Abstract
The Baylis–Hillman reaction using ionic liquid/hydrotalcite clay catalytic system has been observed to be more reactive in terms of yield and reaction rate than DABCO/acetonitrile system. During the process, the reactants enjoy ionic liquid/hydrotalcite clay catalytic system and gives corresponding Baylis–Hillman reaction products in good yield. The application of our catalytic system has been diversifying for the synthesis of lactone ceramide analogue from (S)-Garner aldehyde-methyl acrylate using Baylis–Hillman reaction. Recycling of ionic liquid/hydrotalcite clay catalytic system has also been demonstrated in this report.

The Baylis–Hillman reaction using ionic liquid/HT-clay catalytic system has been shown to be more reactive in terms of yield and reaction rate than DABCO/acetonitrile system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The unique physicochemical properties of ionic liquids[1–3] like, good chemical stability in air and moisture, wide range of solubility for organic/inorganic and also for polymeric materials as well as negligible vapour pressure make them more applicable for different organic transformations as a reaction medium, e.g., aldol reaction,[4a−f] coupling reaction,[5a−c] Knoevenagel condensation,[6] Baylis–Hillman reaction,[7] Diels–Alder reaction,[8] etc.
Heterogeneous catalysts are important to chemical industries not only from an economical viewpoint, but also due to comfortable handling, easy separation and re-cyclability.[9a−c] Hydrotalcites or layered double hydroxide (LDH) has attracted much attention as solid base heterogeneous catalysts[10a−e] for various organic reactions like aldol,[11a] nitro aldol,[11b] cyanoethylation,[11c] Meerwein–Ponndorf–Verley reduction,[11d] epoxidation,[11e] etc. These heterogeneous hydrotalcites offer various attractive advantages over other basic catalysts (organic or inorganic) like easy preparation steps, requirement of cheap raw materials, high catalyst activity, mild reaction conditions, easy recovery and re-usability of the catalyst.[10a−e] In this report, we would like to see the application of hydrotalcite clay and ionic liquid in Baylis–Hillman reaction.
Baylis–Hillman reaction is one of the finest examples of atom-efficient reaction because in this reaction all the atoms from the reagents are converted into a product.[10a−e] This reaction is also useful for the synthesis of highly fictionalized and important organic intermediates in one step.[12a−e] Although Baylis–Hillman reaction has a great importance in the area of organic synthesis but this reaction still suffers from various drawbacks like slow reaction rate, lower yield and higher catalyst loading.[12b−d] Various attempts have been made by other researchers[12b−e] to accelerate the rate of Baylis–Hillman reaction like ultra-sound, microwave and high pressure. Except 1,4-diazabicyclo [2.2.2]-octane (DABCO)[12b] various other catalysts like DBU,[13a] Me2S,[13b] phosphines,[13c] TiCl4,[13d] proline[13e] have been screened to accelerate the rate of reaction to improve the product yield. The additives (Lewis acids) and hydrogen donors (alcohol[13a]) have also been assessed to accelerate the rate of reaction to improve the product yield. Imidazolium-based ionic liquid was also tested as solvent in order to overcome the basic drawbacks of DABCO catalysed Baylis–Hillman reaction.[13f] But the reaction suffers with low yields of Baylis–Hillman reaction adduct due to the direct addition of deprotonated imidazolium salt to the aldehyde.[13g] Different supports like PEG,[14] ionic liquids,[14c] etc. have also been used to anchor the catalyst as well as reactants (aldehydes and acrylates)[14d] in order to reduce the catalyst loading, improve the reaction rate and the recycling of the catalytic system for Baylis–Hillman reaction. Although the above mentioned supported catalysts as well as supported reactants offer some advantages during the Baylis–Hillman reaction, so to say, improved reaction rate, low catalyst loading and recycling of catalyst but these types of above supported systems also suffer with costly starting materials, long preparation steps (3–4 steps), tedious work-up procedure, specific reaction conditions, etc.
In some recent reports heterogeneous catalysts have been successfully tested in ionic liquid medium to catalyse the organic reactions.[15a−e] For instance, proline intercalated montmorillonite clay catalyse aldol reaction in ionic liquid[15a] and ionic liquid mediated hydrotalcite clay catalyses Knoevenagel and nitro aldol condensation reaction.[15b] In both cases, the products of corresponding reactions have been obtained in good yield and selectivity with the added advantage of catalyst re-cyclability. The basic advantages of such ionic liquid immobilized heterogeneous system are; easy preparation steps for catalyst, low catalyst loading, high reaction rate, simple work-up procedure, etc.
