Abstract
Synthesis of a new nano hybrid of 5,10,15,20-mesotetra(4-aminophenyl) porphyrin (TAP) functionalized with multi-walled carbon nanotubes (MWCNTs) through an amide linkage is reported for the first time. This MWCNT-TAP hybrid was characterized by Raman, Fourier transform infrared (FT-IR), Transmission electron microscopy (TEM), Thermogravimetric analysis (TGA), absorption and emission spectroscopy. TGA analysis reveals that there is a \(\sim \)60% weight loss when heated from 150–750\(^{\circ }\mathrm{C}\), which is attributed to the amount of TAP molecules that were attached to MWCNTs. Electronic properties of MWCNTs were improved in the hybrid compared to raw MWCNTs as evidenced by Raman spectra. The absorption and emission spectra of TAP and nano-hybrid indicate strong positive solvatochromism with increasing solvent polarity. Fluorescence quenching of TAP in different solvents were observed in the emission spectra in the MWCNT-TAP hybrid, indicating that covalent functionalization facilitated effective energy or electron transfer from porphyrin moiety to the MWCNT.
Graphical Abstract
SYNOPSIS 5,10,15,20-mesotetra(4-aminophenyl) porphyrin (TAP) was covalently functionalized with MWCNTs through an amide linkage. Fluorescence quenching of TAP by MWCNTs were observed in different solvents. The absorption and emission spectra of this nano-hybrid exhibits strong positive solvatochromism, increasing with the solvent polarity.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon nanotubes (CNTs) have attracted vast attention in recent years owing to their exceptional electrical, chemical and mechanical properties, which make them excellent candidates for various applications.[1,2,3,4,5] Multiwalled carbon nanotubes (MWCNTs) are usually composed of a large number of concentric carbon nanotubes. For this reason, MWCNTs are considered as one of the best candidates among the carbon nanomaterials as a component of electron donor-acceptor (D-A) ensembles.[6, 7] Therefore, significant effort has been devoted to the chemical functionalization of MWCNTs, mainly to enhance their solubility and to improve their compatibility in MWCNT-based nanohybrid systems.[8,9,10] Linking of electron donor such as porphyrin chromophore to MWCNT as acceptor (D-A) through a covalent bond is expected to facilitate the development of hybrid materials with improved solubility and photoelectronic properties through a synergistic effects. [11,12,13] Porphyrins are thermally stable, light harvesting molecules that show excellent nonlinear optical properties owing to their extensive \(\uppi \)-electron delocalization. Incorporation of photoexcited electron donors, such as porphyrins or metal phthalocyanine macrocyles, with carbon nanomaterials would constitute an ideal candidate for photovoltaic and fuel cell applications.[10, 12, 14] In the last few years, numerous studies have been reported in the literature on non-covalent porphyrin-nanocomposites formed due to \(\uppi \)-\(\uppi \) and van der Waals forces for various molecular photoelectronic applications. [15,16,17,18,19] Nevertheless, studies involving covalent incorporation of porphyrin chromophores with carbon nanomaterials are limited.[20,21,22,23,24,25] Moreover, covalently functionalized porphyrin with carbon nanomaterials have two disadvantages; first one is low degree of porphyrin functionalization on the surface of CNTs, and another is that the structure of CNTs is badly damaged during the carboxylation of MWCNTs surface by strong oxidative acid treatment. On the other hand, the reports on the functionalization of CNTs with porphyrin molecules to surmount these two weaknesses all together are scarce. [21] Most of the porphyrin-linked carbon nanomaterials reported to date contain only unsubstituted mesotetraphenyl porphyrin (TPP).[21, 26,27,28] In this paper, we report the synthesis of a new nanohybrid of MWCNT that has been covalently functionalized with 5,10,15,20-mesotetra(4-aminophenyl) porphyrin (TAP) through an amide linkage. TAP has more potential compared to TPP as it has free amino groups (electron donating) that can enhance the optical properties of the nanohybrid. Synthesized MWCNT-TAP has been characterized by thermogravimetric analysis (TGA), Transmission electron microscopy (TEM), Raman, Fourier transform infrared (FT-IR), absorption and emission spectroscopy.
