Abstract
Flavonoids are an important class of secondary metabolites that play various roles in plants such as mediating defense, floral pigmentation and plant–microbe interaction. Flavonoids are also known to possess antioxidant and antimicrobial activities. Coleus forskohlii (Willd.) Briq. (Lamiaceae) is an important medicinal herb with a diverse metabolic profile, including production of a flavonoid, genkwanin. However, components of the flavonoid pathway have not yet been studied in this plant. Chalcone synthase (CHS) catalyses the first committed step of flavonoid biosynthetic pathway. Full-length cDNA, showing homology with plant CHS gene was isolated from leaves of C. forskohlii and named CfCHS (GenBank accession no. KF643243). Theoretical translation of CfCHS nucleotide sequence shows that it encodes a protein of 391 amino acids with a molecular weight of 42.75 kDa and pI 6.57. Expression analysis of CfCHS in different tissues and elicitor treatments showed that methyl jasmonate (MeJA) strongly induced its expression. Total flavonoids content and antioxidant activity of C. forskohlii also got enhanced in response to MeJA, which correlated with increased CfCHS expression. Induction of CfCHS by MeJA suggest its involvement in production of flavonoids, providing protection from microbes during herbivory or mechanical wounding. Further, our in silico predictions and experimental data suggested that CfCHS may be posttranscriptionally regulated by miR34.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
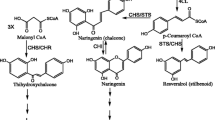
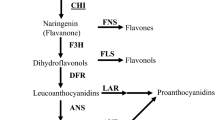
Flavonoids are an important class of secondary metabolites that are involved in a wide array of processes like floral pigmentation, pollination, nitrogen fixation and are produced in response to stress, UV, pathogens, insects etc. (Winkel-Shirley 2002; Cushnie and Lamb 2005; Taylor and Grotewold 2005; Lillo et al. 2008; Päsold et al. 2010; Fini et al. 2011). Several flavonoids have been shown to possess antioxidant, antimicrobial, antiinflammatory or antitumour activities (Cazarolli et al. 2008). Chalcone synthase (CHS) is an important enzyme that channels the flux of phenylpropanoid pathway towards biosynthesis of flavonoids (figure 1). Phenylpropanoid pathway provides precursors for biosynthesis of other important metabolites, such as rosmarinic acid, which has been demonstrated to possess antiviral activity against Japanese encephalitis (Swarup et al. 2007). CHS belongs to type III polyketide synthase (PKS) superfamily (Schröder 1997). It catalyzes the condensation of three molecules of malonoyl-CoA and one molecule of CoA ester of cinnamic acid or its derivative like coumaric acid yielding naringenin chalcone (Martens and Mithöfer 2005). Chalcone isomerase (CHI) then reversibly converts it to flavanone (Moustafa 1967). These intermediates mark the entry point from where pathway diverges into several branches, each resulting in a different class of flavonoids like anthocyanins, phytoalexins and phytoanticipins (Jez et al. 2001; Dao et al. 2011).
CHS has been studied in many plant species including Oryza sativa L. (Poaceae), Aquilaria sinensis (Lour.) Spreng. (Thymelaeaceae), Physcomitrella patens (Hedw.) Bruch & Schimp. (Funariaceae), Psilotum nudum (L.) P. Beauv. (Psilotaceae) etc. (Reddy et al. 1996; Goodwin et al. 2000; Yamazaki et al. 2001; Jiang et al. 2006; Wang et al. 2013). The number of CHS copies varies to a great extent in different plants. A single copy of CHS is present in Antirrhinum majus L. (Plantaginaceae), six copies in Ipomoea batatas (L.) Lam. (Convolvulaceae), eight in Petunia hybrida (Solanaceae), two in Arabidopsis thaliana (L.) Heynh. (Brassicaceae) and Populus trichocarpa Torr. & A. Gray (Salicaceae), whereas three copies of CHS are present in Vitis vinifera L. (Vitaceae) and Viola cornuta L. (Violaceae) (Van den Hof et al. 2008). CHS expression may be induced in response to herbivory, plant pathogens and various abiotic stresses like ultraviolet, wounding or exogenous phytohormone / elicitor treatment (Richard et al. 2000; Schenk et al. 2000). Dao et al. (2011) found that Ipomoea purpurea (L.) Roth (Convolvulaceae) plants with nonfunctional copies of CHS suffered almost twice the intensity of infection from Rhizoctonia solani and about 25% greater damage from herbivorous attack than the wild type, proving the role of CHS in pathogen resistance. Coleus forskohlii (Willd.) Briq. (Lamiaceae) is an important medicinal herb known for the production of forskolin, a potent and reversible activator of adenylate cyclase, responsible for antihypertensive property of the herb (Alasbahi and Melzig 2010). The plant is distributed widely in various regions of Asia such as Sri Lanka, India, Nepal, Bhutan, Thailand and China as well as in Africa and Brazil (Kavitha et al. 2010). The plant has a diverse metabolic profile (Kavitha et al. 2010; Paul et al. 2013), including production of minor compounds such as the flavonoid: genkwanin (7-O-methylapigenin) (Alasbahi and Melzig 2010). However, components of the flavonoid pathway have not yet been studied in this plant. Here, we carried out the isolation of the gene encoding CHS, CfCHS, from leaf of C. forskohlii. Quantitative real time RT-PCR was used to investigate its expression in different tissues and under the effect of various abiotic elicitors.
Materials and methods
Plant material
Coleus forskohlii plants were collected on 17 October 2011 and identified by Dr Y. S. Bedi. A voucher specimen was prepared and submitted to Janaki Ammal herbarium, IIIM (Acronym RRLH, accession no. 22164). Plants were grown and maintained in greenhouse.
RNA isolation and cDNA synthesis
Total RNA was isolated from the young leaves, mature leaves, stem, root and root tip of plants using TRIzolⓇ (Invitrogen, Life Technologies, USA), following manufacturer’s instructions. Quantity and quality of RNA was assessed using NanoDrop spectrophotometer (Thermo Scientific, USA) and agarose gel electrophoresis (2%), respectively. Total RNA was treated with DNase (DNA-freeTM kit; AmbionⓇ TURBO DNA-freeTM, Life Technologies, USA) to remove any potential DNA contamination. cDNA was synthesized by using ImProm-IITM Reverse Transcription System (Promega, Madison, USA) with an anchored oligo-dT12 primer (FirstChoiceⓇ RLM-RACE Kit, AmbionⓇ , Life Technologies, Carlsbad, USA) and 1 μg of DNAse treated RNA as template.
Cloning of full-length CfCHS
Degenerate primers (CfCHSF and CfCHSR; refer table 1 for sequences) were used to obtain partial clone of CfCHS by carrying out PCR amplification from leaf cDNA in a total volume of 20 μL reaction mixture containing 2 μL buffer (10 ×; with MgCl2), 2 μL dNTPs (2 mM), 1 μL each primer (5 μM), 1 unit of Taq DNA polymerase (New England Biolabs, USA) and cDNA template under the following conditions: 10 min at 95 ∘C, 35 cycles of 30 s at 95 ∘C, 30 s at 60 ∘C and 40 s at 72 ∘C, followed by a final extension of 72 ∘C for 10 min. A 569-bp DNA fragment was obtained and gel elution was carried out following manufacturer’s protocol (Qiaquick gel extraction kit; Qiagen, The Netherlands). The eluted product was cloned into the pTZ57R/T plasmid (InsTAcloneTM PCR Cloning Kit; Fermentas, Thermo Fisher Scientific, USA) and sequenced. The sequence thus obtained was used to design gene-specific primers (GSP) for carrying out 3 ′ and 5 ′ RACE.
GSP CfCHS3R and 3 ′ RACE outer primer (provided in the kit) were used for 3 ′ RACE-PCR reaction. Using primary PCR product as template, nested PCR was performed with GSP CfCHS3R forward primer and 3 ′ RACE inner primer. Similar procedure was followed for obtaining 5 ′ end sequence using First ChoiceⓇ RLM-RACE kit (AmbionⓇ, Life Technologies, USA), where GSP CfCHS5R with 5 ′ RACE outer primer and GSP CfCHS5R with 5 ′ RACE inner primer were used for the primary and nested PCR reactions, respectively (table 1). Thermal profile for nested PCR was as follows: 10 min at 95 ∘C, 35 cycles of 30 s at 95 ∘C, 30 s at T m and 1 min at 72 ∘C followed by final extension at 72 ∘C for 10 min. Amplified DNA fragments obtained from 3 ′ and 5 ′ RACE reactions were cloned in pTZ57R/T plasmid (InsTA Clone PCR cloning kit; Fermentas) and sequenced. Full-length coding DNA sequence (CDS) of CfCHS was amplified from leaf cDNA using primers (CDSfwd and CDSrev, table 1) designed from the sequences of 3 ′ RACE and 5 ′ RACE amplicons. These primers were also used with genomic DNA as template for testing the presence of an intron. All primers were obtained from Integrated DNA Technologies, USA.
