Abstract
The circadian clock is an endogenous system designed to anticipate and adapt to daily changes in the environment. Alzheimer’s disease (AD) is a progressive neurodegenerative disease, which is more prevalent in patients with type 2 diabetes mellitus (T2DM). However, the effects of circadian disruption on mental and physical health for T2DM patients are not yet fully understood, even though circadian disruption has been confirmed to promote the progression of AD in population. By housing db/db mice on a disrupted (a 6:18 light/dark cycle) circadian rhythm, we assessed the circadian gene expression, body weight, cognitive ability, and AD-related pathophysiology. Our results indicated that housing in these conditions led to disrupted diurnal circadian rhythms in the hippocampus of db/db mice and contributed to their weight gain. In the brain, the circadian-disrupted db/db mice showed a decreased cognitive ability and an increased hyperphosphorylation of tau protein, even though no difference was found in amyloid protein (Aβ) plaque deposition. We also found that the hyperphosphorylated tau protein exhibited more disruptive daily oscillations in db/db mice hippocampus under the 6:18 light/dark cycle. Circadian alterations could promote the development of AD in T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost all plants and animals exhibit inherent 24-h oscillations to adapt the Earth’s rotation [1]. The main function of circadian rhythms is to prepare an organism for the potential outer opportunities and challenges. In mammals, the circadian timing system is composed of the central clock located in the hypothalamic suprachiasmatic nucleus (SCN) and multiple peripheral clocks. SCN receives photic signals and synchronizes the physiology of the organism to external environmental changes. The light is regarded as the most important zeitgeber. The peripheral clocks mainly get entrained timing signal from SCN. The central molecular mechanism of circadian rhythms is the transcriptional–translational feedback loop (TTFL) [2]. In this circadian TTFL, the typical “clock genes” mainly include circadian locomotor output cycles kaput (CLOCK), brain muscle ARNT-like protein 1 (BMAL1), period (PER1, PER2, and PER3), cryptochrome (CRY1 and CRY2), and the genes encoding the nuclear receptors REV-ERB (REV-ERBα/REV-ERBβ) [3]. These molecular clocks maintain the rhythmic signal output approximately a 24-h period. Many biological processes are related to circadian control, such as sleep-wake cycles, hormone secretion, and blood pressure.
Type 2 diabetes mellitus (T2DM) is a rapidly increasing disease worldwide characterized by hyperglycemia and insulin resistance [4]. According to WHO Executive Summary, diabetes caused 1.5 million deaths and ranked 4th overall in noncommunicable diseases globally in 2012 [5]. Traditionally, researches focused on lifestyle risk factors, such as physical inactivity and poor diet; in recent decades, circadian factors, including sleep-wake behavior and the timing of light exposure, also have been proved important in the development of T2DM [6]. Studies indicated that our main organs of energy metabolism (such as liver, adipose tissue, and pancreas) contained an autonomous clock and showed metabolic rhythms [7]. The activity of the cortisol secretion presented a diurnal rhythm, which had a great effect on glucose and insulin levels [8, 9]. Therefore, a close association between diabetes and circadian clocks is raised. Disruption of circadian clocks has been confirmed to affect glucose metabolism. Loss of Clock was easily developed into metabolic syndrome in mice [10]. Knockdown of circadian Bmal1 will lead to glucose intolerance, fasting, and diurnal hyperglycemia as well as impair glucose-stimulated insulin secretion [11, 12]. Circadian rhythms play a key role in the glucose control.
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is the 5th leading cause of death in the population over 65 [13]. The typical pathophysiological changes of AD are the deposition of extracellular amyloid β (Aβ) plaques and the accumulation of intracellular neurofibrillary tangles (NFT) due to hyperphosphorylated tau proteins [14]. Recent evidence held that circadian disruption could present in the early stage of the disease [15]. Mice with chronic circadian disruption showed a decrease in cognitive flexibility and a loss of dendritic length [16]. Circadian clocks regulated Aβ oscillations in the hippocampus and had an impact on amyloid plaque deposition in AD models [17], which proved that circadian rhythms could directly influence AD pathogenesis. In Tg4510 mice (a model of tauopathy), the expression of Per2 and Bmal1 were evidently disrupted in the hippocampus [18]. Thus, circadian disruption is considered a cause of AD [19].
Reports have demonstrated that patients with T2DM are closely associated with AD, especially in the elderly [13, 20]. AD is even proposed the term “type 3 diabetes” due to the shared similar mechanisms with T2DM, such as oxidative stress, PI3K–GSK3β signaling, and inflammatory pathways [13]. In modern life, patients with T2DM are more easily exposed to environmental circadian disturbance, such as staying up late and transmeridian flight. We would like to confirm whether patients with T2DM under circadian disruption could accelerate the progression of AD.
In the present study, we imitated the circadian disruption condition of modern industry. Disruption was induced by housing db/db mice in the 6:18 light/dark (LD) cycle, whereas controls were maintained in the normal 12:12 LD cycle. We evaluated the circadian gene expression, body weight, cognitive ability, and AD-related pathophysiology to study the association between T2DM and AD after circadian disruption.
Materials and Methods
Animals
The 5-week-old db/db mice (BKS-Leprem2Cd479/Gpt, strain number: T002407) were purchased from the GemPharmatech Co., Ltd. (Jiangsu, China). Mice were given 1 week to habituate to the facility and allowed ad libitum access to normal chow and water. For circadian disruption, one group (n = 16) was kept on the 6:18 LD cycle. The lights were switched at 7 am and off at 1 pm, which were regarded as zeitgeber time (ZT) 0 and ZT6 respectively. The control group (n = 16) remained in the 12:12 LD cycle, with lights on at 7 am (ZT0) and off at 7 pm (ZT12). All animal experiments were approved by the Animal Care and Use Committee of Tongji Hospital in accordance to the Public Health Service Policy on Human Care and Use of Laboratory Animals.
At the age of 14 weeks, behavioral tasks were performed in the db/db mice to assess their cognitive ability. Then, they were sacrificed every 6 h in 1 day (ZT0, ZT6, ZT12, and ZT18) to evaluate the effects of light on diurnal rhythmicity. Three to four mice were sacrificed at each time point to explore the association between circadian disruptions with tau phosphorylation. Reports have demonstrated that Aβ peptide levels exhibited robust daily oscillations in mouse hippocampal interstitial fluid [21]. Thus, we compared overall Aβ deposition status between the two groups (the control group, n = 3; the experimental group, n = 5), rather than at different time points.
Our mice were sacrificed by cervical dislocation to eliminate anesthesia-mediated tau phosphorylation [22]. In the most mice, one hippocampus was used for Western blots to test phosphorylation level of tau protein and another was for qPCR to measure gene expression of circadian clocks. For minority, the brains of mice were removed and sagittally bisected. The right hemisphere was fixed in 4% paraformaldehyde for immunohistochemical analysis; the hippocampus was separated from the left hemisphere and frozen at −80 °C for Western blots and qPCR analysis.
Behavioral Assessment
Open Field Test
For all behavior tests, we adapted the protocols from Volmar et al. [23]. The open field test was performed to test the locomotor activity of db/db mice. Briefly, the open field arena was made up of a standard clear plastic box (45 × 45 × 45 cm) placed in a quiet, well-lit room. Animals were individually put in the center of the box for 10 min. The tracks of mice were recorded using a computerized Ethovision detection system (Noldus, The Netherlands). Total distance and average speed each mouse traveled during that time were automatically recorded. Arena was clean by 70% ethanol between different tests.
Novel Object Recognition Test
A novel object recognition test aimed to assess hippocampal-associated contextual learning in rodents. This test was performed in the same box where the open field test was conducted. First, each mouse was allowed to explore two identical objects in the arena for 5 min and then it was sent back to home cage. After 30-min interval, this mouse was exposed to two different objects (one was the previous object and another was a novel object with different shape and color) in the arena. The frequency and time the mouse explored the novel object were used to evaluate the use of learning and recognition memory. Frequency-related memory index was defined as [(frequency of novel object investigation)/(total frequency of investigation of both objects)*100] and time-related index was calculated as [(novel object investigation time)/(total investigation time of both objects)*100] [23].
Barnes Maze Test
A Barnes maze test was used as a test for spatial learning and memory assessment. Simplified Barnes maze contained “Acquisition” and “Probe” trials.
Barnes Maze Acquisition Trial
We used a dark gray PVC circular platform (100 cm in diameter, elevated 90 cm above the floor) with 20 holes (5 cm in diameter) as the Barnes maze. An escape box was hidden under one of these holes. The aversive cues (the bright light and buzzer sound) were set up in the surroundings to stimulate mice to escape in 5 min. When the mice were exposed to these conditions, they were allowed to escape or were gently guided to the box. From day 1 to day 3, we trained animals for twice with an inter-trial interval of 30 min. On day 4, the mice had a rest for 24 h. Then, on the final day, we conducted the Probe trial.
Barnes Maze Probe Trial
In Probe trial, the escape box was removed from holes. Target zone was the goal hole where the escape box was previously located in the Acquisition test. The errors the mouse made before finding the target zone were used as an evaluation of spatial memory retention.
RNA Isolation and RT-qPCR
Hippocampus samples were collected for RNA isolation at different ZTs. Total RNA was extracted by TRIzol reagent according to the manufacturer’s instructions (Takara Bio, Japan). cDNA was synthesized using the Hifair II Reverse Transcription System (Yeasen, China). Real-time reverse transcriptase–PCR was conducted using the QuantStudioTM 1 system (Thermo Fisher Scientific Biosystem) and SYBR Green qPCR Master Mix (Yeasen, China). Gene expression was normalized to housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase, Gapdh). The primer sequences were referred to previous study [3]. Comparative CT method (2−ΔCT) was used to calculate the relative gene expression.
Western Blots
The total protein was obtained with the cell lysis buffer for western and IP containing a protease and phosphatase inhibitor (Beyotime, China). Subsequently, the samples were centrifