Abstract
Triple-negative breast cancer (TNBC) is a one of the subtypes of breast cancer which accounts for approximately 10–20% of all breast cancers. LncRNA XIST (XIST) is reported to be dysfunctional in numerous tumor types and is involved in the key pathways of cancer initiation, progression and metastasis. Thus, in the present study, we explored the detailed molecular mechanism of XIST in TNBC. XIST was down-regulated in TNBC tissues and cell lines. Overexpressed XIST inhibited cell proliferation, epithelial mesenchymal transition (EMT) and induced apoptosis in vitro as well as suppressed TNBC tumor growth in vivo. MicroRNA (miR)-454 was up-regulated in TNBC tissues and cell lines. Knockdown of miR-454 inhibited TNBC progression by suppressing cell proliferation, EMT and inducing cell apoptosis. Moreover, miR-454 was predicted and confirmed to be a target of XIST, and rescue assay indicated that overexpressed miR-454 could reverse XIST restoration mediated-anti-tumor effects on TNBC cells. In conclusion, XIST interacts with miR-454 to inhibit cells proliferation, EMT and induce apoptosis in TNBC, indicating a promising treatment strategy for TNBC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Triple-negative breast cancer (TNBC) is a one of the subtypes of breast cancer, defined by negative for the estrogen receptor (ER), progesterone receptor (PR) and excess human epidermal growth factor-2 (HER2) protein, accounting for approximately 10–20% of all breast cancers (Savci-Heijink et al. 2015; Li et al. 2017). The typical features of TNBC are : it is more aggressive, has poorer prognosis and higher grade, and is more likely to spread and have recurrence compared to other subtypes of breast cancer (Wang et al. 2015; Navratil et al. 2015; Kumar and Aggarwal 2016). Due to the tumor cells lacking the necessary receptors, common treatments like hormone therapy and drugs that target estrogen, progesterone, and HER-2 are ineffective. Although some medicines are used to successfully treat TNBC, finding certain medications that can interfere with the processes that cause TNBC to grow is still urgent and is of intense interest.
Long non-coding RNA (lncRNA) is a type of RNA molecule with a transcript length of more than 200 nucleotides (Liu and Ding 2017). It does not encode proteins, but it regulates the expression of the gene such as epigenetic regulation, transcriptional regulation, and post-transcriptional regulation (Jarroux et al. 2017). LncRNAs are reported to be dysfunctional in numerous tumor types, such as lung cancer, prostate cancer, breast cancer and TNBC, and are involved in the key pathways of cancer initiation, progression and metastasis, which can play an oncogenic or tumor suppressor role (Peng et al. 2017), and the idea has been consolidated that the expression of lncRNAs have linked to the activation of factors such as p53, c-MYC, E2F, estrogen receptor or androgen receptor (Huarte 2015). In TNBC, several lncRNAs are identified as tumor-driving oncogenic lncRNAs and a few are identified as tumor suppressive lncRNAs: they are all particularly involved in cancer progression (Liu et al. 2015). The X inactivate-specific transcript (XIST), the 19 Kb long lncRNA (17 kb in mouse), is vital for the X-chromosome inactivation in embryogenic development (Penny et al. 1996). XIST has been elucidated the tumor suppressor role in breast cancer (Liu et al. 2015). However, the regulatory functions and molecular mechanisms of XIST on TNBC remain unclear.
MicroRNAs (miRNAs) is a class of small endogenous non-coding RNAs molecules (approximately 22 nt), stimulating translational repression or degradation and playing a vital role in the progress of gene expression (Zhou et al. 2016). Among several candidate miRNAs regulating cell progression, numerous evidences have reported that microRNA-454 (miR-454) expression was associated with tumor growth and dissemination in many cancers (Fan et al. 2017; Fu et al. 2018). In TNBC, miR-454 is involved in poor prognosis and may function as an oncogene via targeting AKT in TNBC (Li et al. 2017a, b; Cao et al. 2016). While, how miR-454 works remains indistinct yet.
