Abstract
Moderately preserved shallow-marine extinct, fossil benthic community has been recovered from a sub-surface Late Palaeocene limestone cave section near Lumshnong in the Jaintia Hills, Meghalaya, NE India. The present contribution focuses on the ecostratigraphic implications of the carbonate microbiofacies based on the evaluated facies gradients. Precise field assessments and microscopic observations led to the identification of three microbiofacies: benthic foraminiferal–algal grainstone, coralline algal framestone and oolitic grainstone–packstone. The microbiofacies distinguished in the study suggest a general shallowing-upward trend from an inner shelf setting to a lagoonal–shoal environment depicting the distinct changes in the benthic community. Presence of coralline alga Distichoplax biserialis and benthic foraminifera Idalina sinjarica, Daviesina khatiyahi, Miscellanea primitiva, Rotalia trochidiformis and Vania anatolica assign the studied carbonates to Early Thanetian (SBZ 3) corresponding to the lower part of the Lakadong Limestone. In this study, ecostratigraphy has facilitated the classification of a single carbonate section corresponding to a solitary shallow benthic zone into multiple microbiofacies attributed to variable environmental depositional conditions. This clearly demonstrates its potential in improving the applicability of biostratigraphy worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Palaeogene marine successions outcropping in Meghalaya, NE India are characterized by multiple carbonate units ascribed to the Sylhet Limestone Group (Jauhri and Agarwal 2001; Matsumaru and Sarma 2010; Sarkar 2015a, b). This region was palaeogeographically located in the eastern part of the relic East Tethys (or Neo-Tethys; Stampfli 2000; Boudagher-Fadel et al. 2015). The Palaeocene–Eocene carbonate facies pertaining to the Meghalaya outcrops are dominated by calcareous algae and benthic foraminifera examined by virtue of several biostratigraphic and palaeoecological analyses (Jauhri 1994, 1997, 1998; Jauhri and Agarwal 2001; Jauhri et al. 2006; Tewari et al. 2010; Sarkar 2015a, b, 2016, 2017a, b, 2018, 2019; Özcan et al. 2018).
Ecostratigraphy (ecosystem stratigraphy) is a very important but lesser investigated sub-discipline of stratigraphy worldwide in comparison to lithostratigraphy, chronostratigraphy and biostratigraphy. The ultimate objective of any branch of stratigraphy is time-correlation of rocks. Ecostratigraphy does not involve any new principles, but attempts to collect data pertinent to taxonomy, biogeography, ecology and evolution of fossils from any bed/lithounit and combine them with inputs from other disciplines like sedimentology. This aims to reconstruct a palaeoecosystem with the prospects of a better eco-environmental correlation of the rocks (Waterhouse 1976; Hoffman 1981; Boucot 1982; Cooper 1999). It is actually ‘the natural development of biostratigraphy’, to provide palaeoecological impetus to the temporal subdivisions of the fossil record aimed at analyzing geobiological evolution (Martinsson 1973; Boucot 1982; Sokolov 1988; Olóriz et al. 2008; Nikitenko et al. 2013; Xue et al. 2018). Understanding the ecostratigraphic trends put forward critical information regarding fluctuations in the biotic composition, and thereby the overall ecology of a particular area or region which is significant in deciphering the factors governing the palaeoenvironmental dynamics and basin evolution (Sokolov 1988; Olóriz et al. 1995, 2008).
Biostratigraphy is well recognized and widely applied in case of fossil studies, mainly demarcating assemblage and acme zones with the objective of time-correlations. However, the biozones (or biofacies) as determined from biostratigraphy cannot always present comprehensive counterparts to the larger-scale lithofacies. A number of correlative fossil communities are often included in a single biozone although each individual community could be treated as a separate zone (Waterhouse 1976). Each fossil community has different limiting factors involved in its development, like water-depth, nutrient regime or substrate morphology. Each phylum or taxon has its own independent geological record and do not necessarily correspond to each other in a common assemblage or biozone. A sequence of strata may be characterized by several flora and fauna of different classes, orders and families, representing remnants of numerous fossil communities. These communities have obviously been subject to various biostratinomic and taphonomic influences that are difficult to estimate on several occasions. This brings possible discrepancy when separate fossil communities are categorized into one biozone only because they share a sufficient number of species to compose a particular assemblage zone or some representative index species are present to designate an entire range zone. Abrupt changes in the palaeontological datasets can be due to human errors as well as naturally imperfect sedimentary records.
However, it should be the aim and objective of every palaeontologist to present time-correlation data with the maximum possible accuracy. Well-defined biozones should definitely include a minimum number of fossil communities, to put forward an adequate representation of biota pertaining to different sub-environments. Substandard sampling and study of limited outcrops or restricted communities only represents indiscrete biomes or floral/faunal provinces, finally leading to an incomplete biozone. This deprives us of a balanced time-correlation and therefore, biostratigraphy must be studied in tandem with ecological data (ecostratigraphy) to fill these gaps in our understanding of the fossil record.