In order to overcome the drawbacks of Baylis–Hillman reaction, in this paper, the advantages of hydrotalcite clay with the merits of ionic liquid have been summarized.
2 Experimental
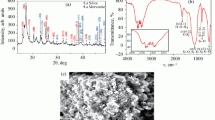
All the chemicals were purchased from Sigma Aldrich and SD fine chemicals. Commercially supplied reagents were used as supplied, except for benzaldehyde, cinnamaldehyde, furfuraldehyde and acrylates which were distilled and stored under an argon atmosphere, protected from light. Organic solvents were dried up as per their the specifications. The work-up and purification procedure were carried out with reagent-grade solvents. NMR spectra were recorded on standard Bruker 300WB spectrometer with an Avance console at 300 and 75 MHz for 1H and 13C NMR, respectively. Trimethylammonium bis-trifluoromethanesulphonamide [tmba][NTf2] was prepared by following reported procedure.[17]
Flash chromatography columns were carried out using MN Kieselgel 60 M gel (40–63 μm, Art. 815381). All eluents had been made distilled prior to use. For thin-layer chromatography (TLC), silica gel plates VWR GL60 F254 were used and compounds were visualized by irradiation with UV light (254 nm). Mg-Al hydrotalcite clay (Mg : Al = 3:1) was prepared as per the original procedure of Miyata[18] which was used without any pre-treatment.
2.1 General experimental procedure
Alkyl acrylate (1.1 mmol) was added to a stirred solution of aldehyde (1 mmol), DABCO (1 mmol for entries 1, 3, 7 and 11 in table 1), dry acetonitrile (2 mL for entries 1 and 2 in table 1), hydrotalcite clay (100 g for entries 2, 4, 8–10, 12–15 in table 1 and for entries 1–13 in table 2) and ionic liquid (200 mg for entries 5–15 in table 1 and for entries 1–13 in table 2), at room temperature for 24 h under argon atmosphere. The reaction medium was diluted with dichloromethane (5 times the volume of solvent used for the reaction) and the resulting solution was further purified on a TLC plate or flash chromatography column (silica gel).
2.2 Experimental procedure for catalyst re-cycling
Methyl acrylate (1.1 mmol) was charged with the solution of p-chlrobenzaldehyde (1 mmol) and [tmba][NTf2]/ hydrotalcite clay (200 mg and 100 mg, respectively) under argon. The reaction mixture was allowed to stir for 24 h at room temperature. The reaction medium was extracted with diethyl ether (5 × 2 mL) and the combined ether extract was further reduced to 2 mL under vacuum. The resulting solution was further purified with TLC plate or by flash column chromatography (silica gel) using n-hexane/ethyl acetate, as eluent, mixture in 9:1 ratio. A new portion of reactants was added to recycle the catalytic system.
2.3 Synthesis of 1-butyl pyridinium bis-trofluromethylsulphonamide [pyc4][\(NTf_{2}^{-}\)]
Distilled pyridine (1 mmol) was stirred with distilled chlorobutane (1 mmol) and acetonitrile (10 mL) at 80 °C for 12 h into the Schlenk tube. The reaction was cooled down at room temperature and washed with diethyl ether (10 × 5 mL) and further dried under reduced pressure to get white hygroscopic solid as 1-butyl pyridinium chloride (10.20 g, 94%). 1H NMR (300 MHz, D2O) δ = 0.91 (t, 3H, J = 7.4), 1.22–1.44 (m, 2H), 1.86–2.10 (m, 2H), 4.60 (t, 2H, J = 7.4), 8.05 (t, 2, H, J = 6.9), 8.52 (t, 1H, J = 7.9), 8.84 (d, 2h, J = 6.2). 13C NMR (75 MHz, D2O) δ = 12.6, 18,6, 32.5, 61.6, 128, 144.4, 145.3. 1-butyl pyridinium chloride (1mmol) was stirred with bis-trifluromethanesulphonamide (1.1 mmol) in 50 mL of distilled water at room temperature for 2 h. The new organic phase immediately appeared which got extracted along with dichloromethane (3 × 5 mL). The organic phase was further washed with water to remove the water soluble impurities. Later organic phase was dried over MgSO4 and concentrated under vacuum. As a result 1-butyl pyridinium bis-trofluromethylsulphonamide was obtained as colourless viscous oil (3.27 g, 98 %). 1H NMR (300 MHz, CDCl3) δ = 0.96 (m 11.2, 3H), 1.40–1.41 (m 14.7, 2H), 1.80–2.12 (m, 2H), 4.60 (m, 16.7, 2H), 8.05 (m, 14.5, 2H), 8.32–8.58 (m, 1H), 8.82 (m, 2H). 13C NMR (75 MHz, CDCl3) δ = 13.2, 13.4, 19.3, 19.4, 33.4, 33.6, 62.6, 122, 128.8, 128.8, 144.5, 145.5.
