930–8
Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201–9
Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am 1995;1:73
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655–61
Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology 2002;62 Suppl 1:64–8
Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235–40
Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122–30
Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: ethanol injection therapy and radiofrequency ablation. Gastroenterology 2004;127:S159–66
Omata M. Treatment of chronic hepatitis B infection. N Engl J Med 1998;339:114–5
Villeneuve JP, Condreay LD, Willems B, et al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology 2000;31:207–10
Yao FY, Terrault NA, Freise C, Maslow L, Bass NM. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology 2001;34:411–6
Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31
Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808–16
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800–7
Yoshida H, Shiratori Y, Moriyama M, et al., for the IHIT (Inhibition of Hepatocarcinogenesis by Interferon Therapy) Study Group. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med 1999;131:174–81
Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346:1051–55
Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med 2003;138:299–306
Kubo S, Nishiguchi S, Hirohashi K, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med 2001;134:963–7
Tateishi R, Shiina S, Yoshida H, et al. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology 2006;44:1518–27
Leung NW, Lai CL, Chang TT, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001;33:1527–32
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, H., Yoshida, H., Goto, E. et al. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int 2, 89–94 (2008). https://doi.org/10.1007/s12072-007-9020-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-007-9020-7