Despite a rich lineage of carbonate systems dating Precambrian to Recent, to date there has been no emphasis on ecostratigraphic evaluation of carbonate systems from India. The present contribution constitutes the first ecostratigraphic analysis of calcareous algal–benthic foraminiferal assemblages from the Late Palaeocene carbonates of the Lakadong Limestone, focussing on the changes in microbiofacies types. This study aims to relate the switching of carbonate microbiofacies types to reconstruct the development of the local study area during the Late Palaeocene. To support the ecostratigraphic interpretations, general biostratigraphic and taphonomic perspectives of the recorded assemblages were also evaluated in brief.
2 Geological setting
The state Meghalaya, situated in northeast India is known for numerous pre-historic caves spanning across the Jaintia, Khasi and Garo Hills. Most of these caves are pristine with respect to negligible micropalaeontological studies carried out to date and constitute an exceptional archaeological heritage. Abundant Palaeogene carbonate successions spread over Meghalaya imply for several unaccounted Palaeocene–Eocene limestone cave sections belonging to the Sylhet Limestone Group that have significant potential to contribute to the regional biostratigraphic data and palaeoenvironmental reconstructions.
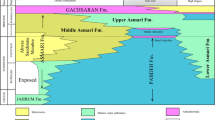
A very widespread and extensive phase of marine transgression regulated the deposition of the Sylhet Limestone Group during the Early Palaeogene (Jauhri and Agarwal 2001). However, intermittent periods of increased clastic supply and shallowing in the Early Eocene interrupted the process of carbonate deposition (Jauhri and Agarwal 2001), resulting in the occurrence of several sandstone and carbonate–siliciclastic mixed beds within the Sylhet Limestone Group all across Meghalaya. The Sylhet Limestone Group includes three carbonate units; Lakadong Limestone, Umlatdoh Limestone and Prang Formation in ascending chronological order intercalated with the Lakadong Sandstone and Narpuh Sandstone units (figure 1). The Sylhet Limestone Group is underlain by the Therria Sandstone and overlain by the Kopili Formation represented by shale/sandstone alternations (Jauhri and Agarwal 2001; Jauhri et al. 2006; Sarma et al. 2014). Nagappa (1959) presented a subdivision of the Sylhet Limestone Group, based on the study of randomly oriented thin-sections emphasizing on the analysis of the larger benthic foraminifera (LBF) for biostratigraphy. He correlated the limestone units of the Sylhet Limestone Group with the Ranikot, Laki and Khirthar (=Kirthar) stages of Pakistan. Several Tethyan LBF (Hottinger 1960; Hottinger and Drobne 1988; Racey 1995; Matsumaru 1996) have been reported from the Sylhet Limestone Group that has resulted in precise correlations with the European stages (Jauhri 1997, 1998; Jauhri and Agarwal 2001).

(modified after Jauhri and Agarwal 2001).
Stratigraphic column highlighting the Sylhet Limestone Group
3 Materials and methods
The present study concentrates in a cave section (92°22.6′E; 25°10.8′N) in the vicinity of Chiehruphi village in Khliehrat block, on the National Highway 44 (NH-44; Jowai–Badarpur road transect) in the Jaintia Hills, Meghalaya (figure 2). This belongs to the Synrang–Pamiang cave system and situated north of the road transect from Chiehruphi village to Musianglamare on the NH-44. Owing to constant water seepage in the cave rocks, the limestones of the studied section suffered from fair degree of leaching. The fossiliferous limestone samples were collected from a measured section pertaining to an outcrop close to the main entrance of the cave. Due to objection from the local village inhabitants, any sampling from interior portions of the cave was avoided. 28 samples were collected (figure 3) and 62 petrographic thin-sections (~3.0–4.0 × 2.0–2.5 cm) were prepared for the evaluation of the carbonate textures and the corresponding microbiofacies components. Two thin-sections were prepared from each sample but six samples characterized by robust algal framestones were used for preparing extra thin-sections to further examine the frame-building corallines. Relative abundance of the skeletal components corresponding to the carbonate microbiofacies was studied in the thin-sections by image analysis and measuring the proportional area occupied by each taxon relative to the total biotic population (Perrin et al. 1995). Carbonate textures have been assigned on the basis of classification schemes proposed by Dunham (1962) and Embry and Klovan (1971). Growth-form terminology for the coralline algae is based on the work of Woelkerling et al. (1993). Taphonomic aspects in the current study have been evaluated following Nebelsick and Bassi (2000) to understand the roles of abrasion (transport and/or sediment agitation), fragmentation (production of maerls or unattached corallines usually formed from original rhodolith branches), disarticulation (phenomenon associated with geniculate corallines pertinent to the post-death decomposition of decalcified genicula) and bioerosion (signatures of bioeroders like gastropods brought about by surface grazing and endophytic bioerosion boring) in the depositional environment. Examination of the thin-sections was carried out under Olympus BX 50 plane light microscope and photography of the microbiofacies types/components was done with Olympus PM-20 Exposure Control Unit.

(modified from Dutta and Jain 1980).