2.4 Synthesis of entry 14 using [tmba][NTf2]/hydrotalcite clay catalytic system
Methyl acrylate (1.1 mmol) was charged in the mixture of Garner aldehyde (1 mmol) and [tmba][NTf2]/ hydrotalcite clay catalytic system under argon. The reaction mixture was allowed to stir at room temperature for 24 h. The reaction mixture was extracted from diethyl ether (5 × 2 mL). Further, the combined ether extract was reduced to 2 mL under vacuum. The resulting solution was further purified with TLC plate or by flash column chromatography (silica gel) using n-hexane/ethyl acetate mixture in 8:2 ratio as eluent to get major product 14a (from the mixture of 14a and 14b as colourless oil (yield 68 %)). 1H NMR (400 MHz, CDCl3): δ = 1.43–1.53 (m, 15H), 3.77 (s, 3H), 3.90 (br s, 1H), 4.13–4.23 (m, 2H), 4.46–4.60 (m, 2H), 5.77 (s, 1H), 6.23 (s, 1H).
3 Result and discussion
We initiated the screening of ionic liquid immobilized hydrotalcite clay (ionic liquid/Hydrotalcite clay catalytic system) in different proportion with respect to the DABCO/CH3CN system for the model Baylis–Hillman reaction between benzaldehyde and methyl acrylate (table 1, scheme 1). In our study, the catalytic activity of DABCO was tested in ionic liquids i.e., [pyc4][NTf2] and [tmba][NTf2] as well as in acetonitrile. Ionic liquid/DABCO system offers the Baylis–Hillman reaction product 1 in good yield (60–65%), while the yield (30 %) of the product was found to be less in acetonitrile/DABCO system. Product 1 was obtained with a very poor yield (10%) while performing the same action with benzaldehyde, methyl acrylate and hydrotalcite clay catalyst in acetonitrile solvent for 24 h. Table 1 (entries 3 and 4) shows that the ionic liquids were found inactive towards Baylis–Hillman reaction in the absence of the catalyst.
After optimizing different parameters like quantity of ionic liquid, the amount of hydrotalcite clay as well as the molar ratio of reactants, the Baylis–Hillman reaction product 1 in good yield (85–86 %) was obtained. Surprisingly hydrotalcite clay shows almost similar activity in both the ionic liquids for Baylis–Hillman reaction in term of reaction rate and yield (table 1, entries 8 and 10). Significant drop in yield of Baylis–Hillman reaction product 1 was found with ionic liquid/ hydrotalcite clay catalytic system while playing with the reaction time (table 1, entries 9 and 10). Temperature effects were also studied by performing three separate reactions using [tmba] [NTf2]/hydrotalcite clay catalytic system and the subsequent results were summarized in table 1 (entries 13–15). Lower yield (10–25%) of product 1 was obtained at 0°C as well as on − 50°C. Such temperature study shows that the Baylis–Hillman reaction works well at room temperature (25–30°C) with ionic liquid/hydrotalcite clay catalytic system. Straight away product isolation was achieved by adding ether, which generate bi-phasic medium with a clear supernatant organic layer containing product and catalyst phase.
After getting wonderful results with our proposed catalytic system, we screened a series of aliphatic and aromatic aldehydes (electron-rich and electron-deficient) with different acrylates for both the ionic liquids (scheme 2, table 2, entries 1–14). We obtained the Baylis–Hillman reaction products 2 in good yield (more than 54% in 24 h). As usual aliphatic aldehydes gave lower yields (table 2, entries 3 and 4, 54–61%) with respect to aromatic aldehydes. In case of aromatic aldehydes, the Baylis–Hillman reaction product was obtained with good yield (64–92%). We also tested our catalytic system for another t-butyl substituted acrylate (table 2, entry 2) with benzaldehyde and we obtained Baylis–Hillman reaction product 1 with good yield (64–68%) while the reaction between methyl acrylate with benzaldehyde gave a very higher yield (more than 85%). Cinnamaldehyde as well as β-phenylcinnamaldehyde were also tested to justify the application of the reported catalytic system, resulted in the corresponding Baylis–Hillman reaction products with good yield (68–82%).[15f]
2-Furfuraldehyde and trimethoxy benzaldehyde also gave their corresponding Baylis–Hillman product in good yield with both the ionic liquids (table 2, entries 8 and 9). On the other hand, we also tested four different electron deficient aryl aldehydes (as a difficult substrate) with methyl acrylate (table 2, entries 10–13) to strengthen the utility of ionic liquid-clay system compared to the existing protocol using DABCO method which requires longer reaction time. Surprisingly, ionic liquid-clay system was found highly active in terms of reaction rate and yield with respect to the conventional DABCO system. We obtained the corresponding Baylis–Hillman reaction products (for four different electron deficient aryl aldehydes with methyl acrylate) in acceptable yield (51–68 %) after 24 h while achieving the same yield more than 48 h are required in case of the conventional DABCO method.