Sequence analysis
The partial nucleotide sequences obtained for the core fragment, 3 ′ and 5 ′ RACE products were assembled using the assembly tool in CLC Genomics Workbench (Qiagen, The Netherlands). Open reading frame (ORF) in the assembled full-length CfCHS sequence was predicted by using ORF finder tool (www.ncbi.nlm.nih.gov/gorf/gorf.html). The nucleotide sequence of CfCHS was translated using ExPASy translate tool (Gasteiger et al. 2003). Computational analysis was carried out to predict potential miRNA-binding sites using psRNATarget tool (Dai and Zhao 2011). For miRNA target prediction, complete nucleotide sequences (with UTRs) of CfCHS and its homologues from Solenostemon scutellarioides (L.) Codd (Lamiaceae), Agastache rugosa (Fisch. & C. A. Mey) Kuntze (Lamiaceae), Perilla frutescens (L.) Britton (Lamiaceae) and Misopates orontium (L.) Raf. (Plantaginaceae) were analysed (maximum expectation was set to 5.0, all other parameters were set as default). Multiple sequence alignment with protein sequences from related plants was performed using the CLC genomic workbench (CLC bio, a Qiagen company, The Netherlands). Percentage similarity and identity among the homologous sequences was calculated using MatGAT tool (Campanella et al. 2003). Sequences of CHS homologues were downloaded from NCBI GenBank: A. rugosa (GenBank acc. no. AFL72079.1), S. scutellarioides (GenBank acc. no. ABP57071.1), P. frutescens (GenBank acc. no. BAA19548.1) and M. orontium (GenBank acc. no. CAJ44127.1). Phylogenetic tree was constructed using MEGA 5.2 using neighbour-joining algorithm (Saitou and Nei 1987; Tamura et al. 2004, 2011). The theoretical isoelectric point (pI) and molecular weight of the protein were predicted using ComputePI tool on ExPASy web server (Gasteiger et al. 2003).
Stress treatment
Gene expression analysis of CfCHS was carried out in plants treated with various elicitors as described earlier for expression study of cytochrome P450 genes in C. forskohlii (Awasthi et al. 2015). A 50 mL solution of salicylic acid (SA) (1 mM), MeJA (100 μM), abscisic acid (ABA) (50 μM) or 2,4-dichlorophenoxyacetic acid (2,4-D) (50 μM) was sprayed on aerial parts of the potted four-weeks old C. forskohlii plants. MeJA-treated plants were covered with a thin, transparent plastic bag and kept in isolation. Plants sprayed with 50 mL of 0.1% ethanol were used as control. Young leaves were sampled for 4 h after treatment. The samples were frozen in liquid nitrogen, and stored at −70 ∘C until further use.
Estimation of flavonoid content and radical scavenging activity were carried out in control and MeJA-treated plants. For this, the plants were treated with MeJA as per the procedure followed for the treatment of plants for gene expression analysis, however, here the treatment was given once a day for three consecutive days, following which the plant samples were harvested and shade-dried. Crude extracts were prepared from 100 mg of dried leaves, stems and roots of C. forskohlii. Tissues of shade dried were powdered and extracted thrice with methanol at 30 ∘C (with sonication) for 3 h. The crude extracts thus obtained were analysed using spectrophotometry.
Expression analysis using qRT-PCR
Quantitative real time RT-PCR (qRT-PCR) was carried out for expression profiling of CfCHS in different tissues and under different elicitor treatments as described earlier (Rather et al. 2015). Briefly, first strand cDNA synthesis was performed using ImProm-IITM Reverse Transcription System (Promega, USA). Primers used for qRT-PCR study were designed using Primer3 software (Untergasser et al. 2012). qRT-PCR assay was carried out using the LightCyclerⓇ 96 Real Time PCR System (Hoffmann-La Roche, Switzerland) according to the manufacturer’s instructions. Each PCR reaction (20 μL) contained 1 × LightCyclerⓇ 480 SYBR Green I Master (Hoffmann-La Roche, Switzerland), 1 μM primers (Integrated DNA Technologies, USA, refer to table 1 for sequences: qRTfwd and CfCHS5R) and cDNA (appropriately diluted). Thermal cycling conditions for the qPCR were: preincubation at 95 ∘C for 10 min, followed by 45 cycles of 3 step amplification (95 ∘C for 10 s, 60 ∘C for 15 s and 72 ∘C for 25 s). The PCR was followed by a dissociation curve analysis (heating to 95 ∘C for 10 s at normal ramping, cooling to 65 ∘C for 60 s at normal ramping followed by slow heating to 97 ∘C for 1 s at reduced ramping rate of 0.2 ∘C/s) to test qPCR reaction specificity. Each assay was carried out in triplicate and a nontemplate negative control was included. Actin gene was chosen as house-keeping internal control for normalization. The threshold cycle (C t ) of the amplification curve was used for the calculations. The relative expression level was analysed using the 2 −ΔΔct method (Livak and Schmittgen 2001; Awasthi et al. 2015), where ΔΔC t = (C t, t a r g e t −C t, a c t i n ) t i m e x −(C t, t a r g e t −C t, a c t i n ) t i m e 0.
