Abstract
Although questioned on several occasions, the existence of cancer stem cells (CSCs) has been confirmed by a number of studies on experimental animal models. Nevertheless, it was shown that CSC hypotheses have several limitations and inconsistencies regarding the explanation of CSC origin, CSC identification and isolation, possible heterogeneity within CSC population, as well as methodology issues in some studies that were carried out in order to prove CSC existence. The aim of this article is to give a short and comprehensive review of recent advances concerning CSC hypothesis and to describe its impact on modern molecular physiology and pharmacology research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, despite significant advances in molecular biology and clinical medicine, cancer still remains a major public health issue. Standard chemotherapy is often ineffective, and unable to eliminate all malignant cells in the body. During the past century, there have been many efforts to develop a theory that could successfully explain cancer pathogenesis and resistance to medications. In 1937, the research of Jacob Furth and Morton Kahn indicated that a single leukaemic cell, after being transplanted into a mouse, was able to produce a new hematopoietic malignancy (Furth and Kahn 1937). This led to the conclusion that within cancer cell population, certain cells might possess the ability to repopulate the cancer tissue and initiate new tumour growth. In the early 1960s, Ernest McCulloch, James Till and Andy Becker demonstrated the existence of self-renewing stem cells after injecting bone marrow into irradiated mice (McCulloch and Till 1960; Becker et al. 1963; Till et al. 1964). These and other experiments set the basis for the so-called cancer stem cell (CSC) hypotheses. The first exact proof of CSC existence was presented in 1994 by John Dick et al., who successfully identified and purified human acute myeloid leukaemia-initiating cells with distinct stem properties (Lapidot et al. 1994).
Today, CSCs are thought to be a small population of cancer cells that have the ability of unlimited growth, self-renewal, as well as differentiation into other, more specialized cancer cell types (Ichim and Wells 2006). When transferred to immunodeficient animal models, CSCs may form new tumour tissue, whereas other cancer cells in most cases do not possess that ability. It was suggested that CSCs are able to survive and regenerate the tumour even after a large percentage of malignant tissue has been destroyed by chemotherapy.
CSC hypothesis is often regarded as an alternative to standard model of clonal evolution (CE). This model states that tumours develop as a consequence of accumulated mutations and selection of clones over period of time, resulting in a heterogeneous group of malignant cell in which cells of the dominant population (selected and expanded because of their growth advantage) have more or less equal ability for tumour regeneration and repopulation (Nowell 1976; Visvader and Lindeman 2008). However, although questioned on several occasions, the existence of CSCs has been confirmed by a number of studies on experimental animal models. CSCs have been identified and isolated in various hematological as well as solid malignancies. In recent years, various research strategies have been focused on detecting specific surface markers and signalling pathways that could in the future lead to development of medicaments capable of targeting CSCs.
On the other hand, it was shown that CSC hypotheses has several limitations and inconsistencies regarding the explanation of CSC origin, CSC identification and isolation, possible heterogenicity within CSC population, as well as methodology issues in some studies that were carried out in order to prove CSC existence. Therefore, many authors today suggest that neither the CSC model nor the CE model should be ruled out when explaining cancer pathogenesis, and that the answer lies in the combination of these two models.
The aim of this article is to give a short and comprehensive review of recent advances concerning CSC hypothesis and to describe its impact on modern molecular physiology and pharmacology research.
2 Cancer stem cells in hematological and solid malignancies: Evidence of CSCs existence and identification of CSC markers
One of the first studies that significantly contributed to formation of CSC hypotheses was published in 1994 (Lapidot et al. 1994). The authors identified a subtype of acute myeloid Leukaemia (AML) cells with specific surface marker phenotype (CD34+ CD38−) that were able to produce large numbers of colony-forming progenitors when transplanted into severe combined immune-deficient (SCID) mice. CD34+ CD38+ and CD34− cells did not have these properties. These experiments confirmed the earlier line of thought that only a fraction of malignant cells has the capability to generate and sustain new tumour tissue upon transplantation. In 1997, Bonnet et al. also demonstrated that the cell capable of initiating human AML in SCID mice possesses the differentiative and proliferative capacities and the potential for self-renewal expected of a leukemic stem cell (Bonnet and Dick 1997).
