Abstract
Despite of promising improvements in treatment of gastric cancer, the mortality rate of this malignancy remains high. Chronic infection by Helicobacter pylori, interfering with intracellular signalling pathways, is the main risk factor for gastric cancer. Some evidence suggests that microRNAs (miRNA), the small noncoding RNA molecules, can play role as oncogenes or tumour suppressors in the cells. MiR-222 is one of the remarkable miRNAs undergoing upregulation in gastric cancer. However, the association between miR-222 upregulation and H. pylori infection in gastric cancer tissues remains unclear. The aim of this study was to analyse the expression level of miR-222 in gastric cancer tissues, evaluating the relationship between miR-222 expression level and H. pylori infection and also finding novel miR-222 targets based on in silico investigations. MiR-222 expression level in 200 patients including 112 H. pylori positive and 88 H. pylori negative was relatively measured using RT-qPCR and compared with 88 healthy samples. In silico enrichment analysis of miR-222 targets was performed by DAVID database to evaluate the possible role(s) of miR-222 in gastric tumourigenesis. We observed upregulated level of miR-222 in gastric cancer tissues compared with normal samples (P < 0.05). However, no significant difference between miR-222 expression in H. pylori-positive and H. pylori-negative cases was observed. Our in silico analyses showed the possible role of p53, p27, PTEN and Elongin B in gastric cancer tumourigenesis. MiR-222 functions as an onco-miRNA and its overexpression can be involved in pathogenesis of gastric cancer, independent of H. pylori infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic infection by Helicobacter pylori, interfering with intracellular signalling pathways is the main risk factor for gastric cancer. Some evidence suggests that microRNAs (miRNAs) can play role as oncogenes or tumour suppressors in the cells. MiR-222 is one of the remarkable miRNAs undergoing upregulation in gastric cancer. However, the association between miR-222 upregulation and H. pylori infection in gastric cancer tissues remains unclear. In this study, we analysed the expression level of miR-222 in gastric cancer tissues. Evaluating miR-222 expression level in 200 patients by RT-qPCR showed the upregulated level of miR-222 in gastric cancer tissues compared with normal samples (P = 0.001). However, no significant difference between miR-222 expression in H. pylori-positive and H. pylori-negative cases was observed. In conclusion, miR-222 functions as an onco-miRNA and its overexpression can be involved in pathogenesis of gastric cancer, independent of H. pylori infection.
Gastric or stomach cancer is a kind of malignancy resulting from the stomach lining. The incidence of gastric cancer is unevenly spreading over two major high-risk regions, eastern Asia, central and eastern Europe and South America. In less-developed countries, stomach cancer among men is the third most frequently diagnosed cancer, and leading cause of cancer death along with liver cancer (Torre et al. 2015). The overwhelming majority of stomach cancer risk factors are infection with H. pylori (González et al. 2013), genetic factors (Sugerman et al. 1995), a diet high in salt, smoked foods (Jakszyn and González 2006; González et al. 2013), smoking (Nomura et al. 1990) and consuming alcohol (González et al. 2013; Thrumurthy et al. 2013). These parameters can cause transformation of normal gastric tissue to superficial gastritis, gastritis with atrophy, intestinal metaplasia, dysplasia and finally gastric cancer (Catalano et al. 2005). Infection by H. pylori, a gram-negative bacteria, is the main risk factor for 65–80% of gastric cancer cases (González et al. 2013). The stomachic epithelium of over 50% of the world’s population is infected by H. pylori (Cotticelli et al. 2005; Noto and Peek 2012). H. pylori expresses a range of risk factors that deregulated host intracellular signalling pathways (Noto and Peek 2012). Among the mediators induced by H. pylori, miRNAs have the main effect on the interactions between bacteria and host cells (Zabaleta 2012). MiRNAs are noncoding RNAs with approximate 22 nucleotides that function as a regulator of posttranscriptional RNA (Chun-Zhi et al. 2010). Various studies show that miRNAs play significant roles in different cell processes including differentiation, development, angiogenesis, cell cycle progression, proliferation and apoptosis (Noto and Peek 2012). In a number of studies, dysregulation of different miRNAs including upregulation of miR-222 in gastric cancer have been explored (Chun-Zhi et al. 2010; Li et al. 2012; Fu et al. 2014). MiR-222 is known as an onco-miRNA and its aberrant regulation has been observed in some diseases; for instance, hepatocellular carcinoma, melanoma, lymphoma, glioblastoma and also colorectal, lung, breast and thyroid cancers (Chun-Zhi et al. 2010; Li et al. 2012). Further, some of the experimentally validated miR-222 target genes are CDKN1C, CDKN1B, PTEN, VGLL4 and RECK.