MicroRNA expression study using RT-PCR
Total RNA of 1 μg was tailed and reverse transcribed by NCodeTM Express SybrⓇ GreenERTM miRNA qRT-PCR kit, universal (Invitrogen, Life Technologies, USA) according to the user’s manual. MiRNA specific primers (table 1) were used to estimate their expression in response to MeJA and ABA. C t values were normalized to 5.8S gene. Relative miRNA expression was calculated using the 2 −ΔΔct method (Livak and Schmittgen 2001).
Quantification of total flavonoid content
Total flavonoid content was quantified using spectrophotometer as described earlier (Kaur et al. 2013). Briefly, dried crude extract (prepared from 100 mg of dried plant material) dissolved in 500 μL of distilled water was mixed with 30 μL of 5% NaNO2 solution, followed by 5 min of incubation at room temperature. After this, 300 μL of 10% AlCl3⋅H 2O solution was added and the sample was further incubated for 6 min. Finally, 200 μL of 1 M NaOH and 200 μL of distilled water were added to the sample and absorbance was read at 510 nm. Total flavonoids were calculated using quercetin as standard (10–100 μg; R 2 = 0.998). The results were expressed as mg quercitin equivalent (mg QAE) per gram dry weight of plant material. The experiment was done in triplicates.
DPPH radical scavenging activity
Measurement of radical scavenging activity of C. forskohlii in response to MeJA treatment was carried out in different tissues (leaves, stem and roots) according to the method described by Blois (1958). Ascorbic acid was used as positive control and per cent inhibition was determined according to the following equation:
where AS is the absorption of the solution when the sample extract was added at a particular concentration and ADPPH is the absorbance of the DPPH solution. Three experimental replicates were taken for the assay. IC50 values were calculated as the concentration of extracts causing 50% inhibition of DPPH radical, a lower IC50 value corresponded to a higher antioxidant activity of sample.
Results and discussion
Cloning of CfCHS and sequence analysis
Degenerate primers were used to obtain a core fragment of 569 bp. Using the sequence information of core amplicon, 5 ′ and 3 ′ RACE primers were designed. RACE-PCR was carried out to obtain the 5 ′ and 3 ′ ends of the cDNA, giving an amplicon size of 842 and 362 bp, respectively (figure 2). The full-length clone of 1598 bp contained an ORF of 1176 bp, starting with an ATG start codon at position 245 and ending with a TGA stop codon at position 1420. The clone was sequenced and designated as CfCHS (NCBI GenBank accession no. KF643243). CDS primers yielded an amplicon of about 1500 bp from the genomic DNA template, suggesting the presence of intron(s) in CfCHS gene (figure 3). Further, an amplification of full-length CDS was attempted from cDNAs of different tissues. In conventional end-point PCR, bands were detected only in young leaf and root tissues and they were of the same size, suggesting absence of alternative splicing (figure 4). Moreover, in general, CHS genes from different plants are known to contain only a single intron flanked by two exons (Van den Hof et al. 2008). Theoretical translation of CfCHS nucleotide sequence encoded a protein of 391 amino acids having a molecular weight 42.75 kDa and pI 6.57 (figure 5). The 5 ′ and 3 ′ untranslated regions (UTR) were 244 and 178-bp long, respectively. Closest homologues of CfCHS protein sequence were identified using BlastP (NCBI) and multiple sequence alignment was carried out (figure 6). CfCHS sequence shared 92.6% identity and 94.9% similarity with the CHS from A. rugosa (figure 6b). A phylogenetic tree was constructed using CHS protein sequences from different organisms, and as expected, CfCHS fell in the clade of dicotyledonous plants showing close phylogenetic similarity with CHS from S. scutellarioides, belonging to the same family (figure 7).
Multiple sequence alignment of CfCHS sequence and its homologues in other plant species. (a) Multiple sequence alignment of CfCHS protein sequence with the homologous proteins from other species A. rugosa (GenBank acc. no. AFL72079.1), S. scutellarioides (GenBank acc. no. ABP57071.1), P. frutescens (GenBank acc. no. BAA19548.1) and M. orontium (GenBank acc. no. CAJ44127.1). (b) Represents percentage similarity and identity among the related CHS proteins of different plant species calculated using MatGAT tool.
Expression analysis
To understand the spatial pattern of distribution, expression profiling of CfCHS was carried out in flower, young leaf, mature leaf, stem, root and root tip using qRT-PCR. Young leaf showed highest expression of CfCHS, followed by flower, while root and root tip showed negligible expression (figure 8a). The data is consistent with the previous studies, where highest expression is observed in aerial parts of the plant (Fritze et al. 1991; Thain et al. 2002). CHS plays an important role in flower pigmentation (Napoli et al. 1990; Vander Krol et al. 1990). Maximum accumulation of CHS was demonstrated in the corolla and young leaves of snapdragon, where pigmentation is intense (Fritze et al. 1991).