Maps of (a) India (in the inset) with part of Meghalaya highlighted to show the location of the study area (not to scale); (b) Geological map of the study area in the Jaintia Hills
4 Results
Based on the field and microscopic observations, three major microbiofacies are recognized in the examined limestone profile: benthic foraminiferal–algal grainstone, coralline algal framestone and oolitic grainstone–packstone. Photomicrographs showing various microbiofacies and important biotic components are displayed in figures 4 and 5. Each microbiofacies is described on the basis of types, abundance and frequency of the biotic components and texture of the rocks. Thereafter, the related sedimentary environment is interpreted to designate the ecostratigraphic characteristics. Major alterations in the microbiofacies types and principal biogenic components within the studied profile enable precise ecostratigraphy. In addition to these major facies types, rare occurrences of mud-supported wackestones and mudstones are also recorded in the study material.
Photomicrographs displaying the major microbiofacies types and important biotic components. (a) Foraminiferal–algal grainstone with Rotalia trochidiformis and multiple filaments of Distichoplax biserialis. Sample no. CH 1. (b) Idalina sinjarica, other benthic foraminifera, geniculate and non-geniculate algal fragments with debris material. Sample no. CH 4. (c) Larger nummulitid with abundant algae, foraminifera and unidentifiable microfossils in grainstone microfacies. Sample no. CH 7. (d) Vania anatolica with coralline algae fragments in grainstone matrix. Sample no. CH 1. (e–f) Oolitic grainstone–packstone with varieties of recrystalized coated grains and algal protuberances in grainstone matrix. Sample no. CH 23. Scale bars: (a–b) 300 μm, (c) 2 mm, (d–f) 500 μm.
Photomicrographs displaying the major microbiofacies types and important biotic components. (a–c) Coralline algae with arborescent habit (Marinella lugeoni?) forming algal framestones. Sample nos. (a) CH 9, (b) CH 14, (c) CH 15. (d) Grainstone showing coralline algae with gastropod and bivalve shells. Sample CH 6. (e) Daviesina khatiyahi, Corallina sp., Jania sp. and other unidentifiable algae/foraminifera in grainstone matrix. Sample CH 5. (f) Distichoplax biserialis, various foraminifera and echinoderm remains in grainstone matrix. Sample no. CH 3. Scale bars: (a) 1 mm, (b–f) 500 μm.
4.1 Microbiofacies 1: Benthic foraminiferal–algal grainstone (figures 4a–d; 5d–f)
This microbiofacies is recorded from the lowermost part of the studied profile (figure 3), measuring ~1.15 m in thickness. Benthic foraminifera < 2 mm (22%; Miscellanea primitiva, Rotalia trochidiformis, Daviesina khatiyahi, Vania anatolica, Idalina sinjarica, Quinqueloculina sp., Triloculina sp., Biloculina sp., Periloculina sp., textulariids and other unidentifiable agglutinated and rotaliid forms) and coralline red algae (18%; lithophylloid Distichoplax biserialis, geniculate Jania sp. and Corallina (?), several unidentifiable geniculate and non-geniculate thalli in form of debris and small branches) represent the main biotic components of this poorly sorted microbiofacies in association with rare bivalve, gastropod, molluscan and bryozoan fragments, larger benthic foraminifera (>2 mm), small echinoderm plates and spines, micritized intraclasts, ooids and some non-recognizable coated grains. The components of the algal debris and the small coralline branches are hard to distinguish even up to the subfamily or generic level. Majority of the corallines (65%) and benthic foraminifera (73%) are influenced by abrasion, fragmentation and disarticulation (applicable only to geniculate coralline algae).
Interpretation:
The biogenic assemblage suggests deposition in a shallow, proximal inner-shelf warm marine environment characterized by moderate- to high-energy conditions. Strong hydrodynamic energy is reflected primarily by the significant quantities of abraded and fragmented coralline algae and benthic foraminifera. Common incidence of fragmentation, abrasion and disarticulation in combination with complete absence of biologically-induced taphonomic processes like bioerosion and encrustation indicate rapid burial of the skeletal grains and also possible allochthonous nature of the deposits (Chelaru et al. 2019). Rare occurrence of larger benthic foraminifera indicates unfavourable physical conditions like depth, wave energy etc. and/or insufficient quantity of available light for the symbiotic association between larger benthic foraminifera and algal endosymbionts (e.g., diatoms). Since there are several records backed up by numerous evidences in support of shallow-marine inner shelf systems characterized by simultaneous occurrence of coralline red algae and larger benthic foraminifera (Zamagni et al. 2008; Sarkar 2015a, 2018), the rare record of LBF in this shallow environment can be due to higher nutrient regime (eutrophication) that possibly did not allow sufficient light to penetrate the water column. Biostratigraphic assessment of the assemblage based on coralline Distichoplax biserialis (Sarkar 2018) and the identified benthic foraminifers reveal that these organisms thrived in this depositional environment during the Early Thanetian (Shallow Benthic Zone 3; Serra-Kiel et al. 1998) representing the lower part of the Lakadong Limestone. Vania anatolica, an agglutinated foraminifer identified by Sirel and Gündüz (1985) from the Thanetian of East Turkey is reported for the first time from the Indian subcontinent and presents an important contribution for the assignment of this microbiofacies to SBZ 3.