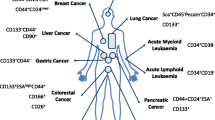
Today, it is known that CSCs are present in various solid tumours as well. Breast CSCs were first identified as CD44+CD24−/low cells in samples of human breast tumours (Al-Hajj et al. 2003). According to these authors, only 100 cells with this phenotype were enough to form tumours in SCID mice, whereas tens of thousands of cells with alternate phenotypes failed to form cancer tissue (Al-Hajj et al. 2003). A recent study on breast cancer cell lines confirmed the presence of functional CSCs with metastatic capacity and a distinct molecular signature (Charafe-Jauffret et al. 2009). Some breast CSCs were identified by detecting the expression of a specific stem cell marker, aldehyde dehydrogenase (ALDH), using fluorescent ALDEFLUOR assay as previously described (Ginestier C et al. 2007). After quantitative RT-PCR assessment of CSC population, it was shown that interleukin 8 (IL8) receptor CXCR1/IL8RA was consistently expressed in the ALDEFLUOR-positive cell population, indicating that the IL8 signalling pathway may be of great importance for CSC function (Charafe-Jauffret et al. 2009). Also, breast CSCs are thought to be stimulated by estrogen through the FGF/Tbx3 signalling pathway that similarly controls normal mammary epithelial stem cell biology (Fillmore et al. 2010).
There is a considerable amount of evidence that brain cancers are also hierarchically organized. Human meduloblastoma CD 133+ cells were shown to be capable of initiating new tumour tissue (consisting of both CD 133+ and CD 133− cells) after intracranial transplantation into SCID mice (Singh et al. 2004). There are also reports that glioma stem cells have some unique properties, such as preferential activation of the DNA damage response, which makes them resistant to radiotherapy (Bao et al. 2006). Those reports suggest that CD133+ tumour cells could be the source of tumour recurrence after radiation (Bao et al. 2006). The exact signalling pathways that regulate brain CSC function (self-renewal, differentiation, etc.) are largely unknown. Two years ago, it was suggested that TGF-beta can induce the self-renewal capacity of glioma stem cells probably via the Smad-dependent induction of leukaemia inhibitory factor (LIF) and the subsequent activation of the JAK-STAT pathway (Peñuelas et al. 2009). Also, there are reports that the ‘stemness’ (stem-like properties) of brain CSCs may depend on T-cell activity (Irvin et al. 2010). We assume that in the next 5 years significant research efforts will be focused on clarifying the relationship between CSCs and immune responses modulation.
The existence of tumorigenic and non-tumorigenic cells within colon cancers has been shown by a number of recent studies (O’Brien et al. 2007; Ricci-Vitiani et al. 2007). All experiments were performed in immunodeficient mice, and CSCs were shown to be CD133-positive. Several research steps have been taken in order to identify a specific signalling pathway that could eventually lead to growth inhibition and destruction of these cells (de Sousa et al. 2011).
Recently, it has been suggested that human melanomas also contain tumour-initiating cells (Schatton et al. 2008). Melanoma CSCs were defined by expression of the chemoresistance mediator ABCB5. These authors stated that specific targeting of this tumorigenic minority population could inhibit tumour growth and impact the prognosis of the disease (Schatton et al. 2008). CD133 and ABCG2 molecules are also associated with melanoma stem cells (La Porta 2009). Still, many issues concerning these markers in melanoma remain unclear. Although CD133 and ABCG2 are sometimes expressed in cells with proportionately higher tumour propagating activity than other malignant cells, a clear demonstration of their involvement in self-renewal capacity in melanoma cancer stem cells still does not exist (La Porta 2009).
All in all, we can conclude that, so far, there is strong evidence that CSCs are present in a majority of solid and hematological malignancies that affect human population. Detection of specific surface markers that characterize CSCs is an important step that may impact future strategies in anti-cancer drugs design and development. Nevertheless, further research efforts are required determine the clinical significance of these findings, and to show whether CSCs are universal property of all cancers.
3 Cancer stem cell hypotheses has several limitations and inconsistencies
When the concept of CSCs was first formulated, many researchers thought that the standard model of CE of cancer needed major revision, and that CSC hypotheses could eventually even replace it as one of the main principles of cancer pathophysiology. However, several questions about CSCs function and role in cancer tissue remained unanswered.