Expression profile of CfCHS. Expression profile of CfCHS (a) in different tissues and (b) under the effect of elicitors. Methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA) and 2,4-diphenylphenoxyacetic acid (2,4-D) at 4 h after treatment. Actin was used as housekeeping control. Expression of CfCHS in stem was used as baseline for calculating fold change in (a). Expression of CfCHS in 0.1% ethanol-treated (control) plants was used as baseline for calculating fold change in (b). Three replicates were used for analysis. *P <0.05 and **P <0.01.
Treatments with chemical elicitors are known to mimic the effects of environmental stresses (Tuteja and Sopory 2008). The changes in expression pattern of CfCHS on treatment with various phytohormones/elicitors like MeJA, SA, ABA and 2,4-D were also determined. CfCHS expression was strongly induced in plants treated with MeJA (figure 8b), which mimics herbivore attack or mechanical wounding (Creelman and Mullet 1997; Kessler and Baldwin 2002). MeJA can diffuse to distal parts of plant (Karban et al. 2000; Ruiz-Medrano et al. 2001), thereby mediating systemic signalling. Wounding exposes the plant to pathogenic bacteria and fungi. Flavonoids are reported to have antimicrobial properties (Cushnie and Lamb 2005; Orhan et al. 2010). Thus, the enhanced expression of CfCHS involved in flavonoid biosynthesis in response to MeJA would probably result in accumulation of flavonoids that may protect the plant from pathogenic microbes. It has been reported that MeJA promoted silymarin production and enhanced CHS activity in cell cultures of Silybum marianum (L.) Gaertn. (Asteraceae) (Sánchez-Sampedro et al. 2005). MeJA also induced CHS expression in Petunia corollas (Tamari et al. 1995). Abscisic acid is another important phytohormone that serves myriad functions ranging from developmental to adaptive stress responses (Cutler et al. 2010). ABA promoted synthesis of flavonoids and CHS expression in Ginkgo biloba (L.) (Ginkgoaceae) (Yan 2002; Shuiyuan et al. 2004; Li et al. 2014). In our studies, we found that ABA treatment marginally induced expression of CfCHS at the concentration of ABA and time-point we tested (figure 8b). 2,4-D is a synthetic auxin herbicide which suppresses anthocyanin (class of flavonoids) synthesis (Takeda et al. 1993; Ban et al. 2003). However, it has also been reported that anthocyanin synthesis once induced by light could not be suppressed by further addition of 2,4-D (Takeda 1990). In our results also, we observed that 2,4-D had a negligible effect on expression of CfCHS (figure 8b). However, CfCHS appeared to be downregulated in response to SA treatment (figure 8b). Our results are in agreement with the previous studies performed in constitutive salicylic acid producing transgenic Nicotiana tabacum cv. Samsun NN (Solanaceae) plants, where CHS was suppressed, relative to the wild-type plants (Nugroho et al. 2002).
Total flavonoid content
Flavonoids significantly contribute to the antioxidant property of the plants (Luo et al. 2002). Total flavonoid content in leaves and roots was found to be significantly increased in response to MeJA treatment, whereas there was negligible change in stem (table 2). Significant increase in CfCHS gene expression as well as the total flavonoid content in leaves of C. forskohlii in response to MeJA treatment suggests that CfCHS could be involved in biosynthesis of flavonoids. MeJA has been earlier reported to enhance flavonoid content and antioxidant activity of Rubus fruticosus L. (Wang et al. 2007).
DPPH radical scavenging activity
DPPH method is a simple, rapid, sensitive and reproducible assay used for measuring the antioxidant activity of plant extracts. In the DPPH assay, an antioxidant scavenges the free radicals and is used to measure the capacity of extracts to scavenge the stable radical DPPH formed in solution by donation of hydrogen atom or an electron (Mishra et al. 2012). On MeJA treatment, antioxidant activity in extract prepared from leaves and root tissues was increased, whereas there was no significant change in antioxidant activity of stem extracts. Among the three types of tissues tested, root extract showed the highest antioxidant activity. The IC50 values for methanolic extracts of leaves, stem and roots of control and MeJA-treated plants are given in table 2. The antioxidant activity of all the extracts was lower than that of ascorbic acid (6.04 ± 0.32), which was used as positive control.
