Abstract
Two different azo molecules functionalized graphene oxide (GO) through an ester linkage have been synthesized for the first time. Chemical structure of the azo-GO hybrids was confirmed by Fourier transform infrared spectroscopy and UV-visible spectroscopy. The GO functionalized with 5-((4-methoxyphenyl)azo)-salicylaldehyde was further characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM). The SEM studies demonstrated that the morphology of the azo-GO hybrid was found to be similar to the GO sheets but slightly more wrinkled. Further, TEM image of azo-GO indicates some dark spots on the GO sheets due to azo functionalization. AFM results also reveal that the azo functionalization increases the thickness of GO sheet to 4–5 nm from 1.2–1.8 nm. Both the azo-hybrids show absorption band around 379 nm due to the π–π* transition of the trans azo units. Photoluminescence spectra of azo-GO hybrids show a strong quenching compared with azo molecules due to the photoinduced electron or energy transfer from the azo chromophore to the GO sheets. It also reveals strong electronic interaction between azo and GO sheets.

Graphene oxide functionalized with two different azo molecules through an ester linkage have been synthesized and characterized. Photoluminescence spectra of azo-GO hybrids show strong quenching compared with azo molecules due to the photo-induced electron or energy transfer from the azo chromophore to the GO sheets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Photochromic compounds, which can easily undergo large conformational changes when exposed to light of appropriate wavelength, are fascinating as molecular switching devices.[1] Azobenzenes are one among the photo switching molecules that fulfill this condition.[2] Due to its clean photochemistry, and substantial change in material properties during light irradiation, these molecules have been employed in a variety of applications such as optical data storage,[3] photo switching technologies,[4] liquid crystals,[5] chemo sensors[6] and as sensitizer in dye sensitized solar cells (DSSC).[7] They are readily synthesized and easily characterized by 1H NMR, UV visible spectroscopy (UV-Vis) and Fourier transform infrared (FT-IR) spectroscopy. When irradiated with an appropriate wavelength of light,[8] azo benzene molecules undergo cis-trans isomerization which enables them to act as photo switchable core because of their thermal stability, detectability, high sensitivity and reversibility. These photochromic molecules undergo cis-trans isomerization with high quantum efficiencies. Materials containing even small amount of azo molecules have been shown to effect a substantial change in optical properties and morphologies.[9, 10]
Graphene[11] as a unique 2D nanocarbon material, has attracted significant interest owing to its distinctive structure, outstanding electronic, thermal and mechanical properties.[12–14] Graphene is one of the best candidates for numerous applications such as nanoelectronics, gas sensors, organic photovoltaics, field-effect transistors and other nanocomposites.[15–19] Though there are numerous reports on the many exceptional properties, and applications of both single and multi-walled carbon nanotubes (CNT)[20] and fullerenes,[21] such intensive research is expected for graphene as a building block for new nanomaterials. However, the solubility and processability of this graphene in variety of solvents are the main issues for many diverse applications of graphene-based materials. This solubility problem can be minimized by using graphene oxide (GO),[10, 22–27] which is the oxygenated form of monolayer graphene platelets. This allows chemical functionalization of GO with variety of organic molecules, which will generate new materials with more interesting properties for further applications.[28, 29] One major advantage of using GO is its bulk synthesis from natural graphite by chemical oxidation and subsequent exfoliation. The hydrophilic surface functional groups such as epoxide, hydroxyl, and carboxyl groups of GO makes it more water soluble and enables further chemical modification of graphene.[23, 30]