A cell culture-based study has reported the association between upregulation of miR-222 and infection with H. pylori in gastric cancer. However, little is known about the effect of H. pylori on the expression level of miR-222 expression in gastric cancer tissues.
The purpose of this study was to evaluate miR-222 expression in gastric cancer tissues to find its possible role in induction of gastric cancer, probably associated with H. pylori infection. Further, possible targets of miR-222 that could be involved in progression of gastric cancer were studied in-silico. Based on the results, we propose miR-222 as a biomarker involving in induction of gastric cancer, independent of H. pylori infection.
Materials and methods
Patients and control samples
Fresh tissue samples were collected after surgery from 200 patients with gastric cancer who attended Al-Zahra Hospital (Isfahan, Iran), including 112 H. pylori-positive and 88 H. pylori-negative. Also 88 healthy samples including 48 H. pylori-positive and 40 H. pylori-negative were taken from endoscopy section of Al-Zahra Hospital during a course of nine months after signing a consent form. To assess H. pylori positivity or negativity in cancer cases, 5 mL of blood was collected in EDTA-containing tubes for ELISA test and in healthy samples an endoscopy biopsy was used for rapid urease test. For all of the samples, standard consent form was taken from individuals. All study protocols and consent forms were approved by institutional review board of Zist-Fanavari Novin Biotechnology Institute.
RNA extraction
Total RNA (including miRNA) was isolated using Hybrid-R TM miRNA kit (GeneAll, Seoul, South Korea). The protocol was used based on the manufacturer’s manual. The extracted total RNA was stored at −70°C.
cDNA synthesis and RT-qPCR
cDNA synthesis for miR-222 was achieved using a Universal cDNA Synthesis kit (Exiqon, Denmark) based on a poly-A tailing method, according to the manufacturer’s instruction. Real-time PCR reactions were performed as triplicate using an ABI PRISM 7500 instrument (Applied Biosystems, Foster City, USA) standard protocols. Total volume of 10 μL comprised of 1 : 50 diluted cDNA products added to a master mix containing 10 pmol/ μL of miR-222 primers using miRCURY LNA TM microRNA PCR, ExiLENT SYBR Ⓡ Green master mix (Exiqon, Vedback, Denmark). The amplification programme was set as 95°C for 10 min followed by 40 cycles of 95°C for 10 s, 60°C for 1 min. The relative expression level of miR-222 was normalized by U6 as a reference gene (Livak and Schmittgen 2001). Real-time data were analysed and reported based on 2 −ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
All statistical tests were executed by SPSS (ver. 20). Nonparametric test (Mann–Whitney U test) was used for comparing the expression levels of miR-222 in tumour and normal samples, as well as H. pylori-positive and H. pylori-negative cases. Due to lack of normal distribution of gene expression, Spearman correlation coefficient test was used to determine the correlation between gene expressions in stages of gastric cancer. Kruskal–Wallis test was performed to study the gene expression levels in different blood groups. For all the tests, a P < 0.05 was considered statistically significant.
Results
Upregulation of miR-222 in gastric cancer
The expression pattern of miR-222 was assessed by qRT-PCR method within three groups including H. pylori-positive (n = 112), H. pylori-negative patients (n = 88) and a group of healthy subjects (n = 88). Based on statistical analysis, miR-222 expression level in patients was significantly higher than controls (P = 0.001) (figure 1). Among the 288 studied tissues, miR-222 overexpression was observed in 56% (112 of 200) of gastric cancer patients, while no healthy sample showed miR-222 overexpression. Among 112H. pylori-positive and 22 H. pylori-negative patients, ˜64% (72 of 112) and 45% (40 of 88) of cases showed miR-222 overexpression, indicating higher rate of miR-222 overexpression in H. pylori-positive specimens. However, there was no significant difference between miR-222 expression in H. pylori-positive and H. pylori-negative patients (Mann–Whitney U test, P = 0.202 for control and P = 0.537 for patient groups) (figure 2, a&b).
Real-time PCR analysis of miR-222 expression in control and patient groups. MiR-222 expression level in patients was significantly higher than controls (Mann–Whitney U test, P = 0.001). The box-plot shows the first and third quartiles in a box, median is represented with the band inside the box and stars represent the outliers.
Real-time PCR analysis of miR-222 expression between H. pylori-positive and H. pylori-negative cases in (a) control and (b) patient groups. No significant differences were observed in both groups (Mann–Whitney U test, P = 0.202 for control and P = 0.537 for patient groups). The box-plot shows the first and third quartiles in a box, median is represented with the band inside the box and stars represent the outliers.