Posttranscriptional regulation of CfCHS
CHS catalyzes the first committed step of flavonoid biosynthesis, which is an important regulatory branch point, channelling flux of phenylpropanoid pathway towards biosynthesis of flavonoids. Several species of Coleus including C. forskohlii are known to produce rosmarinic acid. Rosmarinic acid also utilizes p-coumaroyl-CoA as a precursor. Downregulating CfCHS could help in diverting the metabolic flux towards synthesis of alternate compounds of commercial value such as rosmarinic acid or other phenylpropene phytochemicals. For instance, downregulating the strawberry CHS resulted in enhanced production of phenylpropene aroma compounds like eugenol (Hoffmann et al. 2011). As a result, we carried out an in silico search to find the miRNAs that may target CfCHS, thereby downregulating its expression.
MiRNAs are small noncoding RNAs that regulate the target mRNA levels, by cleavage of target or by suppressing its translation. They are known to play fundamental roles in various biological processes, including development and physiology (Bartel 2004; Sunkar et al. 2007). Our in silico search for mRNAs that may regulate CfCHS expression, identified four miRNAs targeting CfCHS, whose target sequence is conserved in at least three other homologues of CHS from four different plant species (S. scutellarioides, A. rugosa, P. frutescens and M. orontium) that were tested. MiR34, miR166 and miR395 were predicted to regulate CfCHS RNA levels through cleavage, while miR477 may act by translational repression as well as cleavage of target transcripts (figure 9). Earlier study in Phelipanche ramosa (L). Pomel (Orobanchaceae) reported that miR166 was downregulated in response to viroid infection (Ivanova et al. 2014). Flavonoids are already known to be involved in defense responses against pathogen infection (Jasinski et al. 2009; Päsold et al. 2010) and in a study, enhanced accumulation of CHS transcripts was reported in Norway spruce after pathogen infection (Nagy et al. 2004). Expression of miR166, miR395 and miR477 was undetectable in all treatments, whereas miR34 was downregulated and CfCHS was upregulated in MeJA-treated plants (figure 10), thus exhibiting an inverse correlation suggesting that miR34 is a potential regulator of CfCHS and flavonoid biosynthesis.
Conclusion
Full-length clone of CHS gene was obtained from cDNA and genomic DNA of C. forskohlii. CfCHS showed dominant expression in young leaves and flowers. CfCHS was found to be significantly induced by MeJA with concomitant increase in total flavonoid content and antioxidant activity. Our results also indicate that miR34 is a potential posttranscriptional regulator of CfCHS expression and flavonoid biosynthesis.
References
Alasbahi R. H. and Melzig M. F. 2010 Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology—Part 1. Planta Med. 76, 753–765.
Awasthi P., Mahajan V., Rather I. A., Gupta A. P., Rasool S., Bedi Y. S. et al. 2015 Plant omics: isolation, identification, and expression analysis of cytochrome P450 gene sequences from Coleus forskohlii. OMICS 19, 782–792.
Ban T., Ishimaru M., Kobayashi S., Shiozaki S., Goto-Yamamoto N. and Horiuchi S. 2003 Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in “Kyoho” grape berries. J. Hortic. Sci. Biotechnol. 78, 586–589.
Bartel D. P. 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297.
Blois M. S. 1958 Antioxidant determinations by the use of a stable free radical. Nature 181, 1199–1200.
Campanella J. J., Bitincka L. and Smalley J. 2003 MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4, 29.
Cazarolli L. H., Leila Z., Elga H. A., Bonorino Figueiredo M. S. R., Poliane F., Rosangela G. D. et al. 2008 Flavonoids: prospective drug candidates. Mini. Rev. Med. Chem. 8, 1429–1440.
Creelman R. A. and Mullet J. E. 1997 Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 355–381.
Cushnie T. P. T. and Lamb A. J. 2005 Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26, 343–356.
Cutler S. R., Rodriguez P. L., Finkelstein R. R. and Abrams S. R. 2010 Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679.
Dai X. and Zhao P. X. 2011 psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39, 155–159.
Dao T. T., Linthorst H. J. and Verpoorte R. 2011 Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 10, 397–412.
Fini A., Brunetti C., Di Ferdinando M., Francesco F. and Massimiliano T. 2011 Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 6, 709–711.
Fritze K., Staiger D., Czaja I., Walden R., Schell J. and Wing D. 1991 Developmental and UV light regulation of the snapdragon chalcone synthase promoter. Plant Cell 3, 893–905.
Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D. and Bairoch A. 2003 ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788.
Goodwin P. H., Hsiang T. and Erickson L. 2000 A comparison of stilbene and chalcone synthases including a new stilbene synthase gene from Vitis riparia cv. Gloire de Montpellier. Plant Sci. 151, 1–8.
Hoffmann T., Kurtzer R., Kilian S., Kiessling P., Fridman E., Pichersky E. et al. 2011 Metabolic engineering in strawberry fruit uncovers a dormant biosynthetic pathway. Metab. Eng. 13, 527–531.
Ivanova D., Milev I., Vachev T., Vesselin B., Yahubyan G., Minkov G. et al. 2014 Small RNA analysis of potato spindle tuber viroid infected Phelipanche ramosa. Plant Physiol. Biochem. 74, 276–282.
Jasinski M., Piotr K., Rodziewicz P., Marek F. and Maciej S. 2009 Changes in the profile of flavonoid accumulation in Medicago truncatula leaves during infection with fungal pathogen Phoma medicaginis. Plant Physiol. Biochem. 47, 847–853.
Jez J. M., Ferrer J. L., Bowman M. E., Austin M. B., Schröder J., Dixon R. A. et al. 2001 Structure and mechanism of chalcone synthase-like polyketide synthases. J. Ind. Microbiol. Biotechnol. 27, 393–398.
Jiang C., Schommer C. K., Kim S. Y. and Suh D. Y. 2006 Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67, 2531–2540.
Karban R., Baldwin I. T., Baxter K. J., Laue G. and Felton G. W. 2000 Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125, 66–71.
Kaur T., Hussain K., Koul S., Vishwakarma R. and Vyas D. 2013 Evaluation of nutritional and antioxidant status of Lepidium latifolium Linn.: a novel phytofood from Ladakh. PLoS One 8, e69112.
Kavitha C., Rajamani K. and Vadivel E. 2010 Coleus forskohlii: a comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plant Res. 4, 278–285.
Kessler A. and Baldwin I. T. 2002 Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 53, 299–328.
Li L. L., Cheng H., Yuan H. H., Xu F., Cheng S. Y. and Cao F. L. 2014 Functional characterization of the Ginkgo biloba chalcone synthase gene promoter in transgenic tobacco. Genet. Mol. Res. 13, 3446–3460.
Lillo C., Unni S. L. and Ruoff P. 2008 Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 31, 587–601.
Livak K. J. and Schmittgen T. D. 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408.
Luo X. D., Margaret J. B. and Kennelly E. J. 2002 Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple). J. Agric. Food Chem. 50, 1379–1382.
Martens S. and Mithöfer A. 2005 Flavones and flavone synthases. Phytochemistry 66, 2399–2407.
Mishra K., Ojha H. and Chaudhury N. B. 2012 Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem. 130, 1036–1043.
Moustafa E. 1967 Purification and properties of chalcone–flavanone isomerase from soya bean seed. Phytochemistry 6, 625–632.
Nagy N. E., Fossdal C. G., Krokene P., Krekling T., Lönneborg A. and Solheim H. 2004 Induced responses to pathogen infection in Norway spruce phloem: Changes in polyphenolic parenchyma cells, chalcone synthase transcript levels and peroxidase activity. Tree Physiol. 24, 505–515.
Napoli C., Lemieux C. and Jorgensen R. 1990 Introduction of a chimeric chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2, 279–289.
Nugroho L. H., Verberne M. C. and Verpoorte R. 2002 Activities of enzymes involved in the phenylpropanoid pathway in constitutively salicylic acid-producing tobacco plants. Plant Physiol. Biochem. 40, 755–760.
Orhan D. D., Ozçelik B., Ozgen S. and Ergun F. 2010 Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 165, 496–504.
Päsold S., Siegel I., Seidel C. and Müller J. L. 2010 Flavonoid accumulation in Arabidopsis thaliana root galls caused by the obligate biotrophic pathogen Plasmodiophora brassicae. Mol. Plant Pathol. 11, 545–562.
Paul M., Radha A. and Kumar D. S. 2013 On the high value medicinal plant, Coleus forskohlii Briq. Hygeia 5, 69–78.
Rather I. A., Awasthi P., Mahajan V., Bedi Y. S., Vishwakarma R. A. and Gandhi S. G. 2015 Molecular cloning and functional characterization of an antifungal PR-5 protein from Ocimum basilicum. Gene 558, 143–151.
Reddy A. R., Scheffler B., Madhuri G., Srivastava M. N., Kumar A., Sathyanarayanan P. V. et al. 1996 Chalcone synthase in rice (Oryza sativa L.): detection of the CHS protein in seedlings and molecular mapping of the chs locus. Plant Mol. Biol. 32, 735–743.
Richard S., Lapointe G., Rutledge R. G. and Séguin A. 2000 Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol. 41, 982–987.
Ruiz-Medrano R., Xoconostle-Cázares B. and Lucas W. J. 2001 The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 4, 202–209.
Saitou N. and Nei M. 1987 The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sánchez-Sampedro M. A., Fernández-Tárrago J. and Corchete P. 2005 Yeast extract and methyl jasmonate-induced silymarin production in cell cultures ofSilybum marianum (L.) Gaertn . J. Biotechnol. 119, 60–69.
Schenk P. M., Kazan K., Wilson I., Anderson J. P., Richmond T., Somerville S. C. et al. 2000 Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 97, 11655–11660.