CNT and GO have been functionalized with chromophores in order to facilitate photo absorptivity in case of photochemical devices.[8, 17] Solar thermal fuel, composed of azobenzene functionalized with carbon nanotubes has been reported in the literature.[31] It has been reported that the number of photoactive molecules per unit volume (i.e., photo isomer concentration) is significantly increased with respect to a solution of free photo molecules, leading to an increased volumetric energy density of 5–7 orders of magnitude due to the highly ordered array of adsorbed photo molecules with CNT substrate.[31] Hybrids of azo/CNT[8] and azo/GO[32] through noncovalent interaction and an amide linkage, respectively, were also studied for photo switching properties. Graphene azo poly electrolyte multilayer fabricated by electrostatic layer by layer also has been investigated for electrochemical capacitor electrode.[33] Recently, there is a report on the preparation of graphene grafted with azo polymer and its photo responsive properties.[34] In this study, we have functionalized GO with two types of azo chromophoric compounds, 5-((4-methoxyphenyl)azo)-salicylaldehyde (azoGO-I) and 5-((4-ethoxyphenyl)azo)-salicylaldehyde (azoGO-II) through an ester linkage. We have chosen the azo molecules in such a way that one end of this organic molecule has electron donating group (methoxy, ethoxy) and other end contains electron withdrawing group (salicylaldehyde). We anticipate that this will act as push-and-pull-like molecular system which in turn will increase the photoinduced electron or energy transfer from the azo chromophore to the GO sheets. The synthesized azo-GO hybrids were characterized by FTIR and UV-Vis. The azo-GO hybrid (I) has been further characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM), and scanning electron microscopy (SEM). The photophysical properties of azo-GO hybrids I and II are also reported.
2 Experimental
2.1 Materials
Thionyl chloride (SOCl2), 4-methoxyaniline (Aldrich), 4-ethoxyaniline (Aldrich), and salicylaldehyde (Aldrich) are used as received. Solvents such as N, N-dimethyl formamide (DMF), di-methyl sulphoxide (DMSO), ethyl acetate, chloroform, dry terahydrofuran (THF), and ethanol were purified and distilled using standard procedure.
2.2 Characterization and instrumentation
FT-IR spectra were recorded in Thermo Nicolet, iS10 FT-IR spectrometer using KBr disk method. Absorption spectra were carried out using Shimadzu, UV 1800 spectrophotometer. 1H nuclear magnetic resonance (NMR) spectra were measured in JEOL 300 MHz multinuclear spectrometers in CDCl3. Chemical shift was expressed in parts per million (ppm) relative to TMS. SEM images were recorded using F E I Quanta FEG 200-High Resolution Scanning Electron Microscope (HRSEM). Elemental analysis was carried out in PerkinElmer series II CHNS/O elemental analyser 2400. Photo luminescence (PL) spectra were recorded using Shimadzu RF-5301 PC spectro fluoro photometer. Powder X-ray diffractograms were performed at room temperature on a Bruker D8 Focus X-ray diffractometer, using Ni-filtered Cu Kα radiation (λ = 1.541 Å) with a scintillator detector (40 kV, 40 mA). The step time was 1 s at 0.04°/step in a 2θ range of 5–90°. High Resolution Transmission Electron Microscope (HRTEM) image was obtained by using the JEOL JEM 2100 transmission electron microscope. AFM carried out by using NTEGRA Prima-NT-MDT.
2.3 Synthesis of 5-((4-methoxyphenyl)azo)- salicylaldehyde (1a)
4-Methoxyanline (1 g, 8.12 mmol) was added to 3.60 ml of 6.00 M hydrochloric acid at 0–5°C. Sodium nitrite (0.57 g, 8.24 mmol in 3.60 ml of H2O) was added dropwise to the reaction mixture for 30 min under constant mechanical stirring. A solution of salicylaldehyde (0.99 g, 8.12 mmol) in 3.60 ml of 10% NaOH was added slowly to the diazonium salt over the period of 1 h at 0–5°C. Dilute acetic acid was added to the formed brownish orange precipitate. The precipitate was washed with NaHCO3 and dried with anhydrous Na2SO4. Resulting orange colour solution was dried under reduced pressure and purified on silica gel (100–200 mesh) column using ethyl acetate, chloroform mixture (1:9). Yield: 1.56 g, (75%), R f value is 0.86. FT-IR (in cm−1): 1654 (C=O), 1454 (N=N), 2926, 2847 (Aliphatic C–H Str.), 3180 (Aromatic C–H str.), 3426 (–OH str.). NMR data in CDCl3, δ = 10.06 (s, aldehyde H), 3.94 (s, –OCH3), 8.18 (d, aromatic 2H), 7.94 (d, aromatic 2H), 7.16 (d, aromatic 1H), 7.07 (d, aromatic 2H) ppm (for NMR spectra, see supporting information figure S1). C14H12N2O3 (256.26): calcd. C 65.62, H 4.72, N 10.93; found C 65.73, H 4.65, N 10.62. The same procedure was followed for synthesizing (2a) by using 4-ethoxyaniline instead of 4-methoxyaniline.