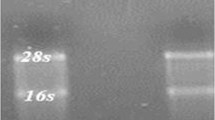
Analysing the relationship between miR-222 expression level and histopathological features of patients, there was no significant correlation between miR-222 expression and progressed stages of gastric cancer (P = 0.0162, r = 0.14). Also, no significant correlation between age and miR-222 expression level was yielded (P = 0.64, r = 0.1). Further, miR-222 expression level was the same for patients with different blood groups (P = 0.273). The specificity of miR-222 and U6 primers was assessed by electrophoresis in 2% agarose gel (figure 3).
Discussion
Infection by H. pylori is the main risk factor in 65–80% of gastric cancer cases (González et al. 2013). H. pylori expresses a range of risk factors resulting in deregulation of host intracellular signalling pathways (Noto and Peek 2012). Among the mediators induced by H. pylori, miRNAs have the main effect on the interactions between bacteria and host cells (Zabaleta 2012). Many efforts have been carried out to identify the potential roles of miRNAs in gastric cancer, leading to the identification of some miRNAs playing role in development of inflammatory response initiated by H. pylori infection, gastric epithelial tumourigenesis, invasion and migration capacities (Bartel 2004). Analysis of miRNAs is a powerful tool to improve the diagnostic and prognostic aspects of gastric cancer. Further, the critical roles of miRNAs in pathogenesis of gastric cancer potentiate them as putative therapeutic targets.
The main aim of this study was to analyse the expression level of miR-222 in both H. pylori-positive and H. pylori-negative gastric cancer fresh tumour samples to evaluate miR-222 as a new diagnostic biomarker for gastric cancer. Fifty gastric tumour samples collected from 112 H. pylori-positive and 88 H. pylori-negative patients as well as twenty-two healthy samples including 48 H. pylori-positive and 40 H. pylori-negative cases were investigated in this study. MiR-222 overexpression was observed in 56% (112 of 200) of gastric cancer patients, while no healthy sample showed miR-222 overexpression. Among 112 H. pylori-positive and 88 H. pylori-negative patients, ˜64% (72 of 112) and 45% (40 of 88) of cases showed miR-222 overexpression, indicating higher rate of miR-222 overexpression in H. pylori-positive specimens. However, no significant difference between expression level of miR-222 and positivity/ negativity of H. pylori status was observed in both patient and control groups. This is inconsistent with the outcomes of Li et al. (2012) study, reporting the significant greater expression level of miR-222 in H. pylori-positive patients. This inconsistency might be due to the analization of miR-222 expression in cell culture-based analysis of the latter study compared with our patient-based investigations. The limited population of our study could also be another parameter leading to the different outcome. In this study, the only meaningful and significant difference was the greater expression level of miR-222 in patient group compared with controls. Upregulated expression level of miR-222 in gastric cancer has also been reported in previous studies (Kim et al. 2009; Li et al. 2011). In 2014, Wang et al. (2014) demonstrated that miR-222 is commonly upregulated in gastric cancer tissue-derived mesenchymal stem cells and cancer tissues.
Functionally, miR-222 targets two important tumour suppressors PTEN and RECK in normal cells and in turn, overexpressed miR-222 can drastically downregulate these proteins resulting in aberrant function of PI 3K-Akt and extracellular matrix proteins in stomach cells (Chun-Zhi et al. 2010; Li et al. 2012). MiR-222 can also promote proliferation by maintaining low expression level of p27 kip1, a cell cycle inhibitor (Le Sage et al. 2007). Moreover, Kim et al. (2009) reported that miR-222 suppresses the Cip/Kip family members of p21 Cip1, p57 Kip2 and the Cdk inhibitors. VGLL4, a tumour suppressor playing a role in miR-222/VGLL4/YAP-TEAD1 regulatory loop, is also a direct target of miR-222 in gastric cancer cells. In fact, upregulated miR-222 can promote proliferation and invasion of gastric cancer cells (Li et al. 2014). Altogether, these studies along with our recent finding reported 56% of overexpression rate of miR-222 in gastric cancer patients, strongly suggesting an onco-miRNA activity of miR-222 in the cells. Therefore, miR-222 is proposed to be valuable diagnostic and prognostic biomarker for gastric cancer.
The main drawbacks of this study were the relatively small-studied population and also extracting small RNA from probably heterogenic tumour samples. Contamination of tumour cells by adjacent normal cells might result in the dilution of miR-222 overexpressing tumour cells and masking the overexpression status of miR-222. It is suggested to evaluate the potential therapeutic molecules with ability to target miR-222 to reduce tumourigenic properties of gastric malignancy.
This study showed that miR-222 functions as an onco-miRNA and its overexpression can be involved in pathogenesis of gastric cancer, independent of H. pylori infection. This report strongly support that miR-222 might be used as a potential diagnostic and prognostic biomarker for gastric cancer.
References
Bartel D. P. 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297.
Catalano V., Labianca R., Beretta G. D., Gatta G., de Braud F. and Van Cutsem E. 2005 Gastric cancer. Crit. Rev. Oncol. Hematol. 54, 209–241.
Chun-Zhi Z., Lei H., An-Ling Z., Yan-Chao F., Xiao Y., Guang-Xiu W. et al. 2010 MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 10, 367.
Cotticelli L., Borrelli M., D’Alessio A., Menzione M., Villani A., Piccolo G. et al. 2005 Central serous chorioretinopathy and Helicobacter pylori. Eur. J. Ophthal. 16, 274–278.
Fu Z., Qian F., Yang X., Jiang H., Chen Y. and Liu S. 2014 Circulating miR-222 in plasma and its potential diagnostic and prognostic value in gastric cancer. Med. Oncol. 31, 1–8.
González C. A., Sala N. and Rokkas T. 2013 Gastric cancer: epidemiologic aspects. Helicobacter 18, 34–38.
Jakszyn P. and González C. A. 2006 Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol 12, 4296–4303.
Kim Y. -K., Yu J., Han T. S., Park S. -Y., Namkoong B., Kim D. H. et al. 2009 Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 37, 1672–1681.
Le Sage C., Nagel R., Egan D. A., Schrier M., Mesman E., Mangiola A. et al. 2007 Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 26, 3699–3708.
Li N., Tang B., Zhu E. -D., Li B. -S., Zhuang Y., Yu S. et al. 2012 Increased miR-222 in H. pylori-associated gastric cancer correlated with tumor progression by promoting cancer cell proliferation and targeting RECK. FEBS Lett. 586, 722–728.
Li N., Yu N., Wang J., Xi H., Lu W., Xu H. et al. 2014 miR-222/VGLL4/YAP-TEAD1 regulatory loop promotes proliferation and invasion of gastric cancer cells. Am. J. Cancer Res. 5, 1158–1168.
Li X., Zhang Y., Zhang H., Liu X., Gong T., Li M. et al. 2011 miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol. Cancer Res. 9, 824–833.
Livak K. J. and Schmittgen T. D. 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25, 402–408.
Nomura M., Nakano S., Kudou J., Ishibashi O. and Niho Y. 1990 Successful treatment of advanced gastric cancer with multiple liver metastasis by combination chemotherapy using mitomycin C, 5-fluorouracil, and high-dose leucovorin: a case report. Gan to kagaku ryoho. Cancer & Chemother. 17, 2097–2100.
Noto J. M. and Peek R. M. 2012 Gastric-to-intestinal transdifferentiation and cancer. Proc. Natl. Acad. Sci. 109, 20173–20174.
Sugerman P., Joseph B. and Savage N. 1995 The role of oncogenes, tumour suppressor genes and growth factors in oral squamous cell carcinoma: a case of apoptosis versus proliferation. Oral Dis. 1, 172–188.
Thrumurthy S. G., Chaudry M. A., Hochhauser D. and Mughal M. 2013 The diagnosis and management of gastric cancer. BMJ 347, 6367.
Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. and Jemal A. 2015 Global cancer statistics, 2012. CA: J. Clin. 65, 87–108.
Wang M., Zhao C., Shi H., Zhang B., Zhang L., Zhang X. et al. 2014 Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 110, 1199–1210.
Zabaleta J. 2012 MicroRNA: a bridge from H. pylori infection to gastritis and gastric cancer development. Front. Genet. 3, 294.
Acknowledgements
We strongly appreciate staffs of Al-Zahra Hospital, blood donors and our colleagues in Zist-Fanavari Novin Biotechnology Institute, for their constructive comments and participations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: J. Gowrishankar
[Noormohammad M., Sadeghi S., Tabatabaeian H., Ghaedi K., Talebi A., Azadeh M., Khatami M. and Heidari M. M. 2016. Upregulation of miR-222 in both Helicobacter pylori-infected and noninfected gastric cancer patients. J. Genet. 95, xx–xx]
Rights and permissions
About this article
Cite this article
NOORMOHAMMAD, M., SADEGHI, S., TABATABAEIAN, H. et al. Upregulation of miR-222 in both Helicobacter pylori-infected and noninfected gastric cancer patients. J Genet 95, 991–995 (2016). https://doi.org/10.1007/s12041-016-0728-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0728-9