Schröder J. 1997 A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 2, 373–378.
Shuiyuan C., Yan W., Junkai L., Yongjun F. and Guicai Z. 2004 Study on the relationship between the endogenous hormones and flavonoids in Ginkgo biloba leaf. Sci. Silvae Sin. 40, 45–49.
Sunkar R., Chinnusamy V., Zhu J. and Zhu J. K. 2007 Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant. Sci. 12, 301–309.
Swarup V., Ghosh J., Ghosh S., Saxena A. and Basu A. 2007 Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 51, 3367–3370.
Takeda J., Abe S., Hirose Y. and Ozeki Y. 1993 Effect of light and 2,4-dichlorophenoxyacetic acid on the level of mRNAs for phenylalanine ammonia-lyase and chalcone synthase in carrot cells cultured in suspension. Physiol. Plant. 89, 4–10.
Takeda J. 1990 Light-induced synthesis of anthocyanin in carrot cells in suspension: II. Effects of light and 2,4-D on induction and reduction of enzyme activities related to anthocyanin synthesis. J. Exp. Bot. 41, 749–755.
Tamari G., Borochov A., Atzorn R. and Weiss D. 1995 Methyl jasmonate induces pigmentation and flavonoid gene expression in Petunia corollas: a possible role in wound response. Physiol. Plant. 94, 45–50.
Tamura K., Nei M. and Kumar S. 2004 Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 101, 11030–11035.
Tamura K., Peterson D., Peterson N., Stecher G., Nei M. and Kumar S. 2011 MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739.
Taylor L. P. and Grotewold E. 2005 Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8, 317–323.
Thain S. C., Murtas G., Lynn J. R., McGrath R. B. and Millar A. J. 2002 The circadian clock that controls gene expression in Arabidopsis is tissue specific. Plant Physiol. 130, 102–110.
Tuteja N. and Sopory S. K. 2008 Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 3, 525–536.
Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., Remm M. et al. 2012 Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115.
Van den Hof K., Van den Berg R. G. and Gravendeel B. 2008 Chalcone synthase gene lineage diversification confirms allopolyploid evolutionary relationships of European rostrate violets. Mol. Biol. Evol. 25, 2099–2108.
Vander Krol A. R., Mur L. A., de Lange P., Mol J. N. and Stuitje A. R. 1990 Inhibition of flower pigmentation by antisense CHS genes: promoter and minimal sequence requirements for the antisense effect. Plant. Mol. Biol. 14, 457–466.
Wang M. -X., Li W. -L., Zhang Z., Wei J. H., Yang Y., Xu Y. H. et al. 2013 Cloning and bioinformatics analysis of chalcone synthase (AsCHS1) gene in Aquilaria sinensis. Zhongguo Zhong Yao Za Zhi 38, 149–153.
Wang S., Bowman L. and Ding M. 2007 Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus Sp.) and promotes antiproliferation of human cancer cells. Food Chem. 107, 1261–1269.
Winkel-Shirley B. 2002 Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 5, 218–223.
Yamazaki Y., Suh D. -Y., Sitthithaworn W., Ishiguro K., Kobayashi Y., Shibuya M. et al. 2001 Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta 214, 75–84.
Yan W. 2002 Studies on the effects of regulating measures on the flavonoids contents in Ginkgo biloba leaves. Hubei Agric. Sci. 5, 103–105.
Acknowledgements
PA and VM are supported by CSIR-Senior Research Fellowship. SGG acknowledges the financial support for this work from CSIR 12th FYP project ‘PMSI’ (BSC0117) of Council of Scientific and Industrial Research (CSIR) and Fast-Track ‘OYS’ project of SERB (DST, New Delhi).
Author information
Authors and Affiliations
Corresponding author
Additional information
Awasthi P., Mahajan V., Jamwal V. L., Kapoor N., Rasool S., Bedi Y. S. and Gandhi S. G. 2016 Cloning and expression analysis of chalcone synthase gene from Coleus forskohlii. J. Genet. 95, xx–xx
Praveen Awasthi and Vidushi Mahajan contributed equally to this work. PA did gene expression profiling using real time PCR, quantification of flavonoid content and measurement of antioxidant activity. VM carried out cloning work and wrote the manuscript. VLJ and NK did the analysis of gene structure and splice variants. YSB and SR provided critical inputs for the study as well as during preparation of manuscript. SGG designed the study, analysed the results and edited the manuscript and figures.
Rights and permissions
About this article
Cite this article
AWASTHI, P., MAHAJAN, V., JAMWAL, V.L. et al. Cloning and expression analysis of chalcone synthase gene from Coleus forskohlii . J Genet 95, 647–657 (2016). https://doi.org/10.1007/s12041-016-0680-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0680-8