2.4 5-((4-Ethoxyphenyl) azo)-salicylaldehyde (2a)
Yield: 1.44 g, (73%), R f value is 0.93. FT-IR (in cm−1): 1661 (C=O), 1472 (N=N), 2976, 2926 (Aliphatic C–H Str.), 3068 (Aromatic C–H str.), 3414 (–OH str.). NMR data in CDCl3, 25°C, TMS: δ = 10.05 (s, aldehyde H), 1.51 (t, alkyl 3H), 4.16 (q, –OCH2), 8.12 (d, aromatic 2H), 7.93 (d, aromatic 2H), 7.15 (d, aromatic 1H), 7.05 (d, aromatic 2H) ppm. C15H14N2O3 (270.29): calcd. C 66.66, H 5.22, N 10.36; found C 65.73, H 5.39, N 10.21.
2.5 Synthesis of acyl chloride functionalized graphene oxide (GOCl)
GO (30 Mg) was refluxed with SOCl2 (20 ml) in the presence of DMF (0.5 mL) at 65–70°C for 24 h under argon atmosphere to form acyl chloride functionalized GO (GOCl). Excess solvent and SOCl2 were removed by distillation.
2.6 Synthesis of 5-((4-methoxyphenyl)azo)- salicylaldehyde functionalized GO (I)
GOCl (100 Mg) and 0.58 mmol of 5-((4-methoxyphenyl)azo)-salicylaldehyde (1a) were dissolved in DMSO (20 ml) and refluxed at 50°C for 65 h under N2 atm. After completion of the reaction, the product was cooled to room temperature. The precipitate was ultra centrifuged and excess azo impurity was removed by washing with ethanol until the solution became colourless.
2.7 Synthesis of 5-((4-ethoxyphenyl)azo)-salicylaldehyde functionalized GO (II)
This azoGO-II hybrid was synthesized by following the above procedure with 5-((4-ethoxyphenyl)azo)-salicylaldehyde (2a) instead of 5-((4-methoxyphenyl)azo)-salicylaldehyde.
3 Results and discussion
3.1 Synthesis and structure of the Azo-GO I and II hybrids
Water soluble GO was prepared from graphite flakes (<20 μm; Sigma Aldrich) using Hummers method.[30] Distinctive powder X-ray diffractograms features of graphite and the synthesized GO are given in the supporting information (figure S2). For the graphite sample, the characteristic peak of hexagonal graphite corresponding to a d-spacing of 0.34 nm was found at 26.66°. Upon conversion of the graphite into GO, the peak position shifted to a lower diffraction angle of 10.52° with an increase in the interlayer spacing of 0.84 nm. Disappearance of graphite diffraction peak at 26.66° confirms the oxidation of graphite to GO.[35] This increase in d-spacing is due to the intercalation of various oxygen species mostly ether ring oxygen and hydroxyl functional groups in between the graphene layers.
The soluble 5-((4-methoxyphenyl)azo)salicylaldehyde-GO (I) and 5-((4-ethoxyphenyl)azo)salicylaldehyde-GO hybrids (II) were synthesized by suitable modification of literature procedure.[32] 5-((4-Methoxyphenyl)azo)-salicylaldehyde (1a) and 5-((4-ethoxyphenyl)azo)-salicylaldehyde (2a) were covalently linked to GO molecules through an ester bond by refluxing appropriate azo compound with GO in DMSO at 50°C for 65 h under N2 atm. The chemical structure of the compound is shown in figure 1. The azo/GO hybrid structures have been confirmed by FT-IR spectra in the range of 500–4000 cm−1 as shown in figure 2. GO contains various functional groups including ether, epoxide, carboxylic acid, phenolate and aldehyde groups. The characteristic absorption peak assigned to carbonyl (–C=O) stretching mode of GO, azo-GO I and II is clearly visible at 1738, 1740 and 1742 cm−1, respectively as shown in figure 2b–d.[32] After functionalization, a new striking peak at 1457 cm−1 in figure 2c and d of hybrids (I) and (II) confirms the stretching mode of (–N=N–) bond.[36] The three peaks in the range of 1020–1162 cm−1 (figure 2c and d) reveal the presence of –C–O– stretching mode of an ester linkage.[37]
Morphologies of GO and azo functionalized GO (I) were investigated by SEM. SEM images of GO and azo-GO (I) sheet morphology over a few micron length scales are given in supporting information (figures S3 and S4). Figure S4 reveals that the graphene sheets in the azo-GO hybrid somewhat are more wrinkled than GO sheet (see figure S3) due to azo functionalization.
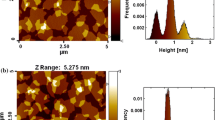
Thickness and morphology of the GO and azo-GO (I) were further probed using AFM. AFM images of GO deposited on freshly cleaved mica surface and its height profile line are given in supporting information (see figure S5 (a) and (b)). From the figure, thickness of the GO layer is found to be ≈1.2–1.8 nm (one to two layers), which is due to conversion of graphene to GO.[38, 39] The AFM images of azo-GO hybrid (I) and its height profile line are given in supporting information (see figure S6 (a) and (b)). This figure indicates that the thickness of azo functionalized GO sheet is ≈4–5 nm (four to six layers). Thickness of the GO sheet increases after functionalization with methoxy azo due to the stacking of the layers, which is similar to other reported results.[40]
TEM images of GO and azo-GO (I) are shown in figures 3 and 4. Figure 4a depicts the TEM images of azo-GO where some dark spots can be observed on the GO sheets due to azo functionalization. Figures 3b and 4b show stacked GO and azo-GO sheets with an interlayer spacing of ≈0.60 and 0.65 nm for GO and azo-GO, respectively. Compared to the pure graphene interlayer distance of 0.34 nm, an interspacing of 0.60 nm indicates fully oxidized graphene.[41] Azo functionalized GO has slightly higher interlayer spacing 0.65 nm compared to GO.