The Garner aldehyde is configurationally stable and reported by several groups as chiral synthon for the stereo-selective synthesis of sphingolipids and their derivatives because it offers 2-amino-1,3-diol subunit as the main backbone of sphingolipids.[16] Drewas et al. reported the synthesis of (S-) Garner aldehyde-methyl acrylate with anticonfiguration using Baylis–Hillman reaction for the synthesis of sphingolipid analogue. Recently, Singh et al. reported the synthesis of N-((E,3S,4R)-5-benzylidene-tetrahydro-4-hydroxy-6-oxo-2H-pyran-3-yl) palmitamide followed by DABCO catalysed Baylis–Hillman reaction adduct (scheme 3, 14b and 14c) using (S-) Garner aldehyde with methyl acrylate under ultrasonication.[18]
We also justified our [tmba][NTf2]/hydrotalcite clay catalytic system for the synthesis of a Baylis–Hillman reaction product (table 2, entry 14, scheme 3, 14a and 14b) using (S-) Garner aldehyde and methyl acrylate at room temperature without using ultrasonication. We obtained the mixture of Baylis–Hillman reaction Baylis– Hillman reaction product 14a and 14b (scheme 3) in good yield (68%) along with justified 1H NMR data.[16]
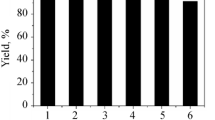
To evaluate the recycling of our catalytic system for Baylis–Hillman reaction, we let p-chlorobenzaldehyde and methyl acrylate to react in our [tmba][NTf2]/ hydrotalcite clay catalytic system for 24 h at room temperature. Afterwards the products were extracted with ethyl ether. A second slot of aldehyde and acrylate was further added to very same used catalytic system and the process was repeated up to 8 times. Ionic liquid/hydrotalcite clay catalytic system was found active up to five recycle after that slight drop in yield was observed (table 3).
4 Conclusion
In summary, ionic liquid/HT-clay catalytic system offers considerable increase in the rate of Baylis–Hillman reaction with respect to acetonitrile/DABCO system. In this report, we provide two different ionic liquids as an alternative solvent for hydrotalcite clay catalyst. As per the result, the reactants enjoy ionic liquid/ hydrotalcite clay catalytic system and gives the corresponding Baylis–Hillman reaction products in good yield. The application of our catalytic system is also diversified by synthesizing lactone ceramide analogue from (S − ) Garner aldehyde-methyl acrylate using Baylis–Hillman reaction. Now, It is important to notice that the recyclability of our ionic liquid/hydrotalcite clay catalytic system was carried out 8 times along with a simple isolation step of product from the reaction mixture.
References
Welton T and Wassersheid P 2008 Ionic liquids in synthesis, 2nd edition (Weinheim: Wiley-VCH) 488
Johnson K E 2007 Electrochem. Soc. Interface 16 38
Wagner M 2004 Chim. Oggi. 22 17
(a) Srivastava V 2010 Cent. Eur. J. Chem. 8 269; (b) Loh T P, Feng L C and Yang H Y 2002 Tetrahedron Lett. 43 8741; (c) Maria M, Toma S, Berkessel A and Koch B 2006 Lett. Org. Chem. 3 437; (d) Guo H M, Cun L F, Gong L Z, Mi A Q and Jiang Y Z 2005 Chem. Commun. 1450; (e) Toma S, Meciarová M and Sebesta R 2009 Eur. J. Org. Chem. 321; (f) Kitazume T, Jiang Z, Kasai K, Mihara Y and Suzuki M 2003 J. Fluorine Chem. 212 205
(a) Prechtl M H G, Scholten J D and Dupont J 2010 Molecules 15 3441; (b) Sowmiah S, Srinivasadesikan V, Tseng M C and Chu Y H 2009 Molecules 14 3780; (c) Karimi B and Zamani A 2012 Org. Biomol. Chem. 10 4531
Hangarge R V, Jarikote D V and Shingare M S 2002 Green Chem. 4 266
(a) Jeong Y and Ryu J S 2010 J. Org. Chem. 75 4183; (b) Rosa J N, Afonso C A M and Santos A G 2001 Tetrahedron 57 4189
Earle M J, McCormac P B and Seddon K R 1999 Green Chem. 1 23
(a) George S M 1995 Chem. Rev. 95 475; (b) Thomas J M and Thomas W J 1996 Principles and practice of heterogeneous catalysis (Weinheim: Wiley-VCH); (c) Hagen J 2006 Industrial catalysis: A practical approach (Weinheim: Wiley-VCH).