First of all, there are issues concerning the methodology used in the studies to identify CSCs. Most of the in vivo experiments have so far been done on animal models (immune-deficient mice) injected with cells obtained from human cancer tissue samples. It is not clear how this xenotransplantation may affect the validity of the obtained data. These issues were addressed in the study by Kelly et al., who isolated primary pre-B/B lymphoma cells from three independent Eμ-myc transgenic mice and injected 10 to 105 cells into non-irradiated congenic animals (Kelly et al. 2007). The results of the experiment showed that regardless of the cell number injected, all animals developed disseminated lymphoma within 35 days, suggesting that the concept of cancer growth being always sustained by a rare cancer stem cell may be wrong (Kelly et al. 2007). Indeed, the main question that we should ask from this congenic transplantation study is, why are so few cells enough for cancer initiation when CSCs are thought to represent only a small fraction of tumour cell population? However, in a response to Kelly’s work, Kennedy et al. indicated that the use of xenotransplantation model is justified, having in mind the development of new, more sensitive xenograft assays that include depletion of residual immune activity, direct injection of cells into bone cavities and transgenic expression of human cytokines (Kennedy et al. 2007). Although these improved assays were able to detect higher percentages of CSCs, the preliminary data suggested that CSCs were still relatively rare in cancer cell population. For example, a novel genetically induced model of human B cell acute lymphoblastic leukaemia (B-ALL) transplanted into in immunodeficient mice showed the frequencies of leukaemia-initiating cells among leukemic blasts of approximately 1% (Barabé et al. 2007; Kennedy et al. 2007). We assume that although xenotransplantation models have certain limitations, especially concerning the effects of murine microenvironment on cancer cell behaviour, for the time being, xenotransplantation will remain a model of first choice for in vivo research, since mouse-to-mouse transfer may require a lot of financial resources and is difficult to carry out.
Secondly, it is speculated that the CSC population itself may in some circumstances show some degree of heterogeneity. For example, recently it was demonstrated that Brca1-deficient mouse mammary cancers contain heterogeneous cell populations (CD44+/CD24− and CD133+) with CSC characteristics (Wright et al. 2008). In acute myelogenous leukaemia, it has been reported that stem cells are rare and heterogeneous when assayed in SCID mice (Sarry et al. 2011). The authors of this study suggested that CSC phenotype possesses a certain degree of plasticity which was not reported in previous investigations (Sarry et al. 2011). There are several possible explanations for CSC heterogeneity. According to one them, some cancer tissues contain cancer progenitor cells that are more differentiated than CSCs but still possess some residual stem cell traits (Fábián et al. 2009).
Also, we should always have in mind that there is a possibility that classical stem cell hierarchy may not be present in all tumours. Some researchers speculate that populations of non-CSCs from various types of tumours have greatly differing susceptibilities to becoming CSCs in response to specific signals (Gupta et al. 2009). This ‘interconversion’ between the two populations, if proven, threatens to relativize the whole CSC concept. According to some other views, CSC assay conditions might have a significant effect on the success rate of transplantation, and thus, it may turn out that CSCs are not as rare as previously thought, particularly having in mind that much depends on recipient microenvironmental/niche factors (Bomken et al. 2010). Having in mind all limitations of CSC hypotheses, many authors agree that a more complex approach that includes both CSC and CE models are required for better understanding of cancer pathophysiology.
4 Future of CSC research
We have reason to believe that in the next decade, main research efforts concerning CSCs will be focused on developing new in vivo and in vitro methods suitable for identification of CSCs and their markers in hematological and solid malignancies (table 1). The very existence of CSCs has been proved on many occasions; however, the present models of cell organization and interaction in tumour tissue will have to be revised if we are to understand the main principles of tumour growth and metastasis. If CSCs are shown to be main (or only) cause of tumour propagation, the primary goal of our research should be to develop a medicament that could specifically target and destroy CSCs, overcoming their documented ability to develop drug resistance. To achieve this goal, various signalling pathways in CSCs will need to be identified and described. Several important steps in this direction have already been made by a number of laboratories. It is, for example, known that standard pathways for self-renewal of normal stem cells, such as Wnt, Notch and Hedgehog signaling, are also present in CSCs and have an important role in their function. Targeting critical steps in those pathways, however, will be complicated by signalling cross-talk as well as other issues (Takebe et al. 2011). Nevertheless, there are already several reports that CSCs can be selectively targeted without inflicting serious damage to normal stem cells (Visvader and Lindeman 2008). For example, parthenolide (PTL), an active component in Feverfew (Tanacetum parthenium), was found to selectively induce programmed cell death (apoptosis) in leukaemic stem cells, possibly by activation of proapoptotic p53 signalling pathway (Guzman et al. 2005). Novel types of monoclonal antibodies directed to the adhesion molecule CD44 can also specifically target leukemic stem cells (Jin et al. 2006). Finally, in some solid tumours, such as gliomas, a combination of targeted antiangiogenic therapy and cytotoxic chemotherapy can selectively reduce the CSC fraction (Folkins et al. 2007). These and other findings give us hope that in the future, novel, more efficient and less toxic anti-cancer medications could be developed. However, to achieve this objective, a multidisciplinary approach to CSC issue is required, including the cooperation between molecular medicine researchers and clinical practitioners.