In the present study, we found XIST was down-regulated in TNBC, and overexpressed XIST inhibited TNBC cell proliferation, epithelial mesenchymal transition (EMT) and induced apoptosis in vitro and suppressed tumor growth in vivo. Moreover, XIST directly bound to miR-454; XIST exerted anti-tumor effects via direct interaction with miR-454. Our study firstly identified the regulatory network of XIST/miR-454 axis in TNBC progression, which may shed new light on the metastasis and therapies in TNBC.
2 Materials and methods
2.1 Patients and specimens
A total of 35 primary TNBC tissues and adjacent normal tissues were obtained from the Suzhou Wuzhong People’s Hospital between September 2017 and January 2018. All specimens were confirmed by two independent pathologists and did not receive any medical treatment before surgery. Specimens were immediately stored at −80°C until experiment. The hospitals and all patients had signed informed consents and the project was approved by the Ethics Committee of the Suzhou Wuzhong People’s Hospital.
2.2 Cell culture and transfection
All human TNBC cell lines MDA-MB-468 and MDA-MB-231 were obtained from American Type Culture Collection (Manassas, VA, USA). The normal breast epithelial cell line MCF-10 A was purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MDA-MB-468 and MDA-MB-231 cells were cultured in DMEM (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Gibco), 100μg/ml streptomycin and 100 U/ml penicillin, and MCF-10A cells in DMEM/F12 medium (Gibco) with 10% FBS (Gibco). All cells were incubated at 37°C with the 5% CO2.
pcDNA3.1-XIST overexpression vector (XIST), pcDNA3.1 empty vector (vector), small interfering RNA (siRNA) against XIST (si-XIST), siRNA negative control (scramble), miR-454 mimic (miR-454), mimic negative control (NC), miR-454 inhibitor (anti-miR-454), inhibitor negative control (anti-NC) were obtained from Genepharma (Shanghai, China). Transfection was conducted by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After 48 h of transfection, the cells were used for subsequent analysis.
2.3 Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA was extracted from TNBC tissues and cells using TRIzol reagent (Invitrogen). For cell RNA extraction, cells were transfer into a centrifuge tube at a concentration of 3×107 and then incubated with 1 mL of Trizol reagent for 5 min at room temperature. As to tissues RNA extraction, 100 mg of tissues were added into a centrifuge tube, followed by adding with 1 ml of Trizol reagent for 5 min at room temperature. Next, each centrifuge tube was added with 0.2 mL of chloroform for 2 min and then centrifuged at 120,000g for 15 min at 4°C. Then the supernatant was collected and interacted with 0.5 mL isopropanol for 10 min, followed by centrifuging at 120,000g for 10 min at 4°C. Immediately, after removing the supernatant, the precipitate was retained and washed with 1 mL of 75% ethanol, and centrifuged at 7500g for 5 min at 4°C. After removing the supernatant and drying the RNA precipitate. RNA precipitate was add with 50 μL DEPC H2O for 5–10 min at 55–60°C. Finally, RNA concentration was qualified using NanoDrop (Thermo Fisher, Waltham, MA, USA).
After that, cDNAs were synthesized from with reverse transcriptase (TermoFisher), qRT-PCR was performed with SYBR Green Real-Time PCR kit (Thermo Fisher). U6 or GAPDH was as an endogenous control and all data were analyzed using the 2−ΔΔCt method. The special primers for miR-454 or U6 were purchased from GeneCopoeia and primers for XIST or GAPDH were listed as follows: XIST, forward 5′-GCATAACTCGGCTTAGGGCT-3′, reverse 5′-TCCTCTGCCTGACCTGCTAT-3′; GAPDH, forward 5′-TCTTTTGCGT- CGCCAGCCGAG-3′, reverse 5′-TGACCAGGCGCCCAATACGAC-3′.
2.4 Cell proliferation and apoptosis
The proliferative capacity of TNBC cells was examined using cell Counting Kit-8 (CCK-8) (Sigma, St. Louis, MO, USA). All cells were digested and seeded into 96-well plates after transfection at a density of 3000/well and maintained at 37°C with 5% CO2 for 24 h. Then CCK-8 reagents were added to the plates and incubated for 2 h. Finally, the absorbance was measured at 450 nm by a microplate reader.
Annexin V-FITC/PI apoptosis detection kit (BD Biosciences, San Jose, CA, USA) was used to analysis apoptosis rate in according to the manufacturer’s protocol. Finally, flow cytometry (BD Biosciences) was performed to detect cell apoptotic rate.
2.5 Western blot
Cells were lysed by RIPA buffer (Thermo Scientific), then a total of 50 μg lysates were separated on 10% SDS-PAGE gel and transferred them onto polyvinylidene difluoride (PVDF) membranes (Sigma), and blocked with 5% nonfat milk for 1 h at room temperature. After incubation with primary antibodies at 4°C overnight, the blots were interacted with HRP-conjugated secondary antibody (Abcam, Cambridge, MA, USA) for 2 h at room temperature after washed with TBST for four times. ECL substrates were used to visualize signals. GAPDH was used as an endogenous protein for normalization.
2.6 Luciferase reporter assay
The binding sequences of XIST and miR-454 were predicted by StarBase software. The wild-type XIST (XIST-wt) or XIST mutant (XIST-mut) containing miR-454 putative target sites were amplified and clone into pGL3 vectors (Promega, Madison, WI, USA) to generate XIST-wt or XIST-mut luciferase reporter vector (pGL3-XIST-wt or -mut), respectively. Then the luciferase reporter vector and miR-454 or miR-NC was co-transfected into MDA-MB-468 and MDA-MB-231 cells using Lipofectamine 2000 (Invitrogen). After 48 h of transfection, cells were lysed and luciferase activity was analyzed though a dual-luciferase assay kit (Promega).
2.7 RNA immunoprecipitation
RNA immunoprecipitation (RIP) assay was conducted by Magna RNA immunoprecipitation kit (Millipore, Billerica, MA, USA). MDA-MB-468 and MDA-MB-231 cells transfected with miR-454 or NC were lysed by RIP buffer and then cell lysis was incubated with magnetic beads coated with anti-Ago2 or IgG antibody (Abcam) at 4°C. Finally, the immunoprecipitated RNA was separated and analyzed by qRT-PCR.
2.8 Pull-down
MiR-454 and miR-NC were biotinylated to be Bio-miR-454, Bio-NC by GenePharma Company (Shanghai, China) and then were transfected into MDA-MB-468 and MDA-MB-231 cells, respectively for 48 h. Sunsequently, cells were collected and lysed and cell lysis was incubated with streptavidin-coated magnetic beads (Invitrogen) for 10 min and washed with buffer. Subsequently, the biotin-coupled RNA complex was pulled down and analyzed by qRT-PCR.
2.9 Murine xenograft assay
BALB/c nude mice (female, four-week-old) were purchased from Vital River Laboratory Animal Technology (Beijing, China) and randomly divided into two groups (6 mice per group). A total of 3×106 cells transfected with the lentivirus containing XIST or negative control (vector) were injected into the flanks of the BALB/c nude mice. The tumor size was regularly monitored and measured. After 40 days, the mice were sacrificed and the tumors samples were weighted and used for further analyzed. The animal experiments were approved by the Animal Research Committee of the Suzhou Wuzhong People’S Hospital and performed in accordance with the guidelines of the National Animal Care and Ethics Institution.
2.10 Statistical analysis
All data were expressed as the mean±SD from at least 3 independent experiments. The differences between two groups were analyzed by student’s t test and multiple comparisons were conducted by one-way analysis of variance (ANOVA). All statistical analyses were performed using GraphPad Prism 7 (GraphPad Inc., San Diego, CA, USA). P value less than 0.05 was considered statistically significant.
3 Results
3.1 XIST is down-regulated in TNBC tissues and cell lines
Firstly, XIST expression in tissues and cells were measured using qRT-PCR. The level of XIST was significantly decreased in TNBC tissues compared with that in adjacent non-tumor tissues (fold change 0.5) (P<0.05, figure 1A). Moreover, the expression of XIST was clearly down-regulated in MDA-MB-468 (fold change 0.3) and MDA-MB-231 (fold change 0.2) cells compared to in the normal breast epithelial cell line MCF-10 A (P<0.05, figure 1B).