4.2 Microbiofacies 2: Coralline algal framestone (figure 5a–c)
This microbiofacies is distributed in the middle part of the studied profile, measuring ~2.45 m in thickness (figure 3). The major biogenic component of this microbiofacies is represented by robust colonies of coralline red algae (~70%) with arborescent and lumpy growth-forms. The commonly applied diagnostic criteria imperative to the identification of fossil coralline algae like type of conceptacles or epithallial cells (Rasser and Piller 1999; Maneveldt et al. 2008, 2016; Kundal 2010, 2011) are not discernible in the algal thalli. However, the growth-forms and the morphological characteristics of the frame-building algae show close affinity to Marinella lugeoni Pfender 1939 (Family Elianellaceae). The in-situ coralline algae constitute the supporting framework of the rock with micritic matrix occurring in the interstices between the fossils. Cavities are mostly absent or very small, giving the microbiofacies a relatively compact structure in comparison to the other two major microbiofacies. Molluscan shells, bryozoan fragments, algal protuberances (warty, lumpy growth-forms), smaller miliolids (Quinqueloculina, Periloculina), rotaliids (Miscellanea primitiva, Rotalia trochidiformis) and coated grains are the secondary constituents of this microbiofacies. Several specimens in this microbiofacies are strongly recrystallized. Numerous wackestone pockets are recorded in this microbiofacies featuring rock debris and multiple bioclasts. Several bioclasts have their interior filled with lime mud.
Interpretation:
The large coralline algal branches and lumps showing close affinity to Marinella lugeoni can be categorized as primary frame-builders based on their high skeletal volume and rigidity of the build-up composing this facies type (Rasmussen and Brett 1985; Geronimo et al. 2002). Dense concentrations of these coralline algae indicate that they thrived in a moderate-energy depositional environment corresponding to proximal inner shelf to an open lagoon (Bosence 1983; Chelaru et al. 2019). This carbonate microbiofacies is autochthonous and supported by a rigid organic framework at the time of deposition that also gives the impression of pseudo-boundstones in the macroscopic carbonate fabric. Recrystallization in several specimens is due to the annihilation of the original microstructure that got dissolved and later on replaced by equivalent calcite cement or were neomorphically transformed to the more stable low-Mg calcite (from original aragonite or high-Mg calcite) without preservation of the original microstructure during the exposure period. Based on the carbonate fabric and biogenic content, a definite decrease in hydrodynamic energy in comparison to microbiofacies 1 can be interpreted. Rare occurrence of Miscellanea primitiva and Rotalia trochidiformis indicate depositional environment corresponding to the Early Thanetian (SBZ 3).
4.3 Microbiofacies 3: Oolitic grainstone–packstone (figure 4e–f)
This microbiofacies is recorded in the upper part of the profile, measuring ~1.65 m in thickness (figure 3). The moderate- to well-sorted grainstone–packstone facies is characterized by abundant proportions of single (35%) and compound radial-fibrous (28%) and tangential (16%) ooids with other coated grains and several peloids too. The ooid coatings are mostly formed around bioclasts (algae, benthic foraminifera, molluscs), other clasts (quartz grains) as well as unidentifiable material (abiogenic?) as the nuclei. Several aggregate grains are also observed in this microbiofacies. Some coralline algal protuberances, rare Distichoplax biserialis, unconsolidated fragments and unidentifiable larger benthic foraminifera (miliolids and rotaliids) are also recorded.
Interpretation:
The depositional environment interpreted for this microbiofacies is protected lagoonal–shoal environment indicating a shallowing-upward trend. Carbonate ooids commonly form in warm, shallow-marine environments featuring high hydrodynamic energy, where they are supplied with nuclei and supersaturated with calcium bicarbonate (Flügel 2004; Duguid et al. 2010; Liu and Zhang 2012). However, the co-occurrence of several aggregate grains in the microbiofacies indicate decreased regime of hydrodynamic energy by virtue of their deposition in a protected lagoon or sandy shoal environment. Several conceptualizations have been proposed regarding the exact process of ooid cortex precipitation and numerous studies provide evidences in favour of the theory of biological origin of ooids (Folk 1993; Folk and Lynch 2001). Role of microbes has also been analysed by several workers (Gerdes et al. 1994; Plee et al. 2008), but Duguid et al. (2010) have argued that the action of microbes like cyanobacteria is limited to altering the chemistry and texture of the ooids, rather than any direct primary role in their formation.
5 Discussion
According to several studies carried out till date, the highly diverse oligotrophic facies dominated by LBF in low-latitude site of Meghalaya, NE India are well comparable to other East Tethyan sites like the Indus Basin and Tibet (Scheibner and Speijer 2008; Afzal et al. 2011; Sarkar 2015a). However, the LBF-dominated facies account for poor records in the low-mid latitude carbonate environments (e.g., NW India, Oman, Libya, Egypt, Adriatic Platform) as summarized in some studies (Scheibner and Speijer 2008; Zamagni et al. 2009; Afzal et al. 2011). Taking into account the current study from Meghalaya, the cave succession under focus appears to be peculiar among the other Lakadong Limestone sections or their counterparts in Tibet or Indus Basin for its near absence of LBF assemblages and occurrence of well-developed algal framestones.
