Abstract
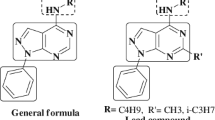
A series of 10 derivatives of 5-(5-amino-1,3,4-thiadiazole-2-yl)-3,4-dihydro-6-methyl-4-phenyl-pyrimidin-2(1H)-one and 10 derivatives of 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-phenyl pyrimidin-2(1H)-one have been synthesized. Among the synthesized derivatives, triazole substituted compounds have shown higher antibacterial inhibition when compared to the thiadiazole derivatives. All the structures of the newly synthesized compounds have been characterized by IR, 1H and 13C NMR, GC-MS and CHN analysis. Most of the compounds have shown promising antibacterial activity when compared with the standard drug ciprofloxacin.

A series of ten derivatives of 5-(5-amino-1,3,4-thiadiazole-2-yl)-3,4-dihydro-6-methyl-4-phenylpyrimidin-2(1H)-one and ten derivatives of 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-phenyl pyrimidin-2(1H)-one have been synthesized and structures characterized. Among the synthesized derivatives, triazole substituted compounds have shown higher antibacterial inhibition when compared to the thiadiazole derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pyrimidine and its derivatives have a wide variety of applications in different fields, prominently for biological treatment process,[1‐8]such as antitumor, anticancer, anti-inflammatory, anti-hypertensive, antibacterial and antifungal agents, etc. The five-membered heterocyclic ring substituted in pyrimidine derivatives have moderate inhibition against the bacterial species.[9] In order to improve the antibacterial activity, we have synthesized twenty pyrimidine derivatives.
The pyrimidine and its derivatives have been synthesized by using various methods. In continuation of this work, novel pyrimidine derivatives were synthesized by using the reported procedure for the first step.[10,11]These pyrimidine derivatives have a large number of reactive sites to produce the substituted derivatives.[12‐18]
Herein, the reaction was carried out only in the 5 th position of the pyrimidine ring, because the ethanolic group which is present in the position 5 is easily removed by boiling in presence of catalyst and hydrazine carbothioamide.[19,20] The synthesized 10 derivatives of 5-(5-amino-1, 3, 4-thiadiazole-2-yl)-3,4-dihydro-6-methyl-4-phenyl-pyrimidin-2(1H)-one and 10 derivatives of 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-phenylpyrimidin-2(1H)-one are subjected to in vitro study of antibacterial activities by using three different species namely, Pseudomonas aeruginosa (Gram –ve), Staphylococcus aureus (Gram + ve) and Escherichia coli (Gram –ve). The inhibition values are compared with standard drug, ciprofloxacin. All the synthesized compounds were characterized by using elemental analysis, mass spectra, 1H and 13C NMR spectra.
2 Experimental
Melting points were determined using open capillary method and are uncorrected. The compounds are checked for homogeneity by TLC on silica gel-G using pet ether and ethyl acetate as eluent in 3:5 ratio. The IR spectra were recorded on FT-IR THERMO NICOLET AVATAR 370 spectrometer using KBr disc. The 1H and 13C NMR were recorded on Bruker Avance-III 400 MHz - NMR spectrometer using DMSO- d 6. Elemental analyses were recorded on elemental vario EL III instrument. The mass spectra were recorded on Joel GC-mate spectrometer. All the compounds gave satisfactory micro analytical results.
2.1 General Procedure
The synthesize 5-(hydrazine carbothioamide)-3,4-dihydro-6-methyl-4-phenyl pyrimidin-2(1H)-one (Scheme 1) 2(a–j), an equimolar mixture of compound 1a (2.61 g, 0.01 mol) and thiosemicarbazide (0.91 g, 0.01 mol) in acetone was refluxed for 10–12 h and allowed to cool. The yellow crude solid was purified by recrystallization from alcohol. M.p.: 139–141 ∘C. [Yield: 2.4 g, 82%] Analysis: Calculated (%) for C13 H 15 O 2 N 5S: C, 51.17; H, 4.94; N, 22.50; S, 10.47. Found (%): C, 51.10; H, 4.85; N, 22.24; S, 10.94. GCMS: m/z 305 [M +]. FT-IR (KBr, cm −1): 3365, 3241, 3116 (NH), 3079 (Ar–H), 2978 (CH), 1724 (C =O), 1385 (C–N), 1219 (C =S), 1089 (N–N). 1H NMR (400 MHz, DMSO- d 6): δ 2.251 (s, 3H), 5.152 (d, J = 3.2 Hz, 1H), 6.501 (s, 2H), 7.213–7.336 (m, 5H), 7.702 (d, J = 2.8 Hz, 1H), 8.175 (d, J = 6.4 Hz, 2H,), 9.149 (s, 1H). 13C NMR (400 MHz, DMSO- d 6): δ 17.72, 59.17, 99.33, 126.21, 127.23, 128.34, 148.25, 151.71, 152.16, 165.33, 178.40.