2 Experimental
2.1 Materials and characterization
Pyrrole, triethylamine, p-nitrobenzaldehyde, and tin(II) chloride dihydrate were purchased from Sigma Aldrich. Pyrrole was purified by vacuum distillation. Other reagents such as chloroform \((\hbox {CHCl}_{3})\), dry tetrahydrofuran (THF, Finar) and N, N- dimethyl formamide (DMF) were purified by using standard procedures. MWCNTs were purchased from Yunnan Great (Group) Co., Ltd. (China). Carboxyl functionalized MWCNTs (MWCNT–COOH) and TAP were prepared by using the literature procedure.[20, 29] Absorption spectrum was measured with SHIMADZU, UV-1800 and SHIMADZU UV-2600 spectrophotometers. Fourier transform infrared spectrum (FTIR) was taken using the KBr pellet method on a Thermo Nicolet, iS10 FTIR spectrophotometer. Raman spectrum was measured using micro Raman spectroscopy (Princeton instrument Acton sp2500). Fluorescence spectrum was recorded using a SHIMADZU RF-6000 computer aided spectrofluorophotometer. Quantum yield of the substances were measured in DMF at 20\(^{\circ }\mathrm{C}\), using 1 cm x 1 cm quartz optical cells under aerobic conditions. For measurements of quantum yields (\(\Phi _{\mathrm{F}})\), TPP in DMF was used as reference (\(\Phi _{\mathrm{F}} = 0.11)\).[30] Thermogravimetric analysis (TGA) was performed using SDT Q600 simultaneous DSC-TGA with a heating rate of 5\(^{\circ }\mathrm{C/min}\) under \(\hbox {N}_{2}\) purge (100 mL/min). JEOL JEM 2100 high resolution transmission electron microscopy (HR-TEM) instrument was used to record the TEM images of nanohybrid.
2.2 Synthesis of TAP–NHCO-MWCNT nanohybrid
MWCNT–COOH (100 mg) was refluxed at 65\(^{\circ }\mathrm{C}\) for 24 h with \(\hbox {SOCl}_{2 }\)(75 mL) under argon atmosphere in the presence of DMF (1.8 mL) to form acyl chloride functionalized MWCNT (MWCNT–COCl). Excess \(\hbox {SOCl}_{2 }\) was removed by vacuum distillation and the remaining residue was washed with dry THF. MWCNT–COCl (100 mg) and TAP (100 mg) were dissolved in DMF (40 mL). Further this mixture was refluxed at 130\(^{\circ }\mathrm{C}\) for 72 h in presence of 1 mL triethylamine (\(\hbox {Et}_{3}\hbox {N}\)) under argon atmosphere. The resultant solution was cooled to room temperature. Excess DMF was removed by using rotary evaporator. 100 mL of ether was added into the reaction mixture in order to precipitate the product. The residue was separated through ultracentrifugation. Finally, the precipitate was purified through three washing cycles each consisting of ultra centrifugation in THF, followed by filtration through nylon filter membrane in order to remove excess TAP and other impurities from the residue. The resultant product was washed with \(\hbox {CHCl}_{3 }\) by following the above procedure and \(\hbox {Et}_{3}\hbox {N}\).HCl was removed from the nanohybrid by washing it with water and it was dried under vacuum.
3 Results and Discussion
3.1 Synthesis of materials
Carboxyl-functionalized MWCNT (MWCNT–COOH) was synthesized by sonicating MWCNT with concentrated solution of \(\hbox {HNO}_{3}/\hbox {H}_{2}\hbox {SO}_{4}\) (1:3 ratio, v/v) at 60\(^{\circ }{\mathrm{C}}\) for 4 h according to a slightly modified literature procedure.[29] Further, this was stirred for 9 h at room temperature. The resultant MWCNT dispersion was filtered through a nylon filter membrane. The final residue was continuosly washed using ultrapure water (18 \(\hbox {M}\Omega \), Milli-Q, Millipore) until the pH of the filtrate was 7 and then dried overnight in a oven at 80\(^{\circ }{\mathrm{C}}\). The synthesis of TAP was carried out by reducing 5,10,15,20-mesotetra(4-nitrophenyl) porphyrin (TNP) using the literature procedure and further purified by soxhlet extraction.[20] Precursor TNP was synthesized from 4-nitrobenzaldehyde and pyrrole by using Alder’s method.[31] TAP (100 mg) was covalently linked to MWCNT through an amide bond by refluxing it with MWCNT–COCl (100 mg) at 130\(^{\circ }{\mathrm{C}}\) for 72 h in DMF solvent under argon in the presence of triethylamine (\(\hbox {Et}_{3}\hbox {N}\)) as shown in the Scheme 1. Though there are four free \(\hbox {NH}_{2}\) groups in TAP, not all could form amide bond with MWCNT, may be due to the tubular nature of the MWCNT. This was confirmed by us by doing the independant experiments with less quantity of TAP (30 mg), we ended up with the poor solubility of the resultant MWCNT-TAP nanohybrid. This may be due to the low degree of functionalization of TAP molecules with MWCNT.
3.2 Spectroscopic and thermal studies
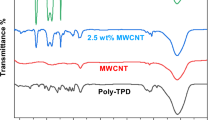
Figure 1 shows the chemical structure of nanohybrid. MWCNT–TAP hybrid was characterized by FT-IR spectrum in the range of 500–4000 \(\hbox {cm}^{-1}\) as shown in Figure 2. In the FT-IR spectrum, MWCNT–COOH has characteristic absorption peak at \(3414\;\hbox {cm}^{-1}\) which corresponds to the O–H vibration frequency. Carbonyl (C=O) stretching vibration of the COOH group appears at \(1709\;\hbox {cm}^{-1}\). Three new striking peaks appeared in the hybrid, after covalent functionalization with porphyrin, at \(1638\;\hbox {cm}^{-1}\), \(1211\;\hbox {cm}^{-1 }\) and \(1597\;\hbox {cm}^{-1}\) which correspond to the formation of C=O, C–N of amide linkage (–CO–NH), and the stretching vibration frequency of porphyrin C=C, respectively.[27, 32] TAP shows two N-H stretching frequencies at \(3336\;\hbox {cm}^{-1}\) and \(3428\;\hbox {cm}^{-1}\) due to N-H stretching of amino group (-\(\hbox {NH}_{2})\) and porphyrin NH group whereas MWCNT-TAP hybrid shows a single broad band at \(3438\;\hbox {cm}^{-1}\) owing to the merging of all three N-H stretchings ((–CO–NH, -\(\hbox {NH}_{2}\) and porphyrin NH groups). These changes in the FT-IR spectra clearly indicate the formation of covalently connected MWCNT–TAP nano hybrid.
TGA curves of MWCNT-TAP, MWCNT-COOH, pure MWCNT and TAP are shown in the Figure 3. The TGA curve of MWCNT–COOH indicates that it is a multi step processes as reported in the literature.[33] The weight loss detected in the range of 50–180\(^{\circ }{\mathrm{C}}\) is attributed to the elimination of physisorbed water on the MWCNT–COOH surface (weight loss of 8%). Further thermal decomposition is observed between 220–520\(^{\circ }{\mathrm{C}}\) (weight loss of 19%). Such a weight loss may be attributed to the decarboxylation, decomposition of –OH that are bound on the surface of MWCNT–COOH, degradation of disordered or amorphous carbon and other metal impurities.[34] The TGA curve of MWCNT-TAP shows that \(\sim \)60% weight loss when heated from 150–750\(^{\circ }{\mathrm{C}}\),[35] whereas the loss is much less <20% for TAP and MWCNT, individually.
Figure 4 shows the Raman spectra of MWCNT, MWCNT–COOH and MWCNT–TAP. All these materials display characteristic G and D bands, with an \(\hbox {I}_{\mathrm{D}}/\hbox {I}_{\mathrm{G}}\) ratio of 0.93, 1.06 and 0.80, respectively.[21] The D band reveals the presence of some disorder in the carbon nano structure. This band is frequently referred to as the defect band. Its intensity relative to that of G band is often used to determine the quality of nanotubes. MWCNT–COOH exhibited enhancement of the D peak compared to the MWCNT. Nevertheless, after functionalization with TAP, the intensity of the D band was significantly reduced compared with pure MWCNT. This indicates that the electronic properties of TAP-functionalized MWCNT were improved in the nanohybrid. Raman spectra of the MWCNT and its hybrid exhibit a band at \(2700\;\hbox {cm}^{-1}\) (G’ band) which is characteristic band of multi-walled carbon nanotube.[36] G peak position of the MWCNT–TAP nanohybrid (1575 \(\hbox {cm}^{-1})\) exhibits a shift to lower frequency (\({\approx }7\;\hbox {cm}^{-1})\) compared with MWCNT (\(1582\;\hbox {cm}^{-1})\), whereas MWCNT–COOH (\(1600\;\hbox {cm}^{-1})\) exhibits a higher frequency shift (\({\approx }8\;\hbox {cm}^{-1})\) compared with MWCNT. In general, hybrid carbon nanomaterials exhibit this type of low frequency shift (softening) when functionalized with an electron donor molecule. It exhibits high frequency shift (stiffening) when hybridized with electron-acceptor molecule.[37] Lower frequency shift in MWCNT–TAP hybrid confirms the charge transfer between TAP and MWCNT, where TAP acts as an electron donor and MWCNT acts as an electron acceptor. Higher frequency shift in MWCNT–COOH compared with MWCNT is possibly owing to the presence of electron withdrawing carboxyl group. This shift reveals the functionalization of MWCNTs with TAP.
Figure 5 shows UV-Vis absorption spectra for TAP and MWCNT–TAP nanohybrid in the range of 300 to 800 nm in DMF solvent. TAP reveals a strong Soret band at 436 nm and weak Q-bands between 500–700 nm. MWCNT–TAP shows a blue shift in the Soret band (429 nm) as compared to TAP. This result supports the covalent functionalization of TAP with MWCNT. Noncovalently attached porphyrins on MWCNTs do not cause shift or broadening of the Soret band.[27] We observed that improved solubility in the case of MWCNT–TAP as compared to GO–TAP. The solutions of TAP, MWCNT–COOH, and MWCNT–TAP in DMF solvent are shown in Figure 6 (under visible light and long UV light 365 nm). It reveals that dispersion of MWCNT–COOH in DMF solvent whereas MWCNT–TAP was completely soluble in DMF solvent. Solution phase UV-Vis-NIR spectra were recorded to demonstrate a linear relationship between the absorbance and concentration of MWCNT and MWCNT-TAP. We have not observed Van Hove singularities (VHS) as in the case of SWCNTs. This finding is similar to the literature report.[38] MWCNT shows a peak at 265 nm in DMF solvent due to \(\uppi \)-\(\uppi \)* transition.[38] The absorbance at 268 nm and 429 nm of MWCNT-TAP was plotted against concentration to get a standard curve (see Figure S1 in Supporting Information, inset A and B, respectively, in mg \(\hbox {L}^{-1})\). Similarly, we plotted the absorbance at 265 nm of MWCNT against concentration to get a standard curve as shown in Figure S2 (Supporting Information). We estimated the effective extinction coefficient of MWCNT and TAP in MWCNT-TAP from the slope of the linear least-squares fit to be \(0.025\;\hbox {L}\;\hbox {mg}^{-1}\;\hbox {cm}^{-1}\) and \(0.1102\;\hbox {L}\;\hbox {mg}^{-1}\;\hbox {cm}^{-1}\), respectively, with an \(\hbox {R}^{2}\) value of 0.998. Similarly, we found that the extinction coefficient of MWCNT from the slope of the linear least-squares fit to be \(0.060\;\hbox {L}\;\hbox {mg}^{-1}\;\hbox {cm}^{-1}\) with an \(\hbox {R}^{2}\) value of 0.999. Absorption coefficient of MWCNT varies due to different excited states.[27] This method shows the much enhanced solubility. We estimated the amount of TAP in the MWCNT-TAP nanohybrid from the absorbance value (A = 0.72) and the extinction coefficient of TAP in DMF solvent. The amount of TAP in 0.5 mg/100 mL of MWCNT-TAP solution is 0.318 mg. That is approximately 64% of hybrid mass is due to TAP. Therefore, the ratio of TAP to MWCNT in the hybrid is 1.7:1 (g/g).
The Soret band peak intensity of the MWCNT–TAP hybrid was suppressed compared to that of pure TAP. This result indicates that there is a charge transfer between TAP and MWCNT in the nanohybrid. This observation is well in accord with the Raman spectra. In order to confirm this, we have done detailed absorption studies in different solvents such as DMSO, DMF, methanol, DCM and THF. MWCNT-TAP nanohybrid shows good solubility in polar solvents such as DMSO, DMF, methanol and acetonitrile. Whereas, it is slightly less soluble in nonpolar solvents such as DCM, THF and ethyl acetate. However, after sonication it disperses well. We observed a bathochromic (or red) shift for both TAP and MWCNT-TAP in the absorption band with increasing solvent polarity as shown in Figure S3 (Supplementary Information). This type of bathochromic or red shift with increasing solvent polarity is usually termed as ‘positive solvatochromism’.[39] This shift is caused by differential solvation of the ground and first excited state of the chromophore. Positive solvatochromism will result if the excited state of the molecule is better stabilized by increasing the solvent polarity compared to the ground state. The Soret band absorption shifts for TAP and MWCNT-TAP with increasing solvent polarity are tabulated in Table 1. Though methanol is more polar than THF and DCM, it shows blue or hypsochromic shift in owing to the ability of forming hydrogen bond with the methanol.
The covalently linked porphyrins on carbon nanomaterials can be employed as energy transporting antennae. Photoluminescent (PL) spectra of TAP and MWCNT–TAP nanohybrid in DMF solvent are shown in Figure 7. The solution of MWCNT–TAP nanohybrid exhibits \(\sim \)65% fluorescent quenching of emission band at 693 nm as compared to that of pure TAP, upon excitation at 450 nm. This peak is red shifted as compared to the reported porphyrin-functionalized carbon nanomaterials.[21, 22] This red shift is owing to the presence of electron donating amino group in the TAP moiety of MWCNT-TAP nanohybrid. Further, the substantial quenching confirms the covalent linking of porphyrins to carbon nanomaterials. This in turn substantiates the electron transfer from the singlet excited porphyrin to MWCNT.[22] Upon excitation at different wavelengths (400, 430, and 550 nm), MWCNT-TAP exhibits similar quenching as shown in Figures S4–S6 (Supplementary Information) which also supports the electron transfer between TAP and MWCNT in nanohybrid. In order to confirm the electron-transfer between TAP and MWCNT in nanohybrid, we carried out detailed photoemission studies in different solvents such as DMSO, DMF, methanol and DCM at different excitation wavelengths (430 and 450 nm). Similar quenching was observed for MWCNT-TAP in all these solvents as in DMF. DMSO exhibits 46% quenching whereas methanol and DCM exhibit 35% and 25%, respectively, as shown in the Figure 8. Further, we observed bathochromic shift with increasing the solvent polarity, except in methanol which shows a blue shift. Generally, this kind of red shift is also termed as ‘strong positive solvatochromism’ which occurs in the dye molecules due to a large change in their permanent dipole moment upon excitation. A positive solvatochromism occurs when the solute dipole moment is higher in the excited state than in the ground state. The compounds with this particular type of behavior exhibit inter- or intramolecular charge transfer absorption. [39] Apart from this dipole moment, the ability of a molecule to make hydrogen bonds with solvent molecules in its ground and excited states determines the extent and sign of solvatochromism. This is the reason for the blue shift in the emission and absorption spectra for both TAP and MWCNT-TAP in methanol. Blue shift in methanol is more pronounced in the absorption spectra compared to emission spectra. Quantum yield (\(\Phi _{\mathrm{F}})\) for the TAP and MWCNT-TAP were measured using TPP as reference at 20\(^{\circ }\hbox {C}\); the measurement details are given in the Supporting Information. We found that the \(\Phi _{\mathrm{F}}\) for TAP is 0.19 whereas the \(\Phi _{\mathrm{F}}\) of MWCNT-TAP nanohybrid is 0.15. The decrease in the \(\Phi _{\mathrm{F}}\) is owing to the charge transfer between TAP and MWCNT in the hybrid.
3.3 Morphology of MWCNTs and MWCNT-TAP
The surface morphology of MWCNT and MWCNT–TAP were studied using HR-TEM images as shown in Figures S7 and S8 (Supplementary Information). These images show the typical surface morphology of MWCNT before and after TAP functionalization. The relatively clear and smooth images of MWCNT surface became bumpy after TAP functionalization as shown in the Figure S8 (b) (Supplementary Information).
4 Conclusions
5,10,15,20-mesotetra(4-aminophenyl) porphyrin (TAP) was covalently functionalized with MWCNT through an amide linkage. The chemical structure of MWCNT-TAP hybrid was confirmed by FT-IR spectroscopy. TGA curve of MWCNT-TAP reveals that there is a \(\sim \)60% weight loss in the range of 150–750\(^{\circ }\hbox {C}\), which is attributed to the amount of TAP molecules that were attached to MWCNTs. The Raman spectroscopy indicates that the intensity of D band in MWCNT-TAP was significantly reduced compared with raw MWCNTs which reveal the improved electronic properties of MWCNTs in the nanohybrid. peak position of G band of the MWCNT–TAP nanohybrid exhibits a lower frequency shift compared with MWCNT. This lower frequency shift in the MWCNT–TAP hybrid confirms the charge transfer between TAP and MWCNT, which is very significant for high photocatalytic activity. Furthermore, emission spectra of nanohybrid show that there is fluorescence quenching of TAP by MWCNT in various solvents. This fluorescence quenching is due to the covalent functionalization of TAP with MWCNT molecules in the nano hybrid that facilitates the effective energy or electron transfer between porphyrin and MWCNT. The absorption and emission spectra of TAP and nanohybrid indicate strong positive solvatochromism with increasing solvent polarity. We found that the \(\Phi _{\mathrm{F}}\) of TAP is 0.19 whereas the \(\Phi _{\mathrm{F}}\) of MWCNT-TAP nanohybrid is 0.15. The decrease in the \(\Phi _{\mathrm{F}}\) is owing to the charge transfer between TAP and MWCNT in the hybrid.
References
Ajayan P M 1999 Nanotubes from Carbon Chem. Rev. 99 1787
De Volder M F L, Tawfisk S H, Baughman R H and Hart A J 2013 Carbon Nanotubes: Present and Future Commercial Applications Science 339 535
Baughman R H, Zakhidov A A and de Heer W A 2002 Carbon Nanotubes—the Route Toward Applications Science 297 787
Wang F, Gu H and Swager T M 2008 Carbon Nanotube/Polythiophene Chemiresistive Sensors for Chemical Warfare Agents J. Am. Chem. Soc. 130 5392
Kong J, Franklin N R, Zhou C, Chapline M G, Peng S, Cho K and Dai H 2000 Nanotube Molecular Wires as Chemical Sensors Science 287 622
Guldi D M, Rahman G M A, Jux N, Balbinot D, Tagmatarchis N and Prato M 2005 Multiwalled carbon nanotubes in donor–acceptor nanohybrids—towards long-lived electron transfer products Chem. Commun. 15 2038
Ehli C, Guldi D M, Herranz M A, Martín N, Campidelli S and Prato M 2008 Pyrene-tetrathiafulvalene supramolecular assembly with different types of carbon nanotubes J. Mater. Chem. 18 1498
Lu W, Li N, Chen W and Yao Y 2009 The role of multiwalled carbon nanotubes in enhancing the catalytic activity of cobalt tetraaminophthalocyanine for oxidation of conjugated dyes Carbon 47 3337