The three microbiofacies represent a pattern of decreasing water depth and hydrodynamic energy from the benthic foraminiferal–algal grainstone to the oolitic grainstone–packstone. From the ecostratigraphic assessment, a schematic palaeoenvironmental model is inferred for this depositional setting representing the stages of community succession in the study locality during the Early Thanetian (figure 6). A community dominated by benthic foraminifera and coralline algae (microbiofacies 1) first inhabited the area that was later colonized by robust, large-sized arborescent and lumpy corallines (microbiofacies 2). Substrate stability is an important parameter for the development of coralline red algae and is a function of multiple ecological factors like substrate composition and hydrodynamic energy. Particular preferences with respect to the substrate types and growth-forms can be associated with specific coralline genera (and also species but difficult to demarcate in fossil material) that constitute an integral part of their palaeoecological studies (Nebelsick and Bassi 2000; Checconi et al. 2007). On basis of dominating arborescent and lumpy corallines in microbiofacies 2, stable substrate with low to medium current activity and deposition above the fair-weather wave base is interpreted. In comparison to microbiofacies 2, the strongly abraded, fragmented and disarticulated corallines in microbiofacies 1 indicate stronger currents and/or wave agitation with a less stable substrate, also possibly signifying complete or partial allochthony. Later on with further shallowing and development of a protected lagoon or sandy shoal environment in the study locality, biogenic ooids and coated grains dominated the niche. Factors like time of exposure and sedimentation rate are expected to show ascending and descending trends respectively when testing the gradient from microbiofacies 1 to 3. In ecostratigraphic terminology, the evolution of microbiofacies 1 depositional environment represents an earlier phase of Early Thanetian and eventually evolves into a shallower environment pertinent to microbiofacies 3 showcasing a later phase of Early Thanetian.
The presence of Distichoplax biserialis, Miscellanea primitiva and Rotalia trochidiformis in both the first and last samples of the studied profile confirm a Early Thanetian age for the entire sequence. Additional records of Daviesina khatiyahi, Vania anatolica and Idalina sinjarica further reaffirm Late Thanetian age corresponding to the Shallow Benthic Zone 3 (SBZ 3). The microbiofacies recorded in the present study yield low diversity and quantities of calcareous algae and benthic foraminifera when compared to some other Lakadong Limestone assemblages recovered from other sites of Meghalaya (Jauhri 1994; Jauhri et al. 2006; Matsumaru and Sarma 2010; Tewari et al. 2010; Sarkar 2015a, 2018, 2019). This low palaeobiodiversity may be attributed to persistent high level of nutrient quantities or eutrophication in the environment that did not allow sufficient amount of light required for the proliferation of the algal–foraminiferal communities by means of increased turbidity in this shallow benthic environment. Distichoplax has been reported from another Lakadong Limestone section in the East Khasi Hills, interpreted to have thrived in an oligotrophic environment (Sarkar 2018). High nutrient regime could well be the cause of unfavourable environment for Distichoplax that could not proliferate. However, high nutrient environment usually is accompanied by significant numbers of bioeroders which is not the case in the present study. Therefore, this needs further analysis. Other important limiting factors responsible for the proliferation of benthic foraminifera are availability of oxygen (Jorissen et al. 1995; van der Zwaan et al. 1999) that could have played a role in their restricted occurrence apart from other physical parameters like temperature, salinity and ocean pH. However, any accurate interpretations on these factors are not within the scope of this present study.
Marinella lugeoni is known only from Upper Jurassic and Lower–Middle Cretaceous limestones to date pertaining to various locations worldwide including Angola, Spain and Brazil (Romanes 1916; Maury 1937; Granier and Dias-Brito 2016). The Early Thanetian algal framestones comprising abundant proportions of corallines showing strong affinity to Marinella lugeoni is an interesting observation from biostratigraphic as well as palaeobiogeographic perspective that needs further investigation. The extreme high abundance and relatively compact structure of these algal thalli does not present the likelihood of pure reworking as a possibility for their presence in this Palaeogene microbiofacies. Similarly, the first occurrence of agglutinated foraminifer Vania anatolica is also highly significant showing strong potential for presence of a biogeographic linkage between Turkey in the Mediterranean region and the East Tethys during the Late Palaeocene, as also has been demonstrated by another case study on the benthic foraminifer Haymanella elongata reported recently from the East Khasi Hills of Meghalaya, NE India (Sarkar 2019). Scarce records of these foraminifera possibly indicate their specialist nature that brought about limited utilisation of the resources available in the habitat and triggered their rapid obliteration from the palaeocommunity amidst other generalist species that show persistent presence over longer geological range presenting signatures of normal reproduction for a prolonged period.
6 Conclusions
This study puts forward the first contribution on ecostratigraphy of a shallow-marine shelf environment based on carbonates from Meghalaya, NE India representing the eastern part of the East Tethys (Neo-Tethys). In the current study, ecostratigraphy has enabled the reconstruction of three sub-environments corresponding to the deposition of distinct microbiofacies that depict the succession of different biotic communities in the study locality within the Early Thanetian time slice. This differentiation would not have been possible applying biostratigraphy alone that would simply categorize all these microbiofacies into one single shallow benthic zone (SBZ 3) on the basis of coralline alga Distichoplax biserialis and the index foraminiferal species recorded in the study section. The succession is mainly characterized by coralline red algae, benthic foraminifera and coated grains. The first records of agglutinated benthic foraminifer Vania anatolica and arborescent-lumpy coralline algae showing close affinity to Marinella lugeoni are important findings from this eastern Tethyan shelf environment that entail further investigations of this succession and possibly other pristine cave sections of Meghalaya to provide critical palaeobiogeographical and biostratigraphic data imperative to the regional geology. The Early Thanetian sedimentary succession evaluated from the Jaintia Hills presents a shallowing-upward trend, developing from a proximal inner shelf to a protected lagoonal–sandy shoal sub-environment.
References
Afzal J, Williams M, Leng M J and Aldridge R J 2011 Dynamic response of the shallow marine benthic ecosystem to regional and pan-Tethyan environmental change at the Paleocene–Eocene boundary; Palaeogeogr. Palaeoclimatol. Palaeoecol. 309 141–160.
Bosence D W J 1983 The occurrence and ecology of recent rhodoliths – a review; In: Coated Grains (ed.) Peryt T M, Springer, Berlin, Heidelberg, pp. 225–242.
Boucot A J 1982 Ecostratigraphic framework for the Lower Devonian of the North American Appohimchi Subprovince; Neues Jahrb. Paläont. Abh. 163 81–121.
BouDagher-Fadel M K, Price G D, Hu X and Li J 2015 Late Cretaceous to early Paleogene foraminiferal biozones in the Tibetan Himalayas, and a pan-Tethyan foraminiferal correlation scheme; Stratigraphy 12 67–91.
Checconi A, Bassi D, Passeri L and Rettori R 2007 Coralline red algal assemblage from the Middle Pliocene shallow-water temperate carbonates of the Monte Cetona (Northern Apennines, Italy); Facies 53 57–66.
Chelaru R, Săsăran E, Tămaş T, Bălc R, Bucur I and Pleş G 2019 Middle Miocene carbonate facies with rhodoliths from the NW Transylvanian Basin (Vălenii Şomcutei Cave, Romania); Facies 65 4.
Cooper R A 1999 Ecostratigraphy, zonation and global correlation of earliest Ordovician planktic graptolites; Lethaia 32 1–16.
Duguid S M A, Kyser T K, James N P and Rankey E C 2010 Microbes and ooids; J. Sedim. Res. 80 236–251.
Dunham R J 1962 Classification of carbonate rocks according to depositional texture; Am. Assoc. Pet. Geol. Mem. 1 108–121.
Dutta S K and Jain K P 1980 Geology and palynology of the area around Lumshnong, Jaintia Hills, Meghlaya, India; Biol. Mem. 5 56–81.
Embry A F and Klovan J E 1971 A late Devonian reef tract on northeastern Banks Island, northwestern Territories; Bull. Can. Pet. Geol. 19 730–781.
Flügel E 2004 Microfacies of Carbonate Rocks; Springer, Berlin, Heidelberg, New York, 976p.
Folk R L 1993 SEM imaging of bacteria and nannobacteria in carbonate sediments and rocks; J. Sedim. Petrol. 63 990–999.
Folk R L and Lynch L 2001 Organic matter, putative nannobacteria and formation of ooids and hardgrounds; Sedimentology 48 215–229.
Gerdes G, Dunajtschik-Piewak K, Riege H, Taher A G, Krumbein W E and Reineck H E 1994 Structural diversity of biogenic carbonate particles in microbial mats; Sedimentology 41 1273–1294.
Geronimo I D, Geronimo R D, Rosso A and Sanfilippo R 2002 Structural and taphonomic analysis of a columnar coralline algal build-up from SE Sicily; Geobios 35 86–95.
Granier B and Dias-Brito D 2016 On the fossil alga Marinella lugeoni Pfender, 1939, nom. cons., and its seven unfortunate avatars; Revision of the Juliette Pfender Collection. Part 2. Revision of the Jesse Harlan Johnson Collection. Part 2, Carnets Geol. 16 231–245.
Hoffman A 1981 The ecostratigraphic paradigm; Lethaia 14 1–7.
Hottinger L 1960 Recherches sur les Alvéolines du Palécène et de lˈEocène; Sch. Pal. Abh. 75/76 1–243.
Hottinger L and Drobne K 1988 Tertiary alveolinids: Problems linked to the conception of species; Rev. Paleobiol. (Benthos’86) 2 665–681.
Jauhri A K 1994 Carbonate buildup in the Lakadong Formation of the South Shillong Plateau, NE India: A micropaleontological perspective; In: Studies on Ecology and Paleoecology of Benthic Communities (eds) Matteucci R et al., Boll. Soc. Pal. Italiana 2 157–169.
Jauhri A K 1997 Post-Cretaceous record of larger foraminifera from the Shillong Plateau, India: An evidence of environmental recovery during Early Cenozoic; Palaeobotanist 46 118–126.
Jauhri A K 1998 Miscellanea (Foraminiferida) from thes Shillong region, NE India; J. Palaeontol. Soc. India 43 73–83.
Jauhri A K and Agarwal K K 2001 Early Palaeogene in the south Shillong Plateau, NE India: Local biostratigraphic signals of global tectonic and oceanic changes; Palaeogeogr. Palaeoclimatol. Palaeoecol. 168 187–203.
Jauhri A K, Misra P K, Kishore S and Singh S K 2006 Larger foraminiferal and calcareous algal facies in the Lakadong Formation of the south Shillong Plateau, NE India; J. Palaeontol. Soc. India 51 51–61.
Jorissen F J, de Stigter H C and Widmark J G V 1995 A conceptual model explaining benthic foraminiferal microhabitats; Mar. Micropal. 22 3–15.
Kundal P 2010 Biostratigraphic, paleobiogeographic and paleoenvironmental significance of calcareous algae; In: Special Issue on Applied Micropaleontology (eds) Kundal P and Humane S, Gond. Geol. Mag. 25 125–132.
Kundal P 2011 Generic distinguishing characteristics and stratigraphic ranges of fossil Corallines: An update; J. Geol. Soc. India 78 570–584.
Liu W and Zhang X 2012 Girvanella-coated grains from Cambrian oolitic limestone; Facies 58 779–787.
Maneveldt G W, Van der Merwe E and Keats D W 2016 Updated keys to the non-geniculate coralline red algae (Corallinophycidae, Rhodophyta) of South Africa; South Afr. J. Bot. 106 158–164.
Maneveldt G W, Chamberlain Y M and Keats D W 2008 A catalogue with keys to the non-geniculate coralline algae (Corallinales, Rhodophyta) of South Africa; South Afr. J. Bot. 74 555–566.
Martinsson A 1973 Ecostratigraphy: Limits of applicability; Lethaia 13 363.
Matsumaru K 1996 Tertiary larger foraminifera (Foraminiferida) from the Ogasawara Islands, Japan; Palaeontol. Soc. Japan, Spec. Paper 36 1–239.
Matsumaru K and Sarma A 2010 Larger foraminiferal biostratigraphy of the lower Tertiary of Jaintia Hills, Meghalaya, NE India; Micropaleontology 56 539–565.
Maury C J 1937 O Cretáceo de Sergipe. Serv. Geol. Mineral. Brasil, Monographia, Rio de Janeiro XI (1936), 283p.
Nagappa Y 1959 Foraminiferal biostratigraphy of Cretaceous–Eocene succession in the India–Pakistan–Burma region; Micropaleontology 5 141–181.
Nebelsick J H and Bassi D 2000 Diversity, growth-forms and taphonomy: Key factors controlling the fabric of coralline algal dominated shelf carbonates; In: Carbonate Platform Systems: Components and Interactions (eds) Insalaco E, Skelton P W and Palmer T J, Geol. Soc. London, Spec. Publ. 178 89–107.
Nikitenko B L, Reolid M and Glinskikh L 2013 Ecostratigraphy of benthic foraminifera for interpreting Arctic record of Early Toarcian biotic crisis (Northern Siberia, Russia); Palaeogeogr. Palaeoclimatol. Palaeoecol. 376 200–212.
Özcan E, Pignatti J, Pereira C, Yücel A O, Drobne K, Barattolo F and Saraswati P K 2018 Paleocene orthophragminids from the Lakadong Limestone, Mawmluh Quarry Section, Meghalaya (Shillong, NE India): Implications for the regional geology and paleobiogeography; J. Micropal. 37 357–381.
Olóriz F, Caracuel J and Rodríguez-Tovar F J 1995 Using ecostratigraphic trends in sequence stratigraphy; In: Sequence Stratigraphy and Depositional Response to Eustatic, Tectonic and Climatic Forcing (ed.) Haq U, Kluwer Academic Publisher, Dordrecht, pp. 59–85.
Olóriz F, Reolid M and Rodríguez-Tovar F J 2008 Taphonomy of fossil macro-invertebrate assemblages as a tool for ecostratigraphic interpretation in Upper Jurassic shelf deposits (Prebetic Zone, southern Spain); Geobios 41 31–42.
Perrin C, Bosence D and Rosen B 1995 Quantitative approaches to palaeozonation and palaeobathymetry of corals and coralline algae in Cenozoic reefs; In: Marine Palaeoenvironmental Analysis from Fossils (eds) Bosence D W J and Allison P A, Geol. Soc. London, Spec. Publ. 83 181–229.
Plee K, Ariztegui D, Martini R and Davaud E 2008 Unravelling the microbial role in ooid formation: Results of an in situ experiment in modern freshwater Lake Geneva in Switzerland; Geobiology 6 341–350.
Racey A 1995 Lithostratigraphy and larger foraminiferal (nummulitid) biostratigraphy of the Tertiary of northern Oman; Micropaleontology 41 1–123.
Rasmussen K A and Brett C E 1985 Taphonomy of Holocene cryptic biotas from St. Croix, Virgin Islands: Information loss and preservational biases; Geology 13 551–553.
Rasser M W and Piller W E 1999 Application of neontological taxonomic concepts to Late Eocene coralline algae (Rhodophyta) of the Austrian Molasse Zone; J. Micropal. 18 67–80.
Romanes M F 1916 XVI. Note on an algal limestone from Angola; Trans. R. Soc. Edinburgh LI 581–584.