Abbreviations
- ALDH:
-
aldehyde dehydrogenase
- AML:
-
acute myeloid leukaemia
- CE:
-
clonal evolution
- CSC:
-
cancer stem cell
- IL8:
-
interleukin 8
- LIF:
-
leukaemia inhibitory factor
- PTL:
-
parthenolide
- SCID:
-
severe combined immune-deficient
References
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ and Clarke MF 2003 Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100 3983–3988
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN 2006 Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature (London) 444 756–760
Barabé F, Kennedy JA, Hope KJ and Dick JE 2007 Modeling the initiation and progression of human acute leukemia in mice. Science 316 600–604
Becker AJ, McCulloch EA and Till JE 1963 Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature (London) 197 452–454
Bomken S, Fiser K, Heidenreich O and Vormoor J 2010 Understanding the cancer stem cell. Br. J. Cancer 103 439–445
Bonnet D and Dick JE 1997 Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3 730–737
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, et al. 2009 Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 69 1302–1313
de Sousa EM, Vermeulen L, Richel D and Medema JP 2011 Targeting Wnt signaling in colon cancer stem cells. Clin. Cancer Res. 17 647–653
Fábián A, Barok M, Vereb G and Szöllosi J 2009 Die hard: are cancer stem cells the Bruce Willises of tumor biology? Cytometry A 75 67–74
Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES and Kuperwasser C 2010 Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. USA 107 21737–21742
Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ and Kerbel RS 2007 Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 67 3560–3564
Furth J and Kahn MC 1937 The transmission of leukaemia of mice with a single cell. Am. J. Cancer. 31 276–282
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, et al. 2007 ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1 555–567
Gupta PB, Chaffer CL and Weinberg RA 2009 Cancer stem cells: mirage or reality? Nat. Med. 15 1010–1012
Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS and Jordan CT 2005 The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 105 4163–4169
Ichim CV and Wells RA 2006 First among equals: the cancer cell hierarchy Leuk. Lymphoma 47 2017–2027
Irvin DK, Jouanneau E, Duvall G, Zhang XX, Zhai Y, Sarayba D, Seksenyan A, Panwar A, Black KL and Wheeler CJ 2010 T cells enhance stem-like properties and conditional malignancy in gliomas. PloS One 5 e10974
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F and Dick JE 2006 Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 12 1167–1174
Kelly PN, Dakic A, Adams JM, Nutt SL and Strasser A 2007 Tumor growth need not be driven by rare cancer stem cells. Science 317 337
Kennedy JA, Barabé F, Poeppl AG, Wang JC and Dick JE 2007 Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science 318 1722
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and Dick JE 1994 A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature (London) 367 645–648
La Porta C 2009 Cancer stem cells: lessons from melanoma. Stem Cell Rev. 5 61–65
McCulloch EA and Till JE 1960 The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiation Res. 13 115–125
Nowell PC 1976 The clonal evolution of tumor cell populations. Science 194 23–28
O’Brien CA, Pollett A, Gallinger S and Dick JE 2007 A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature (London) 445 106–110
Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, et al. 2009 TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 15 315–327
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C and De Maria R 2007 Identification and expansion of human colon-cancer-initiating cells. Nature (London) 445 111–115
Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, et al. 2011 Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J. Clin. Invest. 121 384–395
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, et al. 2008 Identification of cells initiating human melanomas. Nature(London) 451 345–349
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB 2004 Identification of human brain tumour initiating cells. Nature (London) 432 396–401
Takebe N, Harris PJ, Warren RQ and Ivy SP 2011 Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 8 97–106
Till JE, McCulloch EA and Siminovitch L 1964 A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Natl. Acad. Sci. USA 51 29–36
Visvader JE and Lindeman GJ 2008 Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8 755–768
Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV and Varticovski L 2008 Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 10 R10
Acknowledgements
The author is grateful to The Ministry of Education and Science, Republic of Serbia, Research Projects 175059 and 41027.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Pantic I 2011 Cancer stem cell hypotheses: Impact on modern molecular physiology and pharmacology research. J. Biosci. 36 1–5] DOI
Rights and permissions
About this article
Cite this article
Pantic, I. Cancer stem cell hypotheses: Impact on modern molecular physiology and pharmacology research. J Biosci 36, 957–961 (2011). https://doi.org/10.1007/s12038-011-9155-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-011-9155-5