Rayati S and Sheybanifard Z 2016 Catalytic activity of Mn(III) and Fe(III) porphyrins supported onto multi-walled carbon nanotubes in the green oxidation of organic dyes with hydrogen peroxide: A comparative study J. Iran Chem. Soc. 13 541
Wang X, Wang B, Zhong J, Zhao F, Han N, Huang W, Zeng M, Fan J and Li Y 2016 Iron polyphthalocyanine sheathed multiwalled carbon nanotubes: A high-performance electrocatalyst for oxygen reduction reaction Nano Res. 9 1497
Jin J, Dong Z, He J and Li R 2009 Synthesis of Novel Porphyrin and its Complexes Covalently Linked to Multi-Walled Carbon Nanotubes and Study of their Spectroscopy J. Ma. Nanoscale Res. Lett. 4 578
Reddy A L M, Rajalakshmi N and Ramaprabhu S 2008 Cobalt-polypyrrole-multiwalled carbon nanotube catalysts for hydrogen and alcohol fuel cells Carbon 46 2
Wang A J, Fang Y, Long L L, Song Y L, Yu W, Zhao W, Cifuentes M P, Humphery M G and Zhang C 2013 Facile synthesis and enhanced nonlinear optical properties of porphyrin-functionalized multi-walled carbon nanotubes Chem. Eur. J. 15 3882
Stylianakis M M, Konios D, Kakavelakis G, Charalambidis G, Stratakis E, Coutsolelos A G, Kymakis E and Anastasiadis S H 2015 Efficient ternary organic photovoltaics incorporating grapheme -based porphyrin molecule as a universal electron cascade material Nanoscale 7 17827
Rahman G M A, Guldi D M, Campidelli S and Prato M 2006 Electronically interacting single wall carbon nanotube–porphyrin nanohybrids J. Mater. Chem. 16 62
Li H, Zhou B, Lin Y, Gu L R, Wang W, Fernando K A S, Kumar S, Allard L F and Sun Y P 2004 Selective Interactions of Porphyrins with Semiconducting Single-Walled Carbon Nanotubes J. Am. Chem. Soc. 126 1014
Murakami H, Nomura T and Nakashima N 2003 Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites Chem. Phys. Lett. 378 481
Chen J Y and Collier C P 2005 Noncovalent Functionalization of Single-Walled Carbon Nanotubes with Water-Soluble Porphyrins J. Phys. Chem. B 109 7605
Hasobe T, Fukuzumi S and Kamat P V 2005 Ordered Assembly of Protonated Porphyrin Driven by Single-Wall Carbon Nanotubes. J- and H-Aggregates to Nanorods J. Am. Chem. Soc . 127 11884
Yamuna R, Ramakrishnan S, Dhara K, Devi R, Kothurkar N K, Kirubha E and Palanisamy P K 2013 Synthesis, characterization, and nonlinear optical properties of graphene oxide functionalized with tetra-amino porphyrin J. Nanopart. Res. 15 1
Jin J, Dong Z, He J and Li R 2009 Synthesis of Novel Porphyrin and its Complexes Covalently Linked to Multi-Walled Carbon Nanotubes and Study of their Spectroscopy Nanoscale Res. Lett. 4 578
Baskaran D, Mays J W, Zhang X P and Bratcher M S 2005 Carbon Nanotubes with Covalently Linked Porphyrin Antennae: Photoinduced Electron Transfer J. Am. Chem. Soc. 127 6916
Moghadam M, Baltork I M, Tangestaninejad S, Mirkhani V, Kargar H and Isfahani N Z 2009 Manganese(III) porphyrin supported on multi-wall carbon nanotubes: A highly efficient and reusable biomimetic catalyst for epoxidation of alkenes with sodium periodate Polyhedron 28 3816
Xu Y, Liu Z, Zhang X, Wang Y, Tian J, Huang Y, Ma Y, Zhang X and Chen Y 2009 A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property Adv. Mater. 21 1275
Li H, Martin R B, Harruff B A, Carino R A, Allard L F and Sun Y P 2004 Single-Walled Carbon Nanotubes Tethered with Porphyrins: Synthesis and Photophysical Properties Adv. Mater. 16 896