3.2 XIST restoration inhibits TNBC cells proliferation, EMT and induces apoptosis in vitro
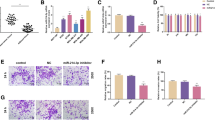
To elucidate the potential effects of XIST on TNBC cells, MDA-MB-468 and MDA-MB-231 cells were transfected with XIST or vector for 48 h, then overexpressed XIST was observed in MDA-MB-468 (fold change 5.0) and MDAMB-231 (fold change 5.2) cells transfected with XIST (P<0.05, figure 2A). Cell proliferation was detected using CCK-8 assays and results showed XIST overexpression inhibited cell proliferation in TNBC (P<0.05, figure 2B). Furthermore, flow cytometry results indicated XIST restoration induced apoptosis of MDA-MB-468 and MDA-MB-231 cells (P<0.05, figure 2C). Subsequently, EMT-related proteins (including E-cadherin, N-cadherin, Snail and Vimentin) were examined via western bolt and the results suggested overexpressed XIST facilitated the level of E-cadherin but suppressed the expression of N-cadherin, Snail and Vimentin both in MDA-MB-468 and MDA-MB-231 cells (P<0.05, figure 2D). These results indicated overexpressed XIST inhibited the proliferation, EMT and induce apoptosis of TNBC cells in vitro.
Biological functions of XIST in TNBC cells. (A) Transfection efficiency of XIST mimic was confirmed by qRT-PCR. Cells proliferation ability (B) and apoptosis rate (C) were detected by CCK-8 assay and Flow cytometry in MDA-MB-468 and MDA-MB-231 cells, respectively. (D) EMT-related proteins (including E-cadherin, N-cadherin, Snail and Vimentin) were examined via western bolt both in MDA-MB-468 and MDA-MB-231 cells. *P<0.05.
3.3 Overexpressed XIST inhibits tumor growth of TNBC in vivo
To further analyze the biological roles of XIST in TNBC tumorigenesis in vivo, MDA-MB-468 and MDA-MB-231 cells stably transfected with XIST or vector were used to establish xenograft model in vivo, respectively. After 5 weeks following the cell inoculation, the results of tumor volume and weight exhibited XIST restoration markedly reduced tumor growth in mice (P<0.05, figure 3A, C). Subsequently, molecular analyses were applied in harvested tumor tissues. The expression of XIST was greatly increased in XIST restoration group compared to control group (P<0.05, figure 3B, D). Therefore, we confirmed that XIST overexpression inhibited TNBC tumorigenesis in vivo.
The effect of XIST interference on tumor growth in vivo. MDA-MB-468 and MDA-MB-231 cells stably transfected with XIST or vector were used to establish xenograft model in vivo, respectively. (A, C) Tumor volume was calculated every week and tumor weight was analyzed in each group after killing mice. (B, D) The expression of XIST was detected in each group at end point using qRT-PCR. *P<0.05
3.4 Knockdown of miR-454 restrains TNBC cells proliferation, EMT and induces apoptosis
The expression of miR-454 was detected and results suggested miR-454 was notably increased in TNBC tissues (fold change 2.5) and cell lines (3.23 with MDA-MB-468 cell; 4.23 with MDA-MB-231 cell) (P<0.05, figure 4A, B). After that, loss-of-function was performed, MDA-MB-468 and MDA-MB-231 cells were transfected with anti-miR-454 or anti-NC for 48 h and a significantly reduction of miR-454 was observed in MDA-MB-468 (fold change 0.31) and MDAMB-231 (fold change 0.26) cells (P<0.05, figure 4C). Subsequently, functional analyses indicated that knockdown of miR-454 induced the inhibition of TNBC cells proliferation and the enhancement of TNBC cells apoptosis (P<0.05, figure 4D, E). Furthermore, western blot results indicated miR-454 inhibition led to increased E-cadherin expression and a decreased levels of N-cadherin, Snail and Vimentin both in MDA-MB-468 and MDA-MB-231 cells (P<0.05, figure 4F). These data suggested that knockdown of miR-454 could suppressed TNBC cells progression.
The biological effects of miR-454 on TNBC cells. The expression of miR-454 was examined in TNBC tissues (A) and cell lines (B) using qRT-PCR. (C) Transfection efficiency of XIST was confirmed by qRT-PCR. Cells proliferation ability (D) and apoptosis rate (E) were detected by CCK-8 assay and Flow cytometry in MDA-MB-468 and MDA-MB-231 cells, respectively. (F) EMT-related proteins (including E-cadherin, N-cadherin, Snail and Vimentin) were analyzed by western bolt both in MDA-MB-468 and MDA-MB-231 cells. *P<0.05.
3.5 XIST directly binds to miR-454
To explore the underlying molecular mechanism of XIST involved in TNBC progression, the potential targets were explored by bioinformatics analysis via StarBase software. MiR-454 was predicted to be a target of XIST with a putative binding site (figure 5A). Subsequently, the luciferase reporter assay results indicated that luciferase activity was significantly reduced in MDA-MB-468 (reduce from 1 to 0.26) and MDA-MB-231 (reduce from 1 to 0.25) cells co-transfected with XIST-wt and miR-454 compared with the NC control, while the inhibition effects of miR-454 mimic were abolished in XIST-mut group (P<0.05, figure 5B). Then RIP assay was used and the data suggested abundance of miR-454 engendered obvious increase of enrichment of XIST after Ago2 RIP in MDA-MB-468 (fold change 4.5) and MDA-MB-231 (fold change 4) cells, whereas its effect was lost in response to IgG RIP (P<0.05, figure 5C). Moreover, bio-miR-454-WT was found could enrich more XIST using RNA pull-down assay in MDA-MB-468 (fold change 6.5) and MDA-MB-231 (fold change 6) cells (P<0.05, figure 5D), indicating the directly interaction between miR-454 and XIST. Subsequently, we found that the level of miR-454 was clearly inhibited by overexpressed XIST, but obviously increased by XIST deletion in MDA-MB-468 (fold change 0.29 and 2.73, respectively) and MDA-MB-231 (fold change 0.35 and 2.57, respectively) cells (P<0.05, figure 5E). Besides that, we also confirmed that mut-XIST had no effects on miR-454 levels (P<0.05, supplementary figure 1), further suggesting XIST regulated miR-454 expression. Taken together, XIST directly bound to miR-454 and negatively modulated miR-454 expression.
XIST directly bound to miR-454. (A) The potential binding sites between XIST and miR-454. Luciferase reporter assay (B), RIP assay (C) and Pull-down assay (D) were used to demonstrate the interaction between XIST and miR-454. (E) The expression of miR-454 was detected by qRT-PCR in MDA-MB-468 and MDA-MB-231 cells transfected with XIST, vector, si-XIST, or scramble. *P<0.05.
3.6 Overexpressed XIST exerts anti-tumor effects via regulating miR-454
To verify whether miR-454 involved in XIST mediated anti-tumor effects on TNBC cells, MDA-MB-468 and MDA-MB-231 cells were transfected with XIST, vector, XIST + miR-NC, or XIST+ miR-454 for 48 h. After transfection, we found XIST restoration repressed the level of miR-454 (fold change 0.35 in MDA-MB-468, 0.3 in MDA-MB-231, respectively), but this suppression was rescued by miR-454 mimic (fold change 2.42 in MDA-MB-468, 2.59 in MDA-MB-231, respectively) (P<0.05, figure 6A). Afterwards, rescue assay indicated overexpressed miR-454 reversed XIST restoration mediated inhibition on proliferation, promotion on apoptosis and stimulation on EMT in MDA-MB-468 and MDA-MB-231 cells (P<0.05, figure 6B–D). Altogether, these data illustrated that XIST exerted anti-tumor effects by directly regulating miR-454 in TNBC.
XIST exerted anti-tumor effects via regulating miR-454. MDA-MB-468 and MDA-MB-231 cells were transfected with XIST, vector, XIST + NC, or XIST + miR-454 for 24 h. (A) The expression of miR-454 was examined by qRT-PCR after transfection. (B) Cells proliferation was detected using CCK-8 assay. (C) Flow cytometry was used to analysis cell apoptosis. (D, E) Western blot was utilized to determine the change of EMT related protein (including E-cadherin, N-cadherin, Snail and Vimentin). *P<0.05.
4 Discussion
LncRNAs are emerging stars in cancer diagnosis and therapy. Emerging evidence has revealed that aberrant expression, mutations and SNPs of lncRNAs are associated with tumorigenesis and metastasis, affecting the proliferation, apoptosis, and EMT of cancer cells (Huarte 2015; Zhang and Tang 2018). Increasing studies have reported that lncRNA expression was significantly altered in breast cancer tissue including TNBC while the effects of lnRNA are controversial. For example, lnRNA MALAT1, LINP1 and NEAT1 increased in TNBC tissues and cell lines, and blocking of them inhibited cell progression (Zhang et al. 2017a; Zhang et al. 2016; Zuo et al. 2017), which act as oncogenes. However, lncRNA GAS5 was found to be down-regulated in breast cancer and acted as a tumor suppressor.
XIST is a non-coding RNA which takes part in the beginning of X chromosome inactivation during early embryogenesis. It was first identified as an oncogene in human glioblastoma stem cells and played important roles in cell progression in glioblastoma (Yao et al. 2015). Studies have been performed to explore the role of XIST in cancers and previous reports have indicated that XIST was highly expressed and exerted as tumor-driving oncogenic in several cancers, including hepatocellular carcinoma (Chang et al. 2017), osteosarcoma (Lv et al. 2018), pancreatic cancer (Wei et al. 2017) and lung cancer (Tantai et al. 2015). However, several studies also confirmed that XIST inhibited tumor process in prostate cancer, gastric cancer and breast cancer (DL et al. 2016; Liu et al. 2015; Du et al. 2017). In the present study, we detected that XIST was significantly down-regulated in TNBC tissues and cells. Functional assay indicated overexpressed XIST inhibited TNBC cell proliferation, EMT and induced apoptosis in vitro as well as suppressed TNBC tumorigenesis in vivo. We firstly verified that XIST functioned as a tumor suppressor in TNBC.
Recently, studies have highlighted an lncRNA-mRNA interaction which is very similar to the miRNA regulation of mRNA and LncRNAs are reported to bind to miRNA and work as a sponge of miRNA (Xiong et al. 2017; Zhang et al. 2017b). XIST has been reported could bind various miRNAs in some common cancers. Wei et al. found XIST promoted pancreatic cancer proliferation by sponging miR-133a to regulate EGFR (Wei et al. 2017). XIST promoted gastric cancer progression through TGF-β1 via derectly interacting with miR-185 (Zhang et al. 2018). XIST interacted with miR-124 to modulate bladder cancer growth, invasion and migration (Xiong et al. 2017). Furthermore, in lung cancer, XIST regulated tumor growth by miR-140/iASPP axis (Tang et al. 2017). MiR-454 is a recently identified cancer-related miRNA. MiR-454 was found to be increased in colorectal cancer, lung cancer and hepatocellular carcinoma, and functioned as a potential oncogene to regulate cancer development (Liang et al. 2015; Yu et al. 2015; Zhu et al. 2016). Besides that, miR-454 was observed to be down-regulated in gastric cancer, glioblastoma, and osteosarcoma, which played anti-tumor effects (Fang et al. 2015; Niu et al. 2015; Song et al. 2017). In this study, the expression of miR-454 was significantly increased in TNBC tissues and cells, knockdown of miR-454 inhibited TNBC cells progression which was consistent with previous study (Lu et al. 2017). After that, miR-454 was predicted and confirmed to be a target of XIST. Thus, we further explored whether XIST/miR-454 responsible for the progression of TNBC, and results indicated that overexpressed XIST exerted anti-tumor effects via direct interaction with miR-454 in vitro.
In conclusion, we confirm that XIST was decreased in TNBC tissues and cell lines, XIST restoration inhibited TNBC cell proliferation and EMT, and induced apoptosis in vitro and suppressed TNBC tumor growth in vivo. Additionally, we first identified the regulatory network of XIST/miR-454 axis in TNBC progression, which may shed new light on metastasis and therapies of TNBC. In the future, we will explore the downstream signaling pathway targeted by XIST/miR-454 axis to provide more valuable information on the molecular regulation mechanisms of TNBC.
Abbreviations
- TNBC:
-
triple-negative breast cancer
- qRT-PCR:
-
quantitative real-time polymerase chain reaction
- EMT:
-
epithelial mesenchymal transition
- ER:
-
estrogen receptor
- HER2:
-
human epidermal growth factor-2
- lncRNA:
-
long non-coding RNA
- XIST:
-
inactivate-specific transcript
- FBS:
-
fetal bovine serum
- NC:
-
negative control
References
Cao ZG, Li JJ, Yao L, Huang YN, Liu YR, Hu X, Song CG, Shao ZM 2016 High expression of microRNA-454 is associated with poor prognosis in triple-negative breast cancer. Oncotarget 7 64900–64909
Chang S, Chen B, Wang X, Wu K and Sun Y 2017 Long non-coding RNA XIST regulates PTEN expression by sponging miR-181a and promotes hepatocellular carcinoma progression. BMC Cancer 17 248
Chen DL, Ju HQ, Lu YX, Chen LZ, Zeng ZL, Zhang DS, Luo HY, Wang F, et al. 2016 Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J. Exp. Clin. Cancer Res. 35 142
Du Y, Weng XD, Wang L, Liu XH, Zhu HC, Guo J, Ning JZ, Xiao CC 2017 LncRNA XIST acts as a tumor suppressor in prostate cancer through sponging miR-23a to modulate RKIP expression. Oncotarget 8 94358–94370
Fan Y, Shi C, Li T and Kuang T 2017 microRNA-454 shows anti-angiogenic and anti-metastatic activity in pancreatic ductal adenocarcinoma by targeting LRP6. Am. J. Cancer Res. 7 139–147
Fang B, Zhu J, Wang Y, Geng F and Li G 2015 MiR-454 inhibited cell proliferation of human glioblastoma cells by suppressing PDK1 expression. Biomed. Pharmacother. 75 148–152
Fu Q, Gao Y, Yang F, Mao T, Sun Z, Wang H, Song B and Li X 2018 Suppression of microRNA-454 impedes the proliferation and invasion of prostate cancer cells by promoting N-myc downstream-regulated gene 2 and inhibiting WNT/beta-catenin signaling. Biomed. Pharmacother. 97 120–127
Huarte M 2015 The emerging role of lncRNAs in cancer. Nat. Med. 21 1253–1261
Jarroux J, Morillon A and Pinskaya M 2017 History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 1008 1–46
Kumar P and Aggarwal R 2016 An overview of triple-negative breast cancer. Arch. Gynecol. Obstetrics 293 247–269
Li Q, Liu J, Meng X, Pang R, Li J 2017 MicroRNA-454 may function as an oncogene via targeting AKT in triple negative breast cancer. J. Biol. Res. 24 10
Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, O’Regan R, Torres MA and Meisel JL 2017 Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 161 279–287
Liang HL, Hu AP, Li SL, Xie JP, Ma QZ and Liu JY 2015 MiR-454 prompts cell proliferation of human colorectal cancer cells by repressing CYLD expression. Asian Pac. J. Cancer Prev. 16 2397–2402
Liu W and Ding C 2017 Roles of lncrnas in viral infections. Front. Cell Infect. Microbiol. 7 205
Liu Y, Sharma S and Watabe K 2015 Roles of lncRNA in breast cancer. Front. Biosci. 7 94–108
Lu L, Mao X, Shi P, He B, Xu K, Zhang S and Wang J 2017 MicroRNAs in the prognosis of triple-negative breast cancer: a systematic review and meta-analysis. Medicine 96 e7085
Lv GY, Miao J and Zhang XL 2018 Long noncoding RNA XIST promotes osteosarcoma progression by targeting Ras-related protein RAP2B via miR-320b. Oncol. Res. 26 837–846
Navratil J, Fabian P, Palacova M, Petrakova K, Vyzula R and Svoboda M 2015 [Triple Negative Breast Cancer]. Klinicka onkologie: casopis Ceske a Slovenske onkologicke spolecnosti 28 405–415
Niu G, Li B, Sun J and Sun L 2015 miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met. Cell Prolif. 48 348-355
Peng WX, Koirala P and Mo YY 2017 LncRNA-mediated regulation of cell signaling in cancer. Oncogene 36 5661–5667
Penny GD, Kay GF, Sheardown SA, Rastan S and Brockdorff N 1996 Requirement for Xist in X chromosome inactivation. Nature 379 131–137
Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J and van de Vijver MJ 2015 Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 150 547–557
Song Z, Li W, Wang L, Jia N and Chen B 2017 MicroRNA-454 inhibits tumor cell proliferation, migration and invasion by downregulating zinc finger Eboxbinding homeobox 1 in gastric cancer. Mol. Med. Rep. 16 9067–9073
Tang Y, He R, An J, Deng P, Huang L and Yang W 2017 lncRNA XIST interacts with miR-140 to modulate lung cancer growth by targeting iASPP. Oncol. Rep. 38 941–948
Tantai J, Hu D, Yang Y and Geng J 2015 Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 8 7887–7895
Wang C, Zhang J, Wang Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B and Xie Y 2015 Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann. Oncol. 26 523–528
Wei W, Liu Y, Lu Y, Yang B and Tang L 2017 LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J. Cell Biochem. 118 3349–3358
Xiong Y, Wang L, Li Y, Chen M, He W and Qi L 2017 The long non-coding RNA XIST interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR). Cell Physiol. Biochem. 43 405–418
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J, Chen L, Xi Z et al., 2015 Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 359 75–86
Yu L, Gong X, Sun L, Yao H, Lu B and Zhu L 2015 miR-454 functions as an oncogene by inhibiting CHD5 in hepatocellular carcinoma. Oncotarget 6 39225–39234
Zhang Y, He Q, Hu Z, Feng Y, Fan L, Tang Z, Yuan J, Shan W et al., 2016 Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat. Struct. Mol. Biol. 23 522–530
Zhang M, Wu WB, Wang ZW and Wang XH 2017a lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 21 1020–1026
Zhang Y, Li Y, Wang Q, Zhang X, Wang D, Tang HC, Meng X and Ding X 2017b Identification of an lncRNAmiRNAmRNA interaction mechanism in breast cancer based on bioinformatic analysis. Mol. Med. Rep. 16 5113–5120
Zhang Y and Tang L 2018 The application of lncRNAs in cancer treatment and diagnosis. Recent Pat. Anticancer Drug Discov. 13 292–301
Zhang Q, Chen B, Liu P and Yang J 2018 XIST promotes gastric cancer (GC) progression through TGF-beta1 via targeting miR-185. J. Cell Biochem. 119 2787–2796
Zhou L, Qu YM, Zhao XM and Yue ZD 2016 Involvement of miR-454 overexpression in the poor prognosis of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 20 825–829
Zhu DY, Li XN, Qi Y, Liu DL, Yang Y, Zhao J, Zhang CY, Wu K et al., 2016 MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed. Pharmacother. 81 79–85
Zuo Y, Li Y, Zhou Z, Ma M and Fu K 2017 Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed. Pharmacother. 95 922–928
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Sorab Dalal.
Corresponding editor: Sorab Dalal
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Hou, L., Yin, L. et al. LncRNA XIST interacts with miR-454 to inhibit cells proliferation, epithelial mesenchymal transition and induces apoptosis in triple-negative breast cancer. J Biosci 45, 45 (2020). https://doi.org/10.1007/s12038-020-9999-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-020-9999-7