Sarkar S 2015a Thanetian–Ilerdian coralline algae and benthic foraminifera from northeast India: Microfacies analysis and new insights into the Tethyan perspective; Lethaia 48 13–28.
Sarkar S 2015b Calcareous algal-rich carbonate sediments from Assam Shelf, NE India: An overview of the palaeoenvironmental implications; In: Petroleum Geosciences: Indian Context (ed.) Mukherjee S, Springer Geology, Switzerland, pp. 175–189.
Sarkar S 2016 Early Eocene calcareous algae and benthic foraminifera from Meghalaya, NE India: A new record of microfacies and palaeoenvironment; J. Geol. Soc. India 88 281–294.
Sarkar S 2017a Microfacies analysis of larger benthic foraminifera-dominated Middle Eocene carbonates: A palaeoenvironmental case study from Meghalaya, NE India (Eastern Tethys); Arab. J. Geosci. 10 21.
Sarkar S 2017b Ecology of coralline red algae and their fossil evidences from India; Thalassas 33 15–28.
Sarkar S 2018 The enigmatic Palaeocene–Eocene coralline Distichoplax: Approaching the structural complexities, ecological affinities and extinction hypotheses; Mar. Micropal. 139 72–83.
Sarkar S 2019 Does specialization imply rare fossil records of some benthic foraminifera: Late Palaeocene examples from the eastern Neo-Tethys (Meghalaya, NE India); Palaeogeogr. Palaeoclimatol. Palaeoecol. 514 124–134.
Sarma A, Ghosh A K and Sarkar S 2014 First record of coralline red algae from the Kopili Formation (late Eocene) of Meghalaya, NE India; Nat. Acad. Sci. Lett. 37 503–507.
Scheibner C and Speijer R P 2008 Late Paleocene–early Eocene Tethyan carbonate evolution – a response to long- and short-term paleoclimatic change; Ear.-Sci. Rev. 90 71–102.
Serra-Kiel J, Hottinger L, Caus E, Drobne K, Ferrandez C, Jauhri A K, Less G, Pavlovec R, Pignatti J, Samso J M and Schaub H 1998 Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene; Bull. Soc. Geol. France 169 281–299.
Sirel E and Gündüz H 1985 Vania, a new foraminiferal genus from the Thanatian of the Van region (east Turkey); Bull. Min. Res. Exp. Inst. Turkey 101/102 20–24.
Sokolov B S 1988 Ecostratigraphy, its place and role in modern stratigraphy; Int. Geol. Rev. 30 3–10.
Stampfli G M 2000 Tethyan oceans; In: Tectonics and Magmatism in Turkey and the Surrounding Area (eds) Bozkurt E, Winchester J A and Piper J D A, Geol. Soc. London, Spec. Publ. 173 1–23.
Tewari V C, Kumar K, Lokho K and Siddaiah N S 2010 Lakadong limestone: Paleocene–Eocene boundary carbonates sedimentation in Meghalaya, northeastern India; Curr. Sci. 98 88–95.
Van der Zwann G J, Duijnstee I A P, den Dulk M, Ernst S R, Jannink N T and Kouwenhoven T J 1999 Benthic foraminifers: Proxies or problems? A review of palecological concepts; Ear.-Sci. Rev. 46 213–236.
Waterhouse J B 1976 The significance of ecostratigraphy and need for biostratigraphic hierarchy in stratigraphic nomenclature; Lethaia 9 317–325.
Woelkerling W J, Irvine L M and Harvey A 1993 Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta); Aust. Syst. Bot. 6 277–293.
Xue K, Liang L, Yi W, Xiaohu K, Zongyan Z and Weihong H 2018 The Changhsingian foraminiferal fauna of the Meishan D Section, Zhejiang, China, and their ecostratigraphic implications; Acta Geol. Sin. (English edn.) 92 1299–1323.
Zamagni J, Mutti M and Košir A 2008 Evolution of shallow benthic communities during the Late Paleocene–earliest Eocene transition in the Northern Tethys (SW Slovenia); Facies 54 25.
Zamagni J, Košir A and Mutti M 2009 The first microbialite-coral mounds in the Cenozoic (Uppermost Paleocene) from the Northern Tethys (Slovenia): Environmentally-triggered phase shifts preceding the PETM? Palaeogeogr. Palaeoclimatol. Palaeoecol. 274 1–17.
Acknowledgements
The author is very much thankful to the Director, Birbal Sahni Institute of Palaeosciences, Lucknow for providing the infrastructure facilities. He also appreciates the very constructive and meticulous reviews by the handling editor Prof Soumyajit Mukherjee and two anonymous reviewers. He is grateful to Dr Abiraman Govindan and Dr Alessandro Vescogni for useful discussions and their help in the identification of some of the microfossil specimens. This study was financially supported by the Science and Engineering Research Board, Department of Science and Technology, Govt. of India (Grant No. SR/FTP/ES-143/2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Soumyajit Mukherjee
Rights and permissions
About this article
Cite this article
Sarkar, S. Ecostratigraphic implications of a Late Palaeocene shallow-marine benthic community from the Jaintia Hills, Meghalaya, NE India. J Earth Syst Sci 129, 10 (2020). https://doi.org/10.1007/s12040-019-1257-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12040-019-1257-8