Abstract
In the present investigation, a new synthetic route for a novel recyclable free [3-MOBdMBn-Ni] and polystyrene-anchored [P-3-MOBdMBn-Ni] nickel complexes is presented. The free and polymer-anchored metal complexes were synthesized by the reaction of nickel (II) with one molar equivalent of unsupported N N′-bis (2-Hydroxy-3-methoxybenzaldehyde) 4-Methylbenzene-1,2-diamine (3-MOBdMBn) or polymer-supported (P-3-MOBdMBn) Schiff-base ligand in methanol under nitrogen atmosphere. The advantages of these polymer-supported catalysts are the low cost of catalyst and recyclability up to six times, due to easy availability of materials and simple synthetic route. The higher efficiency of complexation of nickel on the polymer-anchored 3-MOBdMBn Schiff base than the unsupported analogue is another advantage of this catalyst system. The structural study reveals that nickel(II) complex of 3-MOBdMBn is square planar in geometry. The catalytic activity of nickel complex towards the oxidation of phenol was investigated in the presence of hydrogen peroxide. Experimental results indicate that the reactivity of P-3-MOBdMBn-Ni was dramatically affected by the polymer support compared to free 3-MOBdMBn-Ni. The rates of oxidation (Rp) for unsupported and supported catalysts are 1.37 × 10−6 mole dm−3 s−1 and 2.33 × 10−6 mole dm−3 s−1 respectively.

The catalytic activity of free [3-MOBdMBn-Ni] and polystyrene-anchored [P-3-MOBdMBn-Ni] nickel complexes were tested towards oxidation of phenol and the effect of the H2O2 concentration/phenol concentration/catalyst concentration is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Schiff base catalysts have a great impact on both the scholastic research and polymer industry. Schiff base complexes have shown adaptable applications in different organic transformation reactions such as oxidation,[1,2] epoxidation of olefins,[3,4] and polymerization of ethylene.[5,6] However, it was observed that the homogeneous Schiff-base catalytic systems have two key disadvantages: the lack of control of product which causes the reactor fouling and the limitation of its use in solution processes. Thus, binding these Schiff base metal catalysts onto polymer supports can offer a promising route to prevail over these drawbacks. In general, the heterogeneous Schiff base catalytic system (or supported system) apparently has a lower activity than its corresponding homogeneous analogue. But polymer supported transition metal complexes have shown high catalytic activity[7,8] in comparison to homogeneous and unsupported catalysts. Also, polymer-supported catalysts are easily recovered without any substantial loss in their catalytic activity[9,10] but homogeneous catalysts are not recovered easily.
Further, oxidation of phenol is an industrially important reaction as its products catechol and hydroquinone are widely used as antioxidants, polymerization inhibitors, photography chemicals, flavouring agents, drug intermediates, etc. Since 1970, phenol oxidation, has been widely investigated using various homogeneous and heterogeneous catalysts.[11] Moreover, phenol is an intermediate in the oxidation of many aromatic compounds and a toxic molecule resistant to biotreatment. For the treatment of wastewater containing highly concentrated, toxic or hardly biodegradable compounds, oxidation of such organic pollutants, into non-toxic products in the presence of a catalyst is a promising approach.[12,13]
Oxidation of phenols using various chemical reagents such as, hydrogen peroxide, permanganate, molecular oxygen and ozone, are extensively used.[14] The use of hydrogen peroxide has the benefit of producing oxygen which can be used to supplement biological degradation.[15] Oxidation of phenol using unsupported Schiff base complexes of metal ions is reported,[16] but the oxidation of phenol using polymer-supported transition metal complexes was found to be high[17] in the presence of tert-butyl hydroperoxide (t-BHP). The activity of polymer-supported Schiff base complexes of iron(III), cobalt(II) and nickel(II) ions in the oxidation of phenol[18,19] also showed variation with temperature, time, etc, which might be due to the change in concentration of substrate or catalyst.