3.2 Spectroscopy measurements
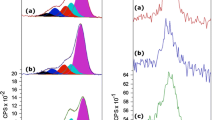
Figures 5 and 6 show UV-vis absorption spectra of 5-((4-methoxyphenyl) azo)-salicylaldehyde (1a), 5-((4-ethoxyphenyl) azo)-salicylaldehyde (2a) and azo-GO hybrids I and II in DMF solvent. UV-vis spectrum of azo salicylaldehyde (1a) and (2a) shows an intense band around 379 nm in DMF solvent which is due to the π–π* transition of the trans azo units.[42] We also confirmed this π–π* transition peak by recording the spectra for (1a) and (2a) in nonpolar DCM solvent; we observed that this peak shifted to shorter wavelength (around 354 nm). The azo-GO hybrids I, II and the pristine azo molecules also exhibit a peak around 450 nm which is absent in the reported azo-GO hybrid and 2-aminoazotoluene.[32] The π–π* transition peak of azo-GO hybrids I, II and the neat azo molecules shows a blue shift of about 22 nm compared to that of reported azo-GO hybrid and the pristine azo chromophore.[32] In figures 5b and 6b, intensity of the peaks are reduced considerably due to covalent functionalization of GO with azo molecules through an ester linkage. This is in agreement with the reported azo-GO hybrid where a similar suppression was observed between the azo-GO compared to pristine azo.[32] This indicates the interaction between the electronic energy level of azo molecules and GO in the azo-GO hybrids.[29]
Photoluminescence spectra of azo compounds (1a), (2a) and azo-GO hybrids (I) and (II) in DMF (excitation wavelength is 400 nm) are shown in figures 7 and 8. Emission spectra of these compounds show two peaks which is similar to the reported azo and azo-GO hybrids.[32] Whereas in the present study, a blue shift was observed in the azo-GO hybrids (I) and (II) compared to pure azo molecules. Emission spectra of these compounds also shows a blue shift compared to the reported azo-GO hybrid and its pristine azo molecule.[32] Figures 7b and 8b show strong quenching compared with pure azo molecules due to the photoinduced electron or energy transfer from the azo chromophore to the GO sheets. This is in agreement with the reported azo-GO hybrid where a similar suppression was observed between the azo-GO compared to pure azo (excitation wavelength is 480 nm).[32] Photoluminescence measurements demonstrate strong electronic interaction between azo group and GO sheets.
4 Conclusion
Thus, we have successfully synthesized two different azo functionalized graphene oxides through an ester linkage for the first time. Chemical structure of the azo-GO hybrids were testified by the FT-IR spectra. A new peak at 1457 cm−1 in IR spectra of hybrids I and II confirms the stretching mode of (–N=N–) bond. SEM morphology studies demonstrated that chemical modification has a strong impact on the structure of GO sheets, forming tiny wrinkles on the graphene film. TEM image of azo-GO indicates some dark spots on the GO sheets due to azo functionalization. AFM results also reveal that the azo functionalization increases the thickness of GO sheet to 4–5 nm from 1.2–1.8 nm. Both the azo-GO hybrids show the absorption band around 379 nm due to the π–π* transition of the trans azo units. Photoluminescence spectra of azo-GO hybrids show strong quenching compared with azo molecules due to the photoinduced electron or energy transfer from the azo chromophore to the GO sheets. It also reveals strong electronic interaction between azo and GO sheets. This result indicates that the azo-GO hybrids can become potential candidates for optoelectronic devices.
Supplementary information
