The distribution and accumulation of the rare earth elements (REE) in the sediments of the Cochin Estuary and adjacent continental shelf were investigated. The rare earth elements like La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu and the heavy metals like Mg, V, Cr, Mn, Fe, Cu, Zn, U, Th were analysed by using standard analytical methods. The Post-Archean Australian Shale composition was used to normalise the rare earth elements. It was found that the sediments were more enriched with the lighter rare earth elements than the heavier ones. The positive correlation between the concentrations of REE, Fe and Mn could explain the precipitation of oxyhydroxides in the study area. The factor analysis and correlation analysis suggest common sources of origin for the REEs. From the Ce-anomalies calculated, it was found that an oxic environment predominates in all stations except the station No. 2. The Eu-anomaly gave an idea that the origin of REEs may be from the feldspar. The parameters like total organic carbon, U/Th ratio, authigenic U, Cu/Zn, V/Cr ratios revealed the oxic environment and thus the depositional behaviour of REEs in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The geochemical properties of rare earth elements (REEs) are useful to understand the processes that mobilise elements during weathering and redistribution of elements between particulate and dissolved phases in the rivers and estuaries (Sholkovitz 1995). The REEs make up a series of 15 elements from the lightest La (LREE) to the heaviest Lu (HREE). These are predominantly trivalent, with two exceptions: Cerium exists as Ce (IV) and Europium as Eu (II) as a function of redox potential (Ahrens 1964; Henderson 1984). Waters with a high pH have the lowest REE concentrations due to depletion by sorption onto particles. Concentrations of heavy REEs are enriched relative to those of the light REEs (Sholkovitz 1995). A large proportion of the dissolved REE inventory in river waters is associated with colloid material which can undergo large scale coagulation in the estuaries. This process results in highly fractionated phases being transported in rivers from the continents to the oceans (Sholkovitz 1995).
Light rare earths (LREEs) are preferentially scavenged on the surface of particles such as Mn-Fe oxyhydroxides and clay minerals or are precipitated as REE phosphates (Byrne and Kim 1990). The formation of both may promote the enrichment of HREEs in seawater. Certain minerals such as aluminosilicates or apatite tend to have high concentrations of REEs while other minerals such as quartz have very low concentrations (Graf 1977). REEs may also be useful indicators of anthropogenic inputs. LREEs are enriched in petroleum cracking catalysis, for the production of light weight hydrocarbons such as gasoline and fuel oil (Olmez et al. 1991). High concentrations of REEs were reported by Borrego et al. (2004) in Tinto and Odiel estuaries in Spain, due to the waste from the fertilizer industries. Dudas and Pawluk (1977) found high concentrations of heavy metals and REEs in cultivated soils and in cereal crops of Alberts, Canada. Phosphogypsum, a by-product from the production of phosphate fertilizers, contains trace elements and rare earth elements.
REEs can be used to understand the geochemical evolution of earth’s crust. REEs mobilise during weathering and undergo modification (non-conservative) in the estuary (Sholkovitz 1995). REEs are sensitive to environmental changes such as redox condition, salinity, chelates, pH, adsorption–desorption, complexation, precipitation, etc. The most important source of REEs is riverine input and hence behaviours of REEs in rivers and estuaries have been used to understand and correlate the geochemical exchange between the crust and the ocean (Elderfield 1988). A large-scale removal of LREEs occurs during estuarine mixing. Ross et al. (1995) studied the positive Eu anomalies among Manso River by REEs normalisation. The REEs enrichment is associated with high pH, while the feldspar and their secondary products are the cause of Eu anomaly (Kurian et al. 2008).
The objectives of this research are:
-
1.
To characterise the distribution and concentration of REEs,
-
2.
To identify the principal sources and sinks of these elements,
-
3.
Use of Ce-anomaly, Eu-anomaly, tetrad effect and other ratios to determine the depositional behaviour.
2 Material and methods
2.1 Study area
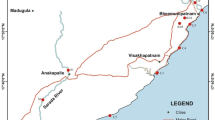
Cochin Estuary is a bar-built micro tidal system connected to the Arabian Sea at two locations – one at Cochin (latitude 9°10′N) and at Azhikode (latitude 10°10′N). The estuary is dividable into two parts: the southern arm stretching from Cochin to the south and the northern arm extending from Cochin to Azhikode. The Cochin bar mouth is about 450 m wide, whereas the Azhikode inlet is relatively narrow. Cochin city receives an annual rainfall of 320 cm, of which 60% occurs during the southwest monsoon period, July–September (Qasim 2003). The estuary receives a high volume of fresh water annually (20 × 109m3 year − 1) from the six rivers in the State of Kerala (Srinivas et al. 2003). Cochin estuarine system is the largest of its kind on the west coast of India with an area of 256 km2. Cochin estuarine system comprises one of the most important harbours and industrial centres in the west coast of India. Kerala is in the southernmost part of Indian peninsula bordered by the Western Ghats on the east and by the Arabian Sea on the west. The continental shelf of Kerala is fairly straight as a result of brake down during late Pliocene. As many as 41 rivers flow towards the sea in the west coast of Kerala. During the southwest monsoon the rainfall is high and the rivers carry maximum sediment load into the continental shelf.
The Arabian Sea is a highly productive area driven by the Asian monsoon, having a pronounced climatic feature of global significance (Nair et al. 1989; Haake et al. 1996). There is an apprehension that environmental characterisation of coastal and estuarine waters will inevitably have consequences for the Arabian Sea’s ecosystem. Marked symptoms were noticed on considerable impact in deterioration of estuarine waters of these coastal areas as well as the shelf system (Naqvi and Jayakumar 2000). The emerging industrial establishments and human settlements along the west coast of India, thus necessitates a critical evaluation of the nature and quantum of inputs to the Arabian Sea as well as their regional assimilative capabilities. If there is a possible threat to the well-being of the living resources of EEZ of India, then the southeastern Arabian Sea is one of the prime locations that is affected the most.
2.2 Sample collection and preservation
Sampling was carried at six stations spread across the Cochin Estuary on May 2007 (figure 1). Sediments were collected from the western continental shelf of India in two different depths (50 and 100 m) during the month of October 2007 onboard Fishery and Oceanographic Research Vessel Sagar Sampada (cruise no. 255). Surface sediments were collected using a Van-veen grab and uncontaminated portions were sampled from the middle of the part using sterile spoons, packed in polyethylene covers and stored at −20°C until analysis (Grasshoff et al. 1999). Before analysis the samples were air dried and homogenised to fine powder.
2.3 REE analysis
Rare earth elements (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu), radioactive elements like Th and U, major elements (Fe, Mn, Mg) and trace elements (Cr, Ni, Co, Zn) were analysed along with texture (sand, silt and clay) and organic carbon. The following sample preparation procedures were adopted for the dissolution of samples in the present investigation.
Open acid digestion methods by Parijat et al. (2007) were adopted for REE detection. A sample of 0.5 g was moistened with mill-Q water then with 10 ml of an acid mixture (7:3:1 HF-HNO3-HClO4). 1 ml of 5 μg/ml Rh solution was added as internal standard and digest at 200°C overnight until crystalline paste was obtained. The remaining residues were dissolved using 10 ml of 1:1 HNO3 and heated gently on a hot plate for 10 minutes at 70°C. Finally, the volume was made up to 250 ml with purified water (18 MΩ) and stored in polyethene bottles. These aliquots were analysed for REEs using a Perkin Elmer SCIEX (Model 6100 ELAN DRCII) ICP-MS at the National Geophysical Research Institute, Hyderabad, India. MAG-1 is used as the reference material for REE analysis. For the analysis of major elements and trace metals, an analytical method suggested by Loring and Rantala (1992) was followed. According to this method, 1 g of dried finely powdered sediment sample was digested with a mixture of HClO4 and HNO3, evaporating to dryness each time until the digestion was complete, then brought into solution in 0.1 M HNO3 (25 ml) and analysed using flame AAS (Perkin Elmer 3110). The precision of the analytical procedure was checked using a triplicate analysis of a certified reference material (BCSS-1) from the National Research Council of Canada and showed a good accuracy and recovery rate. The triplicates of all samples were done analysed and the average values were reported.
Textural characteristics (sand, silt, clay) of the sediments were determined by pipette analysis (Krumbein and Petti John 1938). Total carbon (TC), total nitrogen (TN) and total sulphur (TS) were determined using Vario EL III CHNS Analyzer. Organic carbon (OC) was determined according to the method of Gaudette et al. (1974).
3 Results
The concentration of REE and trace elements abundance of sediments from Cochin Estuary and adjacent continental shelf are presented in tables 1 and 2. The total organic carbon content ranges from 0.14% to 2.5% in the study area. The correlation coefficient values between REE pattern and trace elements are listed in table 3. In present study we adopted the Post-Archean Australian Shale (PAAS) composition for normalising REEs in the study area.
3.1 REE distribution and source
The terrigenous input of REE from the continents, authigenic removal of REE from the water and early diagenesis are major processes that control the enrichment and depletion of metals in the sediments (Sholkovitz 1988). The sediments of Cochin Estuary and the nearby continental shelf regions contain higher concentrations of LREEs than HREEs. It is of the expected behaviour since the REE contents of most shale’s and solid phases are normally enriched in LREEs relative to HREEs (Haskin et al. 1966) even though the absolute concentration in most sediments are similar. The surface sediments have ∑REE concentration close to the shale values and a sample to shale ratio very close to 1 normally indicates a dominant terrigenous source (Piper 1974a; Pattan et al. 2005). The increase of REE concentration indicated an additional supply of REE to the sediment in addition to the detritus source.
La showed higher abundance in station ST-1 (51.45 ppm), ST-2 (45.60 ppm) and ST-5 (52.37 ppm). The average shale was found to be 38 ppm (PAAS). Stations ST-3 (32 ppm), ST-4 (38 ppm), ST-6 (28.5 ppm), ST-7 (13.95 ppm) and ST-8 (21.89 ppm) gave concentrations lower than PAAS. The PAAS of Ce was found to be 79.6 ppm. The studied stations ST-1 (102.81 ppm), ST-2 (96 ppm), ST-4 (80.55 ppm) and ST-5 (102.81 ppm) gave higher concentrations than PAAS and ST-3 (63.8 ppm), ST-6 (50.80 ppm), ST-7 (27.72 ppm) and ST-8 (50.86 ppm) gave much lower concentrations than PAAS.
Pr showed similar distribution trend like other REEs; La and Ce. The highest values were observed in ST-1 (9.91 ppm), ST-2 (9.04 ppm) and ST-5 (10.02 ppm). The PAAS gave 8.83 ppm, other stations have lower concentrations than PAAS.
Nd also gave similar trend like other REEs. The stations ST-1 (38.49 ppm), ST-2 (35.05 ppm) and ST-5 (38.78 ppm) showed distribution higher than PAAS whereas other stations gave lower values. The REEs like Tb, Dy and Ho showed similar trend in all stations. The station ST-1 has concentrations 0.86 ppm for Tb, 4.92 ppm for Dy and 1.03 ppm for Ho; ST-4 had values 0.75 ppm for Tb and ST-5 gave Tb (0.89 ppm), Dy (4.92 ppm) and Ho (1.03 ppm), these concentrations were higher than PAAS. Other stations had lower concentrations than PAAS. The PAAS for Tb, Dy and Ho were found to be 0.774, 4.68 and 0.991 ppm, respectively.
Sm, Eu, Gd gave similar trends in all stations. ST-1, ST-2, ST-4 and ST-5 gave higher concentrations than PAAS. The ST-1 showed 6.93, 1.65 and 5.67 ppm for Sm, Eu and Gd, but in ST-2 Sm, Eu and Gd showed slighter values than ST-1, i.e., 6.02, 1.29 and 4.93 ppm. The ST-4 showed Eu (1.25 ppm), Tb (0.75 ppm) and ST-5 gave Sm (6.91 ppm), Eu (1.59 ppm), Gd (5.65 ppm). PAAS for Sm, Eu and Gd are 5.65, 1.08 and 4.66 ppm, respectively.
In the case of Er, Tm, Yb and Lu, all stations showed values well inside PAAS. The PAAS for Er, Tm, Yb and Lu are 2.85, 0.405, 2.82 and 0.433 ppm, respectively. Stations 1 and 5 showed comparatively higher values with respect to other stations, viz., Er (2.83 ppm), Tm (0.39 ppm), Yb (2.33 ppm), Lu (0.36 ppm) at ST-1 and Er (2.79 ppm), Tm (0.38 ppm), Yb (2.34 ppm), Lu (0.36 ppm) at ST-5, respectively.
The shale normalised REE pattern (figure 2) of stations ST-3, ST-4, ST-6, ST-7 and ST-8 show that the light REE (La, Ce, Pr, Nd) content is ever lower than the shale resulting in a sample to shale ratio lower than 1. The shale-normalised REE patterns of LREE for sediments from stations ST-1, ST-2 and ST-5 have sample to shale ratio 1 or higher that suggest the source to be terrigenous (Piper 1974a). On comparing between the MREE and HREE, the terrigenous contribution is richer in the HREE range than the MREE. REE enrichment is a common feature observed in Fe-Mn crusts and Fe-Mn coating (Grandjean-Lecuyer et al. 1993). Therefore, these results give an idea that the precipitations of oxyhydroxides have a general tendency to develop REE enrichment in the sediments.
3.2 Major and trace metal distribution
Table 2 showed the distribution of major and trace metals along the study area. Fe and Mg in the study area were indicated in terms of percentage. Fe ranged from 1.66% to 6.94%, and Mg from 0.60% to 2.33%. Trace metals like Cu, Cr, Mn, Zn and V, and radioactive metals like U and Th were expressed in ppm levels. Th and U concentration varied from 5.52 to 12.69 ppm and 1.01 to 4.27 ppm in studied stations. In the case of trace metals like Cu, Cr, Mn, Zn and V showed concentrations 4.40 to 28.91 ppm, 23.28 to 111.76 ppm, 40.98 to 244.38 ppm, 14.52 to 361 ppm, and 5.15 to 73.61 ppm, respectively.
4 Discussion
4.1 Relationship between REEs, major and trace metals
The Pearson correlation among the REEs, major and trace metals were generated (table 3). The metal La gave strong positive correlation with Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm, Yb, Lu and including the metal Mn, Fe, Cu, V, U and Th. Metals like Cr and Zn showed no significant correlation with La. Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu showed similar correlation as that of La. Cu gave positive correlation with La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu. In the case of Cr, it indicated no correlation with all REEs, major and trace metals. Fe and Mn gave similar correlation trend as that of Cu. But Fe strongly correlated with Lu and Cu. In the case of Mn, it displayed strong correlation with La, Ce, Pr and Fe, and no correlation with Eu and Cr. Zn showed positive correlation only with Cu and gave no correlation with other REEs, major and trace metals. The metals like Th, U and V revealed similar correlation with other metals. Th recorded no correlation with Cu, Cr and Zn, and gave positive correlation with other metals. U and V showed no correlation with Eu, Cr and Zn, and other metals showed positive correlation. So the correlation analysis indicates a strong relationship between REEs and some of major elements. But the major and trace metals established no correlation with Eu. This may be due to difference in origin of Eu and major and trace metals. Overall strong to moderate correlations of REEs with Fe and Mn indicate that Fe–Mn oxyhydroxides are important carriers of REEs. The transport of iron ore to Cochin Estuary is another reason for the presence of Fe–Mn oxyhydroxides. Taylor and McLennan (1985) suggested that the REEs are enriched in fine-grained sediments, especially clay minerals. According to Deepulal et al. (2011), highly significant correlation between Fe and Mn revealed the formation of Fe–Mn oxyhydroxides.
The factor analysis (FA) was applied to obtain more reliable information about the relationships among the variables (Bartolomeo et al. 2004; Ghrefat and Yusuf 2006). Factor analysis was designed to transform the original variables into new uncorrelated variables called factors, which are linear combination of the original variables. The FA is a data reduction technique and suggests how many varieties are important to explain the observed variances in the data. Principal component analysis was used for extraction of different factors.
Table 4 gave varimax component of two factors in sediment. The two significant components, whose eigenvalues are higher than 1 accounting for 97% of the cumulative variance, were distinguished for the analytical data. Factor 1 accounted for 64.829% of the total variance and is mainly characterised by high levels of Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Fe and Mn. This phenomenon explains that the origin of REEs is from a common source and also the relationship between REEs and Fe–Mn oxyhydroxides. Factor 2 accounted for 31.219% of the total variance including higher values for texture parameters like Fe, silt, clay, total organic carbon and Mg (figure 3). This implies that the organic carbon has strong attachment to lighter fractions like silt and clay. The higher values of Fe in both factors indicate Fe exhibit complex behaviour in Cochin Estuary.
4.2 Fractionation indices
Fractionation indices are represented by (La)n/ (Yb)n, i.e., \(\left[ {{\left( {{{\rm La}_{{\rm sample}} } \mathord{\left/ {\vphantom {{{\rm La}_{{\rm sample}} } {{\rm La}_{{\rm PAAS}} }}} \right. \kern-\nulldelimiterspace} {{\rm La}_{{\rm PAAS}} }} \right)} \mathord{\left/ {\vphantom {{\left( {{{\rm La}_{{\rm sample}} } \mathord{\left/ {\vphantom {{{\rm La}_{{\rm sample}} } {{\rm La}_{{\rm PAAS}} }}} \right. \kern-\nulldelimiterspace} {{\rm La}_{{\rm PAAS}} }} \right)} {\left( {{{\rm Yb}_{{\rm sample}} } \mathord{\left/ {\vphantom {{{\rm Yb}_{{\rm sample}} } {{\rm Yb}_{{\rm PAAS}} }}} \right. \kern-\nulldelimiterspace} {{\rm Yb}_{{\rm PAAS}} }} \right)}}} \right. \kern-\nulldelimiterspace} {\left( {{{\rm Yb}_{{\rm sample}} } \mathord{\left/ {\vphantom {{{\rm Yb}_{{\rm sample}} } {{\rm Yb}_{{\rm PAAS}} }}} \right. \kern-\nulldelimiterspace} {{\rm Yb}_{{\rm PAAS}} }} \right)}} \right]\) ratio (figure 4), which defines relative behaviour of LREE to the HREE. This ratio has been calculated for all the samples in the present study. It ranges from 1.418 to 2.079 indicating HREE are very much depleted in these stations. These results support the studies of Nath et al. (2000), who found significant LREE enrichment in the sediments from the Vembanad lake and the inner shelf sediments in off Cochin.
4.2.1 Ce-anomaly as a redox indicator
The Ce-anomaly is calculated (Wilde et al. 1996) as follows:
where n is the shale normalised concentration. The Ce-anomaly represents its enrichment or depletion compared to its neighbouring elements. Ce-anomaly doesn’t exist when the normalised Ce concentration falls exactly between La and Pr. In this case the REE are generally considered to be of terrigenous origin as reported by Piper (1974a). A depletion of Ce relative to its neighbouring REEs gives rise to a negative Ce-anomaly. This could be resulted from the presence of siliceous organisms and Bementite (Piper 1974a, 1974b; Tlig and Steinberg 1982; Pattan et al. 2005). A positive Ce-anomaly occurs, when Ce is enriched relative to its neighbours and might have resulted from the presence of Fe–Mn oxyhydroxides (Piper 1974b; Pattan et al. 2005).
The Ce-anomalies of the studied area varies from −0.009 to 0.06 (figure 5). Ce-anomaly is positive in stations ST-1, ST-2, ST-4, ST-5, ST-6, ST-7 and ST-8 which suggests sediment deposition and comparatively more bottom water oxic environment.
The positive Ce-anomaly could be attributed to the oxidation of Ce3 + to Ce4 + and incorporation into Mn oxyhydroxide phases as CeO2. Sholkovitz (1995) also reported that the Ce-anomaly in the tropical rivers and estuaries are mainly due to the scavenging of Ce in oxides, principally iron oxyhydroxides. The ST-3 showed slightly negative Ce-anomaly, indicating a suboxic/anoxic depositional condition. The redox condition formed from the Ce-anomaly alone in the sediments are needed to be confirmed by the other depositional parameters sensitive to the redox condition.
4.2.2 Eu-anomaly
\({\rm Eu\text{-}Anomaly}={{\rm Eu}_{\left( {{\rm normalised}} \right)} } \mathord{\left/ {\vphantom {{{\rm Eu}_{\left( {{\rm normalised}} \right)} } {{\rm Eu\ast }}}} \right. \kern-\nulldelimiterspace} {{\rm Eu\ast }}\) (Mascarenhas-Pereira and Nath 2010) where Eu* is \({\left[ {{{\rm Sm}_{\left( {{\rm normalised}} \right)} } \mathord{\left/ {\vphantom {{{\rm Sm}_{\left( {{\rm normalised}} \right)} } {{\rm Gd}_{\left( {{\rm normalised}} \right)} }}} \right. \kern-\nulldelimiterspace} {{\rm Gd}_{\left( {{\rm normalised}} \right)} }} \right]} \mathord{\left/ {\vphantom {{\left[ {{{\rm Sm}_{\left( {{\rm normalised}} \right)} } \mathord{\left/ {\vphantom {{{\rm Sm}_{\left( {{\rm normalised}} \right)} } {{\rm Gd}_{\left( {{\rm normalised}} \right)} }}} \right. \kern-\nulldelimiterspace} {{\rm Gd}_{\left( {{\rm normalised}} \right)} }} \right]} 2}} \right. \kern-\nulldelimiterspace} 2.\)
Eu anomaly is represented as Eu/Eu* where Eu is the concentration of Eu in the sediments normalised with shale value and Eu* is a predicted value obtained by linear interpolation of Sm/Sm* and Gd/Gd*. Value greater than 1 and less than 1 indicate positive and negative anomalies, respectively. A value of 1 indicates no anomaly. Rudick Roberta (1991) suggested that the positive Eu anomaly is mainly due to the effect of areas prominent in hydrothermal vents or due to the feldspar origin.
In the present study, Eu anomaly values vary from 1.037 to 1.841 and is shown in figure 6. All stations showed values close to 1 or greater than 1. This gives an idea that the input may be from hydrothermal vent system or from the feldspar. But in our study area, there is no evidence of hydrothermal vent system, which means that it may have originated from feldspar. Mascarenhas-Pereira and Nath (2010) also quoted the same results.
4.2.3 Tetrad effect
In the past decades, the geochemical behaviour of the REEs has been studied extensively because this group of elements provide a range of geochemical indicators that can be used for the evolution of magmatic and hydrothermal system (Monecke et al. 2007). An additional feature that potentially influences the distribution of the REEs in some geological environments is the lanthanide tetrad effect (Masuda et al. 1987; Monecke et al. 2002). This effect causes a split of the normalised REE patterns into four consecutive curved segments that are referred to as the tetrads (first tetrad: La-Ce-Pr-Nd; second tetrad: (Pm)-Sm-Eu-Gd; third: Gd-Tb-Dy-Ho; fourth: Er-Tm-Yb-Lu). The tetrads are either convex or concave forming M- and W-shaped lanthanide patterns, respectively (Masuda et al. 1987).
In the present study, stations ST-8, ST-4 and ST-2 showed first tetrad (figure 2) and in other stations it is absent. The second tetrad is visible in all stations but it is not taken because of the exclusion of Pm which is a radioactive element. The third is absent in all stations and fourth tetrad is visible in station ST-2. The tetrad effect is a split of REE normalised patterns into four convex-shaped sediments and is caused by complexation of REEs when adsorption, co-precipitation, dissolution, or ligand-exchange reactions occur (Bau 1996). It is believed that the tetrads are mostly taking over from hinterland rocks.
4.2.4 Geochemical parameters indicative of oxygenation condition
A number of parameters such as Mn, total organic carbon, U/Th, authegenic U, Cu/Zn, V/Cr, S compared to organic carbon and all stable isotopes are widely used to understand the depositional environments (Kuspert 1982; MacLeod and Irving 1996; Pattan et al. 2005). Some of the above parameters are used in the present study to understand the depositional behaviour.
Manganese is a redox sensitive element which precipitates as Mn4 + in an oxic environment, whereas it dissolves into pore water as Mn2 + and in turn diffuses upward to reprecipitate as Mn4 + once an oxic environment is encountered (Lynn and Bonatti 1965). Cerium also behaves similar to Mn because of their very similar geochemical behaviour. The Mn concentration in the study area varies from 40 to 244 ppm and appears uniform in almost all stations except ST-6, ST-7 and ST-8. This low Mn concentration in these stations may be due to low sedimentation rates in marine environment, as these stations are purely marine in nature.
The total organic carbon content in the study area varied from 0.15% to 2.59% with an average of 1.28% (figure 7a). Carbon/nitrogen (C/N) ratio in stations ST-6, ST-7 and ST-8 suggest the character of origin as similar to finding of Pattan et al. (2005). These stations showed low organic carbon content and this can be attributed to low sediment accumulation rate, which allows more mineralisation at the sediment–water interface. Continental shelf regions may facilitate extensive mineralisation of organic matter during settling through the water column resulting in low organic carbon content. The low and almost constant organic carbon content suggests a well oxidised environment (Warren 1982).
In oxic water U6 + is presented as Uranyl tricarbonate species, which is highly soluble. In the case of oxygen deficient or reducing environments, U6 + is reduced to U4 + which is particle-reactive and can become fixed onto sediment (Klinkhammer and Palmer 1991). But in the case of Th, it is relatively immobile (Anderson et al. 1983). The low U contents in stations ST-3, ST-4, ST-6, ST-7 and ST-8 indicate the oxic environment and is lower than PAAS (3.1 ppm) (Taylor and McLennan 1985). Higher concentration of U in ST-1 is evident by the presence of Uraninite mineral in this location. Because of the difference in the behaviour of U and Th as in U/Th ratio can be valued as a redox index with high concentrations (>1.25) associated with anoxic environment and low concentrations (0.75) associated with oxic environment as supported by Jones and Manning (1994). The low U/Th ratio (figure 7b) of sediments along the study area is an indicative of prevalence of an oxic environment.
A concentration of U is influenced by primary production because of the degradation of the organic matter reaching the sediments which consumes a sequence of oxidant, i.e., oxygen, nitrate, iron (Fe), and manganese (Mn) oxides and sulphate. Different threshold levels of oxidant consumption trigger a series of reactions that lead to precipitation of U in the sediment (Zheng et al. 2000). Authigenic U precipitation typically starts at the redox potential of Fe-oxide reduction (Zheng et al. 2002). Authigenic U is used as proxies of redox condition in marine sediments because it is conservative in the oxygenated waters but becomes enriched in anoxic sediment (Yamada and Tsunogai 1984). Authigenic U content has been proposed as an index of bottom water anoxia (Wignall and Myers 1988) and is calculated as:
Authigenic U enrichment in sediments tends to be deposited under anoxic conditions that allow large amount of organic matter to accumulate and U to be fixed (Wignall and Myers 1988). The absence of any authigenic U content in the sampled sediment (figure 7c) revealed an oxic environment. Same results have been projected by the study of Jones and Manning (1994), who suggested that authigenic U content of <5 ppm are considered to be indicative of oxic environments.
In oxygenated waters the stable form of vanadium is vanadate, and it is adsorbed onto both Fe and Mn oxyhydroxide phases (Wehrli and Stummm 1989). In the absence of oxygen, vanadate is reduced to vanadyl. Chromium, in contrast, is relatively insoluble and in these conditions it is usually held within the detritus fraction. The mean vanadium (41 ppm) and Cr (69.8 ppm) contents in the study area are lower than PAAS (Taylor and McLennan 1985). The V/Cr ratios of sediment (figure 7d) have also been suggested as an index of bottom water oxygenation and the resulted >4.5 and <2 are suggestive of anoxic and oxic conditions, respectively (Jones and Manning 1994). Hallberg (1976) previously indicated that the Cu/Zn ratio in sediment may reflect redox conditions of the ancient environment and the ratio increased in reduced conditions and decreased in oxidising conditions. The Cu/Zn ratio in the sediments (figure 7e) varied from 0.124 to 0.479 pointing that there is no drastic changes in the depositional environment.
5 Conclusion
The distribution, origin and behaviour of sediments from Cochin Estuary and adjoining continental shelf regions were studied. To understand the behaviour of REEs and their interelemental relationship, correlation analysis were used. Ce-anomaly, Eu-anomaly, authigenic U, U/Th ratio, Mn, total organic carbon, V/Cr ratio and Cu/Zn ratio were used to understand the depositional behaviour and their environmental changes. The Ce-anomaly indicates the oxic environment in bottom waters and the presence of Fe–Mn oxyhydroxides. The positive Eu-anomaly along the study area reveals that the REEs mainly originated from feldspar. The proxies like total organic carbon, Mn, authigenic U, U/Th ratio, V/Cr ratio and Cu/Zn ratios indicate the presence of oxic environment. So the present study indicates the oxic environment in Cochin Estuary. To understand more about the depositional behaviour proxies like stable isotopic studies were needed.
References
Ahrens L H 1964 Aspects of the geochemistry of the rare earths; Progress in the Science and Technology of the Rare Earths (ed.) Eyring L (New York: Pergamon Press).
Anderson R F, Bacon M P and Brewer P G 1983 Removal of 230Th and 231Pa at ocean margins; Earth Planet. Sci. Lett. 66 73–90.
Bartolomeo A D, Poletti L, Sanchini G, Sebastiani B and Morizzi G 2004 Relationship among parameters of lake polluted sediments evaluated by multivariate statistical analysis; Chemosphere 55 1323–1329.
Bau M 1996 Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf and lanthanide tetrad effect; Contrib. Mineral. Petrol. 123 323–333.
Borrego J, Lopez-Gonzalez N, Carro B and Lozano-Soria O 2004 Origin of the anomalies in light and middle REE in sediments of an estuary affected by phosphogypsum wastes (south-western Spain); Mar. Pollut. Bull. 49 1045–1053.
Byrne R H and Kim K H 1990 Rare earth element scavenging in seawater; Geochim. Cosmochim. Acta 54 2645–2656.
Deepulal P M, Gireeshkumar T R, Sujatha C H and Rejomon George 2011 Chemometric study on the trace metal accumulation in the sediments of the Cochin Estuary – Southwest coast of India; Environ. Monit. Assess., doi: 10.1007/s10661-011-2418-7.
Dudas M J and Pawluk S 1977 Heavy metals in cultivated soil and in cereal crops in Alberta; Can. J. Soil. Sci. 57 329–339.
Elderfield H 1988 The oceanic chemistry of the rare earth elements; Phil. Trans. Roy. Soc. London A325 105–126.
Gaudette H E, Flight W R, Toner L and Folger D W 1974 An inexpensive titration method for the determination of organic carbon in recent sediments; J. Sedim. Petrol. 44 249–253.
Ghrefat H and Yusuf N 2006 Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan; Chemosphere 64 2114–2121.
Graf J L 1977 Rare earth elements as hydrothermal tracers during the formation of massive sulphide deposits in volcanic rocks; Econ. Geol. 72 527–548.
Grandjean-Lecuyer P, Feist R and Albarede F 1993 Rare earth elements in old biogenic apatites; Geochim. Cosmochim. Acta 57 2507–2514.
Grasshoff K M, Ehrhardt M and Kremling K (eds) 1999 Methods of Seawater Analysis (Weinheim: Wiley-VCH Verlag GmbH).
Haake B, Rixen T, Reemtsma T, Ramaswamy V and Ittekot V 1996 Processes determining seasonality and interannual variability of settling particles fluxes to the deep Arabian Sea; Particle Flux in the Ocean (eds) Ittekot V et al., Wiley & Sons, pp. 251–270.
Hallberg R O 1976 A geochemical method for investigation of paleoredox condition in sediments; Ambio Spec. Rep. 4 139–147.
Haskin L A, Frey F A, Schmit R A and Smith R H 1966 Meteoritic, solar and terrestrial rare earth distributions; Physics and Chemistry of the Earth (eds) Ahrens L H, Press F, Runcorn S K and Urey H C (Oxford: Pergamon Press), pp. 169–321.
Henderson P 1984 Rare Earth Element Geochemistry; Oxford: Elsevier.
Jones B and Manning D A C 1994 Composition of geochemical indices used for the interpretation of paleoredox conditions in ancient mudstones; Chem. Geol. 111 111–129.
Klinkhammer G P and Palmer M R 1991 Uranium in the oceans: Where it goes and why?; Geochim. Cosmochim. Acta 55 1799–1806.
Krumbein W C and Petti John F J 1938 Manual of sedimentary Petrology; New York: Appleton.
Kurian S, Nath B N, Ramaswamy V, Naman D, Rao T G, Kamesh Raju K A, Selvaraj K and Chen C T A 2008 Possible detrital, diagenetic and hydrothermal sources for Holocene sediments of the Andaman back arc basin; Mar. Geol. 247(3–4) 178–193.
Kuspert W 1982 Environmental changes during oil shale deposition as deduced from stable isotope ratios; In: Cyclic and Event Stratification (eds) Einsele G and Seilacher A (Springer-Verlag: Berlin), pp. 482–501.
Loring D H and Rantala R T T 1992 Manual for the geochemical analyses of marine sediments and suspended matter; Earth Sci. Rev. 32 235–283.
Lynn D C and Bonatti E 1965 Mobility of managanese in diagenesis of deep sea sediments; Mar. Geol. 3 457–474.
MacLeod K G and Irving A J 1996 Correlation of cerium anomalies with indicators of paleoenvironment; J. Sedim. Res. 66(5) 948–955.
Mascarenhas-Pereira M B L and Nath B N 2010 Selective leaching studies of sediments from a seamount flank in the central Indian Basin: Resolving hydrothermal, volcanogenic and terrigenous sources using major, trace and rare-earth elements; Mar. Chem. 121(1–4) 49–66.
Masuda A, Kawakami O, Dohomoto Y and Takenaka T 1987 Lanthanide tetrad effects in nature: Two mutually opposite types, W and M; Geochem. J. 21 119–124.
Monecke T, Kempe U, Monecke J, Sala M and Wolf D 2002 Tetrad effect in rare earth element distribution patterns: A method of quantification with application to rock and mineral samples from granite-related rare metal deposits; Geochim. Cosmochim. Acta 66 1185–1196.
Monecke T, Dulski P and Kempe U 2007 Origin of convex tetrad in rare earth element patterns of hydrothermally altered siliceous igneous rocks from the Zinnwald Sn-W deposit, Germany; Geochim. Cosmochim. Acta 71 335–353.
Nair R R, Ittekot V, Manganini S J, Ramaswamy V, Haake B, Degens E T, Desai B N and Honjo S 1989 Increased particles flux to the deep ocean related to monsoons; Nature 338 749–751.