Krishna M B M, Kumar V P, Venkatramaiah N, Venkatesan R and Rao D N 2011 Nonlinear optical properties of covalently linked graphene-metal porphyrin composite materials Appl. Phys. Lett. 98 081106-1
Guo Z, Du F, Ren D, Chen Y, Zheng J, Liu Z and Tian J 2006 Covalently porphyrin-functionalized single-walled carbon nanotubes: a novel photoactive and optical limiting donor–acceptor nanohybrid J. Mater. Chem. 16 3021
Liu Z B, Tian J G, Guo Z, Ren D M, Du F, Zheng J Y and Chen Y S 2008 Enhanced Optical Limiting Effects in Porphyrin-Covalently Functionalized Single-Walled Carbon Nanotubes Adv. Mater. 20 511
Goyanes S, Rubiolo G R, Salazar A, Jimeno A, Corcuera M A and Mondragon I 2007 Carboxylation treatment of multiwalled carbon nanotubes monitored by infrared and ultraviolet spectroscopies and scanning probe microscopy Diamond Relat. Mater. 16 412
Silva S, Pereira P M R, Silva P, Paz F A A, Faustino M A F, Cavaleiro J A S and Tome J P C 2012 Porphyrin and phthalocyanine glycodendritic conjugates: Synthesis, photophysical and photochemical properties Chem. Commun. 48 3608
Adler A D, Longo F R, Finarelli J D, Goldmacher J, Assour J and Korsakoff L 1967 A Simplified Synthesis for meso-Tetraphenylporphin J. Org. Chem. 32 476
Qu K, Xu H, Zhao C, Ren J and Qu X 2011 Amine-linker length dependent electron transfer between porphyrins and covalent amino-modified single-walled carbon nanotubes RSC Adv. 1 632
Tan J M, Karthivashan G, Arulselvan P, Fakurazi S and Hussein M Z 2014 Characterization and in vitro studies of the anticancer effect of oxidized carbon nanotubes functionalized with betulinic acid Drug Des. Devel. Ther. 8 2333
Datsyuk V, Kalyva M, Papagelis K, Partenios J, Tasis D and Siokou A 2008 Chemical oxidation of multiwalled carbon nanotubes Carbon 46 833
Karousis N, Sandanayaka A S D, Hasobe T, Economopoulos S P, Sarantopouloua E and Tagmatarchis N 2011 Graphene oxide with covalently linked porphyrin antennae: Synthesis, characterization and photophysical properties J. Mater. Chem. 21 109
Zdrojek M, Gebicki W, Jastrzebski C, Melin T and Huczko A 2004 Studies of multiwall carbon nanotubes using Raman spectroscopy and atomic force microscopy Solid State Phenomena 99 1
Chen Y, Huang Z H, Yue M and Kang F 2014 Integrating porphyrin nanoparticles into a 2D graphene matrix for free-standing nanohybrid films with enhanced visible-light photocatalytic activity Nanoscale 6 978
Han T K, Fen L B, Nee N M, Ahmad R and Johan M R 2011 Optical studies on multiwalled carbon nanotubes via modified Wolff-Kishner reduction process Adv. Mater. Res. 194-196 618
Reichardt C 1994 Solvatochromic Dyes as Solvent Polarity Indicators Chem. Rev. 94 2319
Acknowledgements
We gratefully acknowledge the financial support from COE-AMGT (MHRD, New Delhi) for this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prabhavathi, G., Arjun, M. & Yamuna, R. Synthesis, characterization and photoluminescence properties of tetra(aminophenyl) porphyrin covalently linked to multi-walled carbon nanotubes. J Chem Sci 129, 699–706 (2017). https://doi.org/10.1007/s12039-017-1295-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1295-1