Figures S1–S6 as supplementary information can be seen in www.ias.ac.in/chemsci website.
References
Willner I 1997 ACC. Chem. Res. 30 347
Renner C and Moroder L 2006 Chem. Bio. Chem. 7 868
Kanis D R, Ratner M A and Marks T J 1994 Chem. Rev. 94 195
Feringa B L, van Delden R A, Koumura N and Geertsema E M 2000 Chem. Rev. 100 1789
Ikeda T and Tsutsumi O 1995 Science 268 1873
Bhardwaj V K, Singh N, Hundal M S and Hundal G 2006 Tetrahedron 62 7878
Mikroyannidis J A, Tsagkournos D V, Balraju P and Sharma G D 2011 J. Power Sources 196 4152
Simmons J M, In I, Campbell V E, Mark T J, Leonard F, Gopalan P and Eriksson M A 2007 Phys. Rev. Lett. 98 086802
Yager K G and Barrett C J 2001 Curr. Opin. Solid State Mater. Sci. 5 487
Yager K G and Barrett C J 2006 J. Photochem. Photobiol. A182 250
Nikolaev A V, Bibikov A V, Avdeenkov A V, Bodrenko I V and Tkalya E V 2009 Phys. Rev. B79 045418
Tsoukleri G, Parthenios J, Papagelis K, Jalil R, Ferrari A C, Geim A K, Novoselov K S and Galiotis C 2009 Small 5 2397
Rafiee M A, Rafiee J, Srivastava I, Wang Z, Song H, Yu Z-Z and Koratkar N 2010 Small 6 179
Zhou X, Wu T, Ding K, Hu B, Hou M and Han B 2010 Chem. Commun. 46 386
Li X, Wang X, Zhang L, Lee S and Dai H 2008 Science 319 1229
Geim A K and Novoselov K S 2007 Nat. Mater. 6 183
Liu Z, Liu Q, Huang Y, Ma Y, Yin S, Zhang X, Sun W and Chen Y 2008 Adv. Mater. 20 3924
Gilje S, Han S, Wang M, Wang K L and Kaner R B 2007 Nano Lett. 7 3394
Hao R, Qian W, Zhang L and Hou Y 2008 Chem. Commun. 0 6576
Ajayan P M 1999 Chem. Rev. 99 1787
Martin N, Sanchez L, Illescas B and Perez I 1998 Chem. Rev. 98 2527
Niyogi S, Bekyarova E, Itkis M E, McWilliams J L, Hamon M A and Haddon R C 2006 J. Am. Chem. Soc. 128 7720
Stankovich S, Piner R D, Nguyen S T and Ruoff R S 2006 Carbon 44 3342
Si Y and Samulski E T 2008 Nano Lett. 8 1679
Qian W, Cui X, Hao R, Hou Y and Zhang Z 2011 ACS Appl. Mater. Interfaces 3 2259
Qian W, Hao R, Hou Y, Tian Y, Shen C, Gao H and Liang X 2009 Nano Res. 2 706
Cui X, Zhang C, Hao R and Hou Y 2011 Nanoscale 3 2118
Gomez-Navarro C, Burghard M and Kern K 2008 Nano Lett. 8 2045
Yamuna R, Ramakrishnan S, Dhara K, Devi R, Kothurkar N, Kirubha E and Palanisamy P K 2013 J. Nanopart. Res. 15 1
Hummers W S and Offeman R E 1958 J. Am. Chem. Soc. 80 1339
Kolpak A M and Grossman J C 2011 Nano Lett. 11 3156
Zhang X, Feng Y, Lv P, Shen Y and Feng W 2010 Langmuir 26 18508
Wang D and Wang X 2010 Langmuir 27 2007
Wang D, Ye G, Wang X and Wang X 2011 Adv. Mater. 23 1122
Yeh T-F, Syu J-M, Cheng C, Chang T-H and Teng H 2010 Adv. Funct. Mater. 20 2255
Albayrak C, Gumrukcuoglu I E, Odabasoglu M, Iskeleli N O and Agar E 2009 J. Mol. Struct. 932 43
Deng Y, Li Y, Dai J, Lang M and Huang X 2011 J. Polym. Sci. Part A: Polym. Chem. 49 4747
Akhavan O 2010 Carbon 48 509
Subrahmanyam K S, Vivekchand S R C, Govindaraj A and Rao C N R 2008 J. Mater. Chem. 18 1517
Geng J and Jung H-T 2010 J. Phys. Chem. C 114 8227
Lahaye R J W E, Jeong H K, Park C Y and Lee Y H 2009 Phys. Rev. B79 125435
Gokulnath S, Prabhuraja V, Sankar J and Chandrashekar T K 2007 Eur. J. Org. Chem. 2007 191
Acknowledgements
The authors RY and RD thank the Council of Scientific and Industrial Research (CSIR), (Project No: 01(2256)/08/EMR-II), New Delhi, India for funding and Amrita Vishwa Vidyapeetham for infrastructure.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DEVI, R., PRABHAVATHI, G., YAMUNA, R. et al. Synthesis, characterization and photoluminescence properties of graphene oxide functionalized with azo molecules. J Chem Sci 126, 75–83 (2014). https://doi.org/10.1007/s12039-013-0536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0536-1