Naqvi S W A and Jayakumar D A 2000 Ocean biogeochemistry and atmospheric composition: Significance of the Arabian Sea; Curr. Sci. 78(3) 289–299.
Nath B N, Kunzendorf H and Pluger W L 2000 Influence of provenance, weathering and sedimentary processes on the elemental ratios of the fine-grained fraction of the bedload sediments from the Vembanad Lake and the adjoining continental shelf, southwest coast of India; J. Sedim. Res. 70(5) 1081–1094.
Olmez I, Sholkovitz E R, Hermann D and Eganhouse R P 1991 Rare earth elements in sediments of southern California: A new anthropogenic indicator; Environ. Sci. Technol. 25 310–316.
Parijat Roy, Vjsetti Balaram and Anil Kumar 2007 New REE and trace element data on two kimberlitic reference materials by ICP-MS; Geostd. Geoanalyt. Res. 31(3) 261–273.
Pattan J N, Masuzawa T, Borole D V, Parthiban G, Jauhari P and Yamamoto M 2005 Biological productivity, terrigenous influence and noncrustal elements supply to the central Indian Ocean Basin: Paleoceanography during the past 1 Ma; J. Earth Syst. Sci. 114 63–74.
Piper D Z 1974a Rare earth elements in the sedimentary cycle: A summary; Chem. Geol. 14 285–304.
Piper D Z 1974b Rare earth elements in ferromanganese nodules and other marine phases; Geochim. Cosmochim. Acta 38 1007–1022.
Qasim S Z 2003 Cochin backwaters and Vembanad; In: Indian Estuaries (ed.) Qasim S Z (Mumbai: Allied Publishers Pvt. Ltd), pp. 305–382.
Ross G R, Guevara S R and Arribere M A 1995 Rare earth geochemistry in sediments of the Upper Manso River basin, Rio Negro, Argentina; Earth Planet. Sci. Lett. 133 47–57.
Rudick Roberta L 1991 Restites, Eu anomalies, and the lower continental crust; Geochim. Cosmochim. Acta 56 963–970.
Sholkovitz E R 1988 Rare earth elements in the sediments of the North Atlantic Ocean, Amazon delta, and East China Sea: Reinterpretation of terrigenous input pattern to the oceans; Am. J. Sci. 288 236–281.
Sholkovitz E R 1995 The aquatic chemistry of rare earth elements in rivers and estuaries; Aquat. Geochem. 1 1–34.
Srinivas K, Revichandran C, Maheswaran P A, Mohammed Ashraf T T and Nuncio M 2003 Propagation of tides in the Cochin estuarine system, southwest coast of India; Indian J. Mar. Sci. 32 14–24.
Taylor S R and McLennan S M 1985 The Continental Crust: Its Composition and Evolution (Oxford: Blackwell).
Tlig S and Steinberg M 1982 Distribution of rare earth elements (REE) in size fraction of recent sediments of the Indian Ocean; Chem. Geol. 37 317–333.
Warren B A 1982 The deep water of the central Indian basin; J. Mar. Res. 4 520–529.
Wehrli B and Stummm W 1989 Vanadyl in natural waters: Adsorption and hydrolysis promote oxygenation; Geochim. Cosmochim. Acta 53 69–77.
Wignall P B and Myers K J 1988 Interpreting the benthic oxygen levels in mudrocks: A new approach; Geology 16 452–455.
Wilde P, Quinby-Hunt M S and Brebd-Dieter Erdtmann 1996 The whole-rock cerium anomaly: A potential indicator of eustatic sea-level changes in shale of the anoxic facies; Sedim. Geol. 101 43–53.
Yamada M and Tsunogai S 1984 Post depositional enrichment of uranium in sediment from the Bering Sea; Mar. Geol. 54 263–276.
Zheng Y, Anderson R F, Van Geen A and Kuwabara J S 2000 Controls of authigenic Mo formation in marine sediment: Linkage to pore water sulphide; Geochim. Cosmochim. Acta 64 4165–4178.
Zheng Y, Anderson R F, Van Geen A and Fleisher M 2002 Remobilization of authigenic uranium from marine sediments by bioturbation; Geochim. Cosmochim. Acta 66 1759–1772.
Acknowledgements
The authors would like to thank the Captain and crew of FORV Sagar Sampada for their assistance in the cruise no. 255 during which the samples were taken. They gratefully acknowledge the facilities and support provided by the Director, School of Marine Sciences, Dean, Faculty of Marine Sciences and Head, Department of Chemical Oceanography, Cochin University of Science and Technology. The help offered by Dr. Balaram, Head Geochemistry division for ICP-MS analysis is highly appreciated. They also like to thank the Director, CMLRE, Kochi, Ministry of Earth Science, Govt. of India, for providing sample and giving travelling birth to cruise no. 255.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DEEPULAL, P.M., KUMAR, T.R.G. & SUJATHA, C.H. Behaviour of REEs in a tropical estuary and adjacent continental shelf of southwest coast of India: Evidence from anomalies. J Earth Syst Sci 121, 1215–1227 (2012). https://doi.org/10.1007/s12040-012-0223-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12040-012-0223-5