(a) Misra C and Perrotta A J 1992 Clays Clay Minerals 40 145; (b) Othman M R, Helwani Z and Fernando W J N 2009 Appl. Organomet. Chem. 23 335; (c) Kannan S 2006 Catal. Surv. Asia 10 117; (d) Debecker D P, Gaigneaux E M and Busca G 2009 Chemistry 15 3920; (e) Srivastava V 2012 Bull. Catal. Soc. India 11 33
(a) Kantam M L, Choudary B M, Reddy C K V, Rao K K and Figueras F 1998 Chem. Commun. 1033; (b) Bulbule V J, Deshpande V H, Velu S, Sudalai A, Sivasanka S and Sate V T 1999 Tetrahedron 55 9325 and references cited therein; (c) Kumbhar P S, Valente J S and Figueras F 1998 Chem. Commun., 1091; (d) Kumbhar P S, Valente J S and Figueras F 1998 Chem. Commun. 535; (e) Ueno S, Yamaguch K, Yoshida K, Ebitani K and Kaneda K 1998 Chem. Commun. 295
(a) Basavaiah D and Raghavaiah G V 2012 Chem. Soc. Rev. 41 68; (b) Singh V and Batra S 2008 Tetrahedron 64 4511; (c) Shi M, Wang F, Zhao M-X and Wei Y 2011 Chemistry of the Morita–Baylis–Hillman reaction RSC Catalysis Series; (d) Basavaiah D, Reddy B S and Badsara S S 2010 Chem. Rev. 110 5447; (e) Basavaiah D, Rao K V and Reddy R J 2007 Chem. Soc. Rev. 36 1581
(a) Aggarwal V K and Meeru A 1999 Chem. Commun. 2311; (b) Kataoka T, Iwama T, Kinoshita H, Surukami S, Iwwamura T and Watanabe S 1999 Synlett 197; (c) Hayase T, Shibata T S, Soai K and Wakatsuki Y 1998 Chem. Commun. 1271; (d) Li G, Wei H-X, Gao J J and Caputo T D 2001 Tetrahedron Lett. 41 1; (e) Chen S H, Hong B C, Su C F and Sarshar S 2005 Tetrahedron Lett. 46 8899; (f) Rosa J N, Afonso A M and Santos A G 2001 Tetrahedron 57 4189; (g) Aggarwal V K, Emme I and Mereu A 2002 Chem. Cummun. 1612
(a) Aravind A, Sanil George G and Kumar S K 2007 Chem. Central J. 1 435; (b) Giacalone F, Gruttadauria M, Marculescu A M, D’Anna F and Noto R 2008 Catal. Commun. 9 1477; (c) Hullio A A and Mastoi G M 2001 Jordan J. Chem. 7 125; (d) Sammelson R E and Kurth M 2001 J. Chem. Rev. 101 137
(a) Srivastava V, Gaubert K, Pucheault M and Vaultier M 2009 Chem. Cat. Chem. 98 94; (b) Khan F A, Dash J, Satapathy R and Upadhyay S K 2004 Tetrahedron Lett. 45 3055; (c) Srivastava V 2013 J. Chem. 2013 1 (Article ID 439673); (d) Srivastava V 2013 J. Chem. 2013 1 (Article ID 954094); (e) Srivastava V 2012 Asymmetric Organocatalysis 1 2; (f) Kim K H, Lee, H S, Kim Y M and Kim J N 2011 Bull. Korean Chem. Soc, 32 1087
Kumar G, Kaur S and Singh V 2011 ARKIVOC 148
Verron J, Joerger J M, Pucheault M and Vaultier M 2007 Tetrahedron Lett. 48 4055
Miyata S 1975 Clays Clay Minerals 369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SRIVASTAVA, V. Recyclable hydrotalcite clay catalysed Baylis–Hillman reaction. J Chem Sci 125, 1207–1212 (2013). https://doi.org/10.1007/s12039-013-0472-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0472-0