2.2 General procedure for synthesis of 5-(5-amino-1, 3, 4-thiadiazole-2-yl)-3, 4-dihydro-6-methyl-4-phenyl- pyrimidin-2(1H)-one (Scheme 2), 3(a–j)
Hydrazine carbothioamide 2a (3.05 g, 0.01 mol) was dissolved in 5 mL conc. H2 SO 4. This solution was stirred at RT and left overnight. It was then poured in crushed ice. The resulting suspension was kept in ammoniacal water for 2 h, filtered and purified by recrystallization from alcohol as white crystals. M.p.: 174–176 ∘C. [Yield: 2.7 g; 81%]. 1H NMR (400 MHz, DMSO- d 6): δ 2.258 (s, 3H), 4.004 (s, 2H), 5.159 (d, J = 3.2 Hz, 1H), 7.227–7.347 (m, 5H), 7.701 (d, J = 2 Hz, 1H), 9.151 (s, 1H). 13C NMR (400 MHz, DMSO- d 6): δ 17.03, 59.15, 99.30,126.20, 127.20, 128.34, 144.82, 148.27, 152.10, 165.32. FT-IR (KBr, cm −1): 3354, 3227, 3110 (NH), 3027 (Ar–H), 2976 (CH), 1689 (C =O), 1460 (C =N), 1225 (C–S), 1378 (C–N), 1098 (N–N). GCMS: m/z 287 [M +]. Analysis: Calculated (%) for C13 H 13 ON 5S: C, 54.38; H, 4.56; N, 24.39; S, 11.13. Found (%): C, 54.35; H, 4.56; N, 24.64; S, 11.68.
2.3 General procedure for Synthesis of 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-phenyl pyrimidin-2(1H)-one (Scheme 3) 4(a–j)
The carbothioamide 2a (3.05 g, 0.01 mol) was added into (8 g in 100 mL) 8% NaOH; it was refluxed for 4 h. The reaction mixture was cooled to room temperature and acidified with dilute acetic acid, then filtered and washed well with water and purified by recrystallization from alcohol as shiny crystals. M.p.:119–121 ∘C, [Yield: 2.42 g; 80%]. 1H NMR (400 MHz, DMSO- d 6): δ 2.304 (s, 3H), 3.217 (s, 1H), 5.507 (d, J = 3.6 Hz, 1H), 6.975 (s, 1H), 7.268–7.338 (m, 5H), 7.766 (d, J = 2.4 Hz, 1H), 9.217 (s, 1H). 13C NMR (400 MHz, DMSO- d 6): δ 17.74, 59.14, 99.24, 126.22, 127.21, 128.33, 144.84, 148.29, 152.15, 155.11, 165.32. FT-IR (KBr, cm −1): 3423 (NH), 3027 (Ar–H), 2968 (CH), 2235 (SH), 1654 (C =O), 1590 (C =N), 1373 (C–N), 1057 (N–N). GCMS: m/z 287 [M +]. Analysis: Calculated (%) for C13 H 13 ON 5S: C, 54.38; H, 4.56; N, 24.39; S, 11.13. Found (%): C, 54.41; H, 4.22; N, 24.35; S, 11.53.
3 Results and Discussion
Compounds 3(a–j) and 4(a–j) were synthesized as per the Schemes 1, 2 and 3. The final compound 3a was prepared by the reaction of hydrazine carbothioamide 2a, conc.H2 SO 4 and NH3, whereas 4a was prepared by refluxing hydrazine carbothioamide 2a with NaOH. Hydrazine carbothioamide 2a was synthesized by reaction of pyrimidine ethyl ester 1 with thiosemicarbazide in acetone, followed by condensation reaction.
The pyrimidine ethyl ester 1a was prepared by the reaction of benzaldehyde, ethylacetoacetate and urea or thiourea in the presence of mineral acid, followed by Biginelli reaction. The structures of the synthesized compounds were confirmed by IR, 1H and 13C NMR, GC-MS and CHN analysis. Formation of 2a was confirmed by the presence of N–H stretching peaks at 3365, 3241 cm −1 and 3116 cm −1 and C =O stretching peaks at 1724 cm −1 in IR and singlet at δ 6.50 for NH2 group in 1H NMR spectrum.