Although the oxidation of phenol in the presence of metal complexes of salen and hydrogen peroxide as the oxidant is reported earlier[8] the catalytic activity of metal complexes of N, N’-bis (2-hydroxy-3-methoxybenzaldehyde) 4-Methylbenzene-1,2-diamine Schiff base (3-MOBdMBn) is not reported in the literature. Hence, in the present investigation an attempt has been made to prepare polymer-supported nickel complex of 3-MOBdMBn Schiff base and to characterize them for their structures and catalytic activity towards the oxidation of phenol in the presence of hydrogen peroxide as an oxidant. Here the substituent 4-methyl and 3-methoxy play a significant role towards the stability as well as in the catalytic activity of the Ni(II) complex.
2 Experimental
2.1 Materials
Divinyl benzene cross-linked chloromethylated polystyrene beads were obtained from Ion Exchange India Ltd., Mumbai, India and were used to anchor synthesized 3-MOBdMBn Schiff base complexes of metal ions. The anhydrous chloro salts of nickel(II) ions were purchased from Ranbaxy, Mumbai, India and used without further purifications. The phenol, hydrogen peroxide (30.0 wt%), 2-hydroxy-3-methoxybenzaldehyde (3-MOBd) and 4-Methylbenzene-1,2-diamine (MBn) were procured form E. Merck, India. Other chemicals and solvents were of analytical grade (>99.0 wt%) and used after drying with standard methods.[20]
2.2 Characterization of 3-MOBdMBn Schiff base and its metal complexes
IR spectra of 3-MOBdMBn Schiff base and its nickel complexes were recorded on KBr pellet using PerkinElmer 1600 FTIR Spectrophotometer. The electronic spectra of 3-MOBdMBn Schiff base and its nickel complexes were recorded with Shimadzu 1601 PC UV–Vis Spectrophotometer by using sample mull in a cuvette. Thermogravimetric analysis (TGA) of 3-MOBdMBn Schiff base and its nickel complexes were carried out using Perkin-Elmer Pyris, Diamond Thermal Analyzer under nitrogen atmosphere at a heating rate of 5∘C/min. The loading of metal ions on 3-MOBdMBn Schiff base was determined by analyzing the solution with PerkinElmer 3100 Atomic Absorption Spectrometer at λ max of nickel ion. The amount of 3-MOBdMBn Schiff base anchored on polymer beads and its composition were estimated using Haraeus Carlo Ebra 1108 Elemental Analyzer. The1H-NMR spectra of 3-MOBdMBn Schiff base and its nickel complexes were recorded on an FT-NMR-Brucker 300 MHz Spectrometer using DMSO- d 6 as a solvent and tetramethylsilane (TMS) as an internal reference. The magnetic moment (μ) of nickel complexes were measured using Vibrating Sample Magnetometer-155. The molecular weight of 3-MOBdMBn Schiff base and its nickel complexes were determined using a Vapor Pressure Osmometer (Merk VAPRO 5600, Germany).
2.3 Synthesis of 3-MOBdMBn Schiff base and its nickel complex
The 3-MOBdMBn Schiff base was synthesized by the modified procedure reported in the literature.[7] The reaction mixture containing 2-hydroxy-3-methoxybenzaldehyde (20 mmol, 3.043 g) and 4-Methylbenzene-1,2-diamine (10 mmol, 1.22 g) in methanol was refluxed at 60∘C for about 1 h. On cooling at low temperature the reaction mixture produced light orange red coloured crystals, which were filtered and recrystallized with methanol. The metal complexes of 3-MOBdMBn Schiff base were prepared by taking 100 mL methanolic solution of mixture of Schiff base (20 mmol, 7.81 g) and 20 mmol of Nickel metal ions in a round bottom flask and refluxing at 60∘C. After 8 h, the solution was cooled and crystalline metal complexes were separated from the mother liquor. Finally, metal complexes were recrytallized in methanol and dried in a vacuum desiccator.
2.4 Synthesis of polymer-anchored 3-MOBdMBn Schiff base and its nickel complex
To prepare polymeranchored nickel complexes of 3-MOBdMBn Schiff base, the N, N’-bis (4-amino-2-hydroxy-3-methoxybenzaldehyde) 4-Methylbenzene-1,2-diamine (A-3-MOBdMBn) Schiff base was prepared by carrying out nitrosation and reduction reactions of 3-MOBdMBn Schiff base and then reacting resulted A-3-MOBdMBn Schiff base with cross-linked chloromethylated polystyrene beads. The nitrosation of 3-MOBdMBn Schiff base was carried out using 20 mmol (7.81 g) of 3-MOBdMBn Schiff base with sodium nitrite (20 mmol) in 1.0 N hydrochloric acid (100 mL) in an ice bath. The resultant N, N’-bis (4-nitroso-2-hydroxy-3-methoxybenzaldehyde) 4-Methylbenzene-1,2-diamine (NO-3-MOBdMBn) was filtered and washed with hot and cold water to remove reaction impurities. The reduction of NO-3-MOBdMBn was carried out using 20 mmol of nitrosated Schiff base in 1.0 N hydrochloric acid (50 mL) in the presence of metallic iron, which produced A-3-MOBdMBn Schiff base. To immobilize the prepared A-3-MOBdMBn Schiff base on cross-linked chloromethylated polystyrene, the methanol swollen polymer beads (5 g) were refluxed in 50 mL methanol containing 20 mmol (8.41 g) A-3-MOBdMBn Schiff base. After 8 h, the 3-MOBdMBn Schiff base anchored polymer beads were separated and dried in a vacuum desiccator. The amount of 3-MOBdMBn Schiff base loaded on polymer beads was estimated with elemental analysis. Subsequently, the nickel ion was loaded by keeping 3-MOBdMBn loaded polymer beads (5 g) for 10 h in an aqueous solution (50 mL) of nickel ion (20 mmol). Finally, the polymer beads were separated and dried at 70∘C in a vacuum oven after washing with hot and cold water. The loading of nickel ion on polymer bead was determined by analysing the solution with an Atomic Absorption Spectrometer. The loading of nickel ion on free and polymer-supported 3-MOBdMBn Schiff base was calculated as complexation of nickel ion using the amount of 3-MOBdMBn Schiff base taken initially and the amount of nickel ion loaded on polymer beads.
2.5 Catalytic activity of metal complexes towards oxidation of phenol
To evaluate the catalytic activity of free and polymer- supported nickel complexes of 3-MOBdMBn Schiff base, the oxidation of phenol was carried out using hydrogen peroxide as the oxidant at fixed ionic strength (0.10 M) and hydrogen ions concentration (pH7.0) in the reaction mixture. To carry out these reactions, a calculated amount of metal-anchored polymer beads were taken in a two-necked roundbottomed flask containing 0.05 M phenol (4.7 g). The oxidation of phenol was carried out adding 5.67 g (0.05 M) hydrogen peroxide (30 wt%) in the reaction mixture and 2 mL chlorobenzene as an internal standard. The water condenser and supply of nitrogen were attached with reaction flask before starting the heating and stirring (1200 rpm) of reaction mixture. Gas chromatography was used to follow these reactions at different time intervals. The retention time of standards was used to identify the reaction products, and product selectivity was measured using peak areas of reaction products in the chromatograms. The reactions were also carried out taking different molar ratios of substrates, hydrogen peroxide, and catalyst. The rate of oxidation for the oxidation of phenol was determined by studying reactions at different temperatures at constant molar ratios of substrate to H2O2 and catalyst. The reactions were also studied without using catalysts to compare the effect of catalyst towards the oxidation of phenol.
3 Results and Discussion
Investigations have revealed that polymer-supported metal complexes are sometimes more catalytic and efficient than free and unsupported analogues.[18,19] The activity of catalysts also showed dependence on properties of polymer supports and amount of loaded catalysts. Thermal stability of catalysts is required to be high, if these catalysts have to be applied for high temperature reactions. Therefore, thermal analysis of supported metal complexes was carried out to evaluate their possible applications in high temperature reactions and to provide a proof for complexation of metal ions with polymer-anchored 3-MOBdMBn Schiff base. The TGA of polymer supported 3-MOBdMBn Schiff base (figure 1) showed a weight loss of 39.1 wt% at 500∘C, but its nickel(II) ion complex showed a weight loss of 35.1 wt% at the same temperature. This was a clear indication that nickel(II) ion complex was more stable than its Schiff base ligand.[21] Also, the nickel complex of P- 3- MOBdMBn Schiff base was more stable than the polymer supported nickel complex synthesized by Gupta et al.[19] (showed a weight loss of 45 wt% at 500∘C), may be due to the presence of 4-methyl and 3-methoxy substituent in the Schiff base.
Further, polymer-supported 3-MOBdMBn Schiff base and its nickel complex were also characterized by IR and UV techniques to provide a proof for the complexation of metal ions and to decide the structures and geometry of the complex on the basis of elemental analysis and magnetic properties.
3.1 Characterization of 3-MOBdMBn Schiff base
The yield of 3-MOBdMBn Schiff base was found to be 91.6 wt% (scheme 1). The IR spectrum of 3-MOBdMBn Schiff base showed absorption bands at 1609 cm1 (>C =N), 1263 cm1 (>C–O) phenolic (SI). The elemental analysis of 3-MOBdMBn Schiff base proved (wt%): C = 69.82, N = 7.03 and H = 6.31; Caltd (%): C = 70.75, N = 7.17 and H = 5.68, which corresponds to C23 H 22 N 2O4 empirical formula of 3-MOBdMBn Schiff base.[7,22]
The molecular weight of Schiff base was 389.37 g mol−1 (Caltd 390.43 g mol−1). The electronic spectra of 3-MOBdMBn Schiff base showed absorption bands at 289 nm and 347 nm, which were assigned to π→π ∗ and n →π ∗ transitions. The1H-NMR spectrum of 3-MOBdMBn Schiff base (figure S3) showed signals at δ/ppm = 2.79(3H), 3.81(6H), 5.13(2H), 6.80(2H), 6.98(3H), 7.25(2H), 7.41(2H) and 8.61(2H) which corresponded to the structure of 3-MOBdMBn Schiff base as shown in scheme 1.
3.2 Synthesis and characterization of A-3-MOBdMBn Schiff base and its anchoring on polymer beads
The nitrosation of 3-MOBdMBn Schiff base produced 87.5% yield of NO-3-MOBdMBn (scheme 2). It is observed from the elemental analysis of NO-3-MOBdMBn that (wt%): C = 62.01, N = 12.17, and H = 4.95; Caltd (wt%): C = 61.60, N = 12.49 and H = 4.50, which corresponds to C23 H 20 N 4O6 formula of nitrosated Schiff base. The molecular weight of NO-3-MOBdMBn was 447.02 g mol1 (Caltd 448.43 g mol1). The IR spectrum of NO-3-MOBdMBn showed absorption bands at 1604 cm1 (>C=N), 1260 cm−1 (>C–O) phenolic, and 1533 cm−1 and 131 cm−1 for N–O group. The NO-3-MOBdMBn Schiff base showed a shift in NMR signals in comparison to NMR signals observed for 3-MOBdMBn Schiff base. The NO-3-MOBdMBn Schiff base showed proton signals at δ/ppm = 2.82(3H), 3.75(6H), 5.15(2H), 7.15(2H), 7.36(3H), 7.66(2H) and 8.64(2H), which corresponded to the structure of nitrosated 3-MOBdMBn Schiff base as shown in scheme 2. The protons ortho to nitroso group in 3-MOBdMBn Schiff base were deshielded. Hence, their signals appeared at 7.15 and 7.66 ppm instead of 6.8 and 7.41 ppm respectively, as observed for pure 3-MOBdMBn Schiff base. The proton signal at 7.25 ppm was missing due to the substitution of nitroso group in the benzene ring. The reduction of NO-3-MOBdMBn produced 81.3 wt% yield of A3-MOBdMBn Schiff base as shown in scheme 2. The A3-MOBdMBn Schiff base was characterized by different techniques. The elemental analysis of A3-MOBdMBn Schiff base showed (wt%): C = 63.92 N = 13.21 and H = 7.02, Caltd (wt%): C = 65.7 N = 13.33, and H = 5.75, which corresponded to C23 H 24 N 4O4 empirical formula of Schiff base. The molecular weight of A-3-MOBdMBn Schiff base was found to be 418.23 g mol1 (Caltd 420.46 g mol1). The IR spectrum of A-3-MOBdMBn Schiff base showed absorption bands at 1600 cm1 (>C= N), 1255 cm1 (>C–O) phenolic, and a band between 1641 and 1619 cm1 for >C–N group. The1H-NMR spectrum of A-3-MHBdMBn Schiff base showed proton signals at δ/ppm = 2.81(3H), 3.72(6H), 4.15 (4H), 5.15(2H), 6.16(2H), 6.46(2H), 7.36(3H) and 8.63(2H), which corresponded to the structure of A-3-MOBdMBn Schiff base as shown in scheme 2.
The synthesized A-3-MOBdMBn Schiff base was anchored on cross-linked chloromethylated polystyrene beads by refluxing A-3-MOBdMBn Schiff base with polymer beads in DMF for 8 h at 60∘C. The amount of A-3-MOBdMBn Schiff base anchored on polymer beads was 881 wt% (scheme 3). The anchoring of A-3-MOBdMBn Schiff base on polymer beads was confirmed by comparing the IR spectrum of 3-MOBdMBn Schiff base anchored polymer beads with IR spectrum of pure polymer beads. The IR spectrum of polymer-anchored Schiff base showed new absorption bands at 1594 cm−1 (>C=N), 1246 cm−1 (>C–O) phenolic, and a broad band between 1644 cm−1 (>C=N), which were absent in the IR spectrum of pure polymer beads, but were present in free Schiff base. The IR spectrum of pure polymer beads showed absorption band at 1262 cm−1, which is attributed to C–Cl bond of chloromethyl in cross-linked polymer beads.[7] The decrease in the intensity of absorption band at 1262 cm1 in polymer- anchored 3-MOBdMBn Schiff base than pure polymer beads was an evidence for anchoring of 3-MOBdMBn Schiff base on polymer beads. The appearance of new absorption bands and shift in characteristic absorption bands of 3-MOBdMBn Schiff base were also used as evidence for anchoring of 3-MOBdMBn Schiff base on polymer beads.
3.3 Characterization of free and polymer-anchored metal complexes of 3-MOBdMBn Schiff base
The loading of nickel ion on free and polymer-supported 3-MOBdMBn Schiff base was carried out by refluxing free Schiff base (scheme 4) and polymer-anchored Schiff base in solution of metal salt at 60∘C for 6 h (scheme 5). The metal complexes of free Schiff base (3-MOBdMBn-Ni) and polymer-anchored Schiff base (P-3-MOBdMBn -Ni) after separation and purification were analyzed for their structures and loading of nickel ion. The complexation of nickel(II) ion on free 3-MOBdMBn Schiff base and polymer-anchored Schiff base was 86.34 and 89.88 wt% respectively (table 1). These results have clearly suggested that the loading of nickel ion on polymersupported 3-MOBdMBn Schiff base was higher than free 3-MOBdMBn Schiff base.
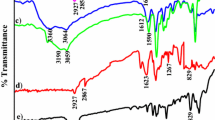
The complexation of nickel ion with 3-MOBdMBn Schiff base showed significant variations in IR bands for >C=N and >C–O groups and new absorption bands appeared due to the formation of M–O and M–N bonds in metal complex. The disappearance of phenolic broad band in the IR spectrum of 3-MOBdMBn Schiff base after the complexation of nickel ion was an evidence for the complexation of metal ions with 3-MOBdMBn Schiff base. The polymer-supported Schiff base showed absorption bands at low frequency in comparison to free Schiff base (SI) On complexation with nickel(II) ion, the frequency of >C=N absorption band of free Schiff base has decreased from 1609 to 1595 cm−1, whereas polymer- supported Schiff base showed variation from 1594 to 1582 cm−1 (figure 2).[7]
These variations in IR band corresponding to >C= N group of 3-MOBdMBn Schiff base were used as evidence for interaction of metal ions with azomethine nitrogen (>C= N) of 3-MOBdMBn Schiff base. The complexation of nickel(II) ions showed new absorption band at 424 cm−1 with free Schiff base and at 417 cm−1 with polymer-anchored Schiff base, which was due to the formation of M–N bond between nickel(II) ion and Schiff base. The complexation of nickel(II) ion showed another new band due to the formation of bond between nickel and phenolic oxygen (–O–M) with polymer-supported 3-MOBdMBn Schiff base at 518 cm−1 (figure 2).
The complexation of metal ions with Schiff base was further confirmed by comparing electronic spectra of nickel complex and pure 3-MOBdMBn Schiff base. The complexation of nickel(II) ion with 3-MOBdMBn Schiff base showed variation in π→π ∗ transition from 289 nm to 280 nm and (table 2) the n →π ∗ transition of 3-MOBdMBn Schiff base was changed from 347 nm to 299 nm. The charge transfer (CT) and d → d transitions were also used as evidence for complexation of nickel with Schiff base. These electronic transitions corresponded to t\(_{\mathrm {2g}}^{\mathrm {6}}\) e\(_{\mathrm {g}}^{\mathrm {2}}\) configurations for nickel(II) ion in this complex. The magnetic moment (μ) of nickel Schiff base complex was found to be 0.83BM, which indicated that nickel(II) ion complex was diamagnetic in nature with zero unpaired electron. The magnetic moment (μ) and electronic configurations have suggested a square planar structure with dsp2 hybridization for nickel complex (scheme 6).
3.4 Oxidation of phenol
The catalytic activity of free and polymer-supported nickel complex of 3-MOBdMBn Schiff base was evaluated by studying the oxidation of phenol in the presence of hydrogen peroxide. The gas chromatographic analysis was used to determine the product selectivity and to estimate the percent conversion of phenol. The catechol (CTL) was a major reaction product in the oxidation of phenol (scheme 7). The formation of reaction products was attributed to enzymatic behaviour of metal complexes of 3-MOBdMBn Schiff base.
The polymer support has facilitated the decomposition of the intermediates. Hence, % conversion of phenol was more with polymer-supported nickel complex (figure 3) in comparison to free complex of 3-MOBdMBn Schiff base (SI). The conversion of phenol increased up to 240 min and after that the conversion became almost constant due to substantial decrease in the concentration of oxidant and substrate in the reaction mixture for both supported and unsupported catalysts (figure 3). The oxidation reaction also had shown dependence on the type of catalyst. The high activity of polymer supported metal complexes was due to more facile interactions of the catalyst with substrate than with unsupported catalysts. The low activity of catalyst in solution was due to the formation of inactive dimers or multimers of metal complexes in the solution. The amount of phenol oxidized with hydrogen peroxide was almost equal to the sum of amount of CTL and hydroquinone (HQ) produced, which indicates the formation of other reaction products such as polymeric phenols is almost none.
The amount of CTL and HQ was equal to phenol conversion, but reaction showed high selectivity for CTL as determined from the area under the GC chromatograms. The supported catalysts were recycled and also further evaluated for their catalytic activity after their applications in oxidation reactions. The efficiency of supported catalysts remained almost constant up to six recycles and after that the efficiency decreased (table 3) which may be due to either decomposition of the catalyst in the reaction media or their extraction to the organic solvent during product isolation.[23] The product selectivity for CTL remained unaffected on using recycled catalysts, which was an indication for the structural stability of polymer supported metal complexes as confirmed by comparing IR spectra of recycled catalysts with IR spectra of freshly prepared catalysts. The activity of metal complexes in substrate conversion and product selectivity was evaluated at different molar ratios of substrate to hydrogen peroxide and catalyst.
The rate of phenol conversion was higher (2.33 × 10−6 mole dm−3 s−1) in the presence of polymer-supported 3-MOBdMBn Schiff base complexes of nickel(II) ion than its unsupported Schiff base analogue (1.37 × 10−6 mole dm−3 s−1) (table 4). The turnover number (TON) for the oxidation of phenol was higher (15.82 g mol−1Ni hr−1) in the presence of polymer supported Schiff base complex in comparison to unsupported Schiff base complex (9.33 g mol−1Ni hr−1) at a molar ratio of 1:1:1 of H2O2 to phenol and catalyst (table 4). The rate of substrate conversion and TON in the oxidation of phenol varied significantly with molar ratio of hydrogen peroxide but product selectivity in both cases remained almost constant (table 4).
3.5 Effect of the H2O2 concentration/phenol concentration/catalyst concentration on the oxidation of phenol
The oxidation of phenol was studied with the variation of the molar ratio of H2O2 to phenol from 0.5 to 2.0 at a constant molarity of the substrate and catalyst (0.05 M) in acetonitrile (2.0 mL). The reaction temperature was maintained at 70∘C. When the molar ratio of H2O2 was varied from 0.5 to 1.0, the oxidation of phenol increased in the presence of supported 3-MOBdMBn Schiff base complex of nickel(II) (figure 4). However, when the molar ratio of H2O2 was further increased (>1) in the reaction mixture, the oxidation of phenol showed a decreasing trend, and a similar trend was observed with unsupported 3-MOBdMBn-Ni Schiff base complex analogue. The decreasing trend in the conversion of phenol was due to the decrease in molar ratio of phenol and catalyst with respect to molar ratio of H2O2.
The catalytic efficiency of the [3-MOBdMBn-Ni] complexes towards the oxidation of phenol was evaluated at different molar ratios of phenol in the reaction mixture, whereas the molar ratio of H2O2 to the catalyst was kept constant. The molar ratio of phenol was varied from 0.5 to 2.0 with respect to the molar ratio of H2O2 to the catalyst. During the molar ratio variation of phenol, the concentration of H2O2 and the catalyst was kept constant (0.05 M). When the molar ratio of phenol was increased from 0.5 to 1.0 in the reaction mixture, the conversion (%) of phenol increased substantially with polymer supported nickel complex of the Schiff base (figure 4), but when the molar ratio of phenol increased further (>1), the conversion (%) of phenol showed a decreasing trend, which was due to the significant decrease in the molar ratio of H2O2 to the catalyst in the reaction mixture in comparison with the molar ratio of phenol. The oxidation of phenol was also evaluated at different molar ratios of polymer-supported 3-MOBdMBn Schiff base complex of nickel at a constant molar ratio (1: 1) of the substrate and oxidant. The molar ratio of Schiff base complex of nickel(II) ions was varied from 0.5 to 2.0 at a constant molarity (0.05 M) of phenol and H2O2 in the reaction mixture. The conversion (%) of phenol also showed the same trend as variation of substrate concentration.
3.6 Mechanism for oxidation of phenol
Considering the experimental findings for the oxidation of phenol with free and supported 3-MOBdMBn Schiff base complex of nickel ion, the following reaction steps are proposed (scheme 8). The free and polymer-supported Schiff base complexes of nickel ion (3-MOBdMBn-Ni) have produced active species (Ni3-MOBdMBn-HOO-) through fast interactions with H2O2 and 3-MOBdMBn Schiff base. The intermediate (Ni3-MOBdMBn-Ph-HOO-) has facilitated the nucleophilic attack of-OOH species on ortho and para position of phenol to produce hydroxy-substituted phenols (scheme 7). The reaction products, catalyst and hydroxyl ions were formed through decomposition of intermediates (scheme 8, step 4) and finally the hydroxyl ions reacted with hydrogen ions, which were produced in step 1.
4 Conclusion
The unsupported and polymer-supported nickel complexes of 3-MOBdMBn Schiff base were synthesized and characterized successfully for their structures and catalytic activity towards the oxidation of phenol. The polymer supported 3-MOBdMBn Schiff base nickel complex showed high catalytic activity than its free analogue. The oxidation of phenol showed high selectivity for catechol. The supported catalysts showed higher rate of oxidation and TON than unsupported catalysts, which clearly suggested that polymer support has played a significant role in increasing the rate for oxidation of phenol in the presence of nickel complexes of 3-MOBdMBn Schiff base. Concentration ration of H2O2/phenol/catalyst on oxidation of phenol has a great effect in the presence of polymer-supported nickel complex. The oxidation of phenol was maximum at a molar ratio of 1:1:1 of phenol to hydrogen peroxide and catalyst.
References
Gupta K C and Sutar A K 2008 Coord. Chem. Rev. 252 1420
Chang Y, Zha F, Su B and Wang Y 2006 J. Macromol. Sci. Pure Appl. Chem. 43 923
Ding K, Wang Z, Wang X, Liang Y and Wang X 2006 Chem. Eur. J. 12 5188
Thomas S R and Janda K D 2000 J. Am. Chem. Soc. 122 6929
Sutar A K, Maharana T, Dutta S, Chen C-T and Lin C-C 2010 Chem. Soc. Rev. 39 1724
Vatankhah-Varnoosfaderani M, Pourmahdian S and Afshar-Taromi F 2011 Iran. Poly. J. 20(11) 897
Grivani G and Akherati A 2013 Inorg. Chem. Comm. 28 90
Gupta K C, Sutar A K and Lin C C 2009 Coord. Chem. Rev. 253 1926
Yoo D W, Han J H, Nam S H, Kim H J, Kim C and Lee J K 2006 Inorg. Chem. Commun. 9 654
Yue C, Fei Z, Bitao S and Yupu W 2006 J. Macromol. Sci. Part A Pure Appl. Chem. 43 (6) 923
Kumar A and Srinivas D 2013 J Mol Catal A: Chem. 368 112
Keav S, de los Monteros A E, Barbier Jr. J and Duprez D 2014 Appl. Catal. B: Environ. 150 402
Bellardita M, Augugliaro V, Loddo V, Megna B, Palmisano G, Palmisano L and Puma M A 2012 Appl. Catal. A: Gen. 441 79
Hernmert C, Renz M and Meunier B 1999 J. Mol. Catal. A. Chem. 137 205
Walling C 1975 Acc. Chem. Res. 8 125
Gupta K C and Sutar A K 2008 J. Mol. Catal. A Chem. 280 173
Owsik I, Kolarz B N and Jezierska J 2006 J. Catal. Lett. 107 197
Gupta K C and Sutar A K 2007 J. Mol. Catal. A Chem. 272 64
Gupta K C and Sutar A K 2007 J. Macromol. Sci. Part A: Pure Appl. Chem. 44 1171
Vogel A I 1978 In Textbook of practical organic chemistry (London: ELBS and Longman)
Fraile J M, Mayoral J A, Royo A J, Salvador R V, Altava B, Luis S V, Burguete M I 1996 Tetrahedron 52(29) 9853
Kowalak S, Weiss R C, Balkus K J 1991 J. Chem. Soc. Chem. Commun. 57 57
Buijsman R C, van Vuuren E and Sterrenburg J G 2001 Org. Lett. 3(23) 3785
Acknowledgements
The authors are thankful to Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and University Grants Commission (UGC), New Delhi, India for funding. The authors are also grateful to Ravenshaw University, KIIT University and National Institute of Technology, Raipur for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
FTIR spectrum of unsupported 3-MOBdDMBn Schiff base and polymer-supported 3-MOBdDBn Schiff base,1H-NMR spectrum of 3-MOBdMBn Schiff base, time variation data for conversion of phenol by unsupported and polymer-supported nickel complex (Ni-3-MOBdMBn) are provided in supporting information (SI) available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SUTAR, A.K., MAHARANA, T., DAS, Y. et al. Polymer supported nickel complex: Synthesis, structure and catalytic application. J Chem Sci 126, 1695–1705 (2014). https://doi.org/10.1007/s12039-014-0728-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0728-3