Treatment of compound 2a with conc. H2 SO 4 and NH3, furnished 5-(5-amino-1,3,4-thiadiazol-2-yl)-3,4-dihydro-6-methyl-4-phenyl pyrimidin-2(1H)-one (3a). The structure of 3a was elucidated on the basis of C-S linkage in the thiadiazole ring, which causes a sharp absorption band at 1225 cm −1 in its IR spectrum. 1H NMR spectrum shows a singlet at δ 4.00 due to NH2 functional group of the compound 3a. The mechanism for compounds 3(a–j) is shown in Scheme 4.
The IR and 1H NMR spectral data reveal that the carbonyl absorption band at 1689 cm −1 of NH–CO–NH group, N–N stretching band at 1098 cm −1, aliphatic C–H and aromatic C–H stretching at 2976 cm −1 and 3027 cm −1, in the pyrimidine compound 3a. Mass spectrum also supports the proposed structure by the presence of molecular ion peak at m/z 287 M +.
The structure of (4a) was elucidated on the basis of C–N linkage in the triazole ring, which causes an absorption band at 1373 cm −1 in its IR spectrum. 1H NMR spectrum shows a singlet at δ 3.21 due to SH functional group of compound 4a. The IR and 1H NMR spectral data reveal that the carbonyl absorption band at 1654 cm −1 of NH–CO–NH group, N–N stretching band at 1053 cm −1, aliphatic C–H and aromatic C–H stretching at 2968 cm −1 and 3027 cm −1, in the group of pyrimidine compound (4a). Molecular ion peak at m/z 287 M + in the mass spectrum also supports the proposed structure. The mechanism of compounds 4(a–j) is shown in Scheme 5.
All these compounds were screened for antibacterial activity by pseudomonas aeruginosa (Gram –ve), staphylococcus aureus (Gram + ve) and escherichia coli (Gram –ve). Ciprofloxacin was used as standard drug. Most of the synthesized compounds showed moderate to good inhibition at a concentration of 10 μg/mL.
3.1 Antibacterial studies
The newly synthesized pyrimidine derivatives were screened for their antibacterial activity in vitro against pseudomonas aeruginosa, staphylococcus aureus and escherichia coli, using agar well disk diffusion method. The test compounds were dissolved in DMSO to get a solution of 10 μg/mL concentration. The inhibition zones were measured in millimeters at the end of an incubation period of 18 h at 37 ∘C. Ciprofloxacin was used as a standard drug and the results are shown in Tables 1 and 2. The investigation of antibacterial screening data reveals that, all the tested compounds show moderate to good inhibition at 10 μg/mL concentration.
3.2 Comparison of anti bacterial activity
Comparison of antibacterial activity for all the synthesised compounds are shown in Figures S1–S3 (in Supplementray Information). Based on the comparative studies, 5-(5-amino-1,3,4-thiadiazol-2-yl)-3,4-dihydro-6- methyl-4-phenylpyrimidin-2(1H)-one, 3(a–j) compounds have less inhibition than the 3,4-dihydro-5-(5-mercapto- 4H-1,2,4-triazol-3-yl)-6-methyl-4-phenylpyrimidin-2(1H)- one 4(a–j) compounds at 10 μg/mL concentration. A few compounds showed very good inhibition, which are closer to the standard drug.
4 Conclusions
The investigation of antibacterial screening data for synthesized compounds reveal that, the 4(a–j) triazole substituted compounds have higher inhibition than the 3(a–j) thiadiazole substituted compounds, because the triazole ring which is substituted in the pyrimidine, enhances the inhibition of the compound, against the three species of pseudomonas aeruginosa, staphylococcus aureus and escherichia coli.
References
Mohd A, Kumar A, Ali I and Khan S A 2009 The synthesis of pharmaceutically important 1,3,4-thiadiazole and imidazolinone derivatives: Antimicrobial studies of heterocyclic compounds Indian J. Chem. 48 1288
Xiang Li, He-Mei Liu, Xui-Zhang and Zhi-Hui 2012 Synthesis and evaluation of antitumor activities of Novel chiral 1,2,4-triazole derivatives: A study of Schiff bases bearing γ-butenolide moiety Org. Med. Chem. Lett. 2 1
Karaarslan M, Kopair P, Cansiz A, Orek C and Sap O 2012 Synthesis and antimicrobial activity of some new 5-(Pyridin-4-yl)-3-thioacetamido-1,2,4-triazole derivatives: Antimicrobial studies of substituted triazole Chem. Sci. Trans. 1 226
Merchant J R, Suneel Y and Dike 1978 Synthesis of some 2,4-diamino-pyrrolo-pyrimidines: A study of substituted pyrimidine J. Chem. Sci. 87 229
EI-Sayed R 2006 Synthesis, antibacterial and surface activity of 1,2,4-triazole derivatives: Antibacterial studies of triazole Indian J. Chem. 45 738
Andrews B and Ahmed M 2013 Novel synthesis and characterization of some pyrimidine derivatives of oxadiazoles, triazoles and thiadiazoles: Synthesis of substituted pyrimidine derivatives Asian J. Chem. 25 2070
Andrews B and Ahmed M 2015 An efficient synthesis, characterization and anti-bacterial activity of pyrimidine bearing 1,3,4-thiadiazole derivatives: A study of pyrimidine moiety Indian J. Chem. 54 406
Ilangovan A, Saravanakumar S and Umesh S 2015 T3P as an efficient cyclodehydration reagent for the one-pot synthesis of 2-amino-1,3,4-oxadiazoles: A study of substituted oxadiazole J. Chem. Sci. 127 797
Kushal R, Lanjewar M. S and Binda D 2009 Synthesis and antimicrobial activity of 5-(2-aminothiazol-4-yl)-3,4-dihydro-4-phenyl pyrimidin-2(1H)-one: Synthesis of pyrimidine compound Indian J. Chem. 48 1732
Kape C O 2000 Dihydropyrimidine synthesis Eur. J. Med. Chem. 35 1043
Kape C O 1993 Biginelli dihydropyrimidine synthesis Tetrahedron 49 6937
Nagarajan S, Tanveer M, Shaikh and Kandasamy E 2015 Synthesis of 1-alkyl triazolium triflate room temperature ionic liquids and their catalytic action: Studies in multi-component Biginelli reaction J. Chem. Sci. 127 1539
Manjula A, Rao B V and Neelakantam P 2004 An inexpensive protocol for Biginelli reaction: Synthesis of pyrimidine compound Synth. Commun. 34 2665
Andrew J, Zych Hong-Jun Wang, Samuel A and Sakwa 2010 Synthesis and Suzuki-Miyaura reaction: Synthesis of dihydropyrimidine-2(1H)-one Tetrahedron Lett. 51 5103
Garima, Srivastava V P and Yadav L D S 2010 Biginelli reaction: Starting directly from alcohol Tetrahedron Lett. 51 6436
Reddy Y T, Reddy P N, Kumar B S and Rajitha B 2005 Bismuth oxide perchlorate catalysed: Synthesis of dihydropyrimidine-2 compound Indian J. Chem. 44B 1304
Bose D S, Fathima L and Mereyala H B 2003 Synthesis of 5-alleoxycarbonyl-4-aryl-3,4-dihydropyrimidine-2(1H)-one: Synthesis of multi-compounds J. Org. Chem. 68 587
Sergey V, Ryabukhin Andrey S, Plaskon Semen S, Bondarenko Eugeniy N, Ostapchuk Oleksandr O, Grygorenko Oleg V and Tolmachev 2010 Acyl pyruvates as synthos: Biginelli reaction Tetrahedron Lett. 51 4229
Rosenani A, Haque and Salam M A 2015 Synthesis, structural characterization and biological activities of organotin(IV) complexes with methoxybenzaldehyde: Synthesis of thiosemicarbazone J. Chem. Sci. 127 1589
Ramchander J, Rameshwar N, Reddy T S, Raju G and Reddy A R 2014 Synthesis and photophysical properties of 1,4-disubstituted naphthloxymethyl-N-alkyl naphthimide-1,2,3-triazole: Synthesis of substituted triazole J. Chem. Sci. 126 1063
Acknowledgements
The authors are thankful to the Principal and Research Department of Chemistry, Islamiah College, Vaniyambadi, Vellore district, Tamil Nadu, India for constant encouragement and providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
All additional information pertaining to characterization of the compounds using FT-IR spectra (Figures S1 to S10 and S31 to S40), 1H NMR spectra (Figures S11 to S20 and S41 to S50), 13C NMR spectra (Figures S21 to S30 and S51 to S60), antibacterial screening data (Tables S1, S2) and antibacterial activities comparative diagram (Figure S1, S2 and S3) are given in the supporting information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
ANDREWS, B., KOMATHI, K. & MOHAN, S. Synthesis and comparing the antibacterial activities of pyrimidine derivatives. J Chem Sci 129, 335–341 (2017). https://doi.org/10.1007/s12039-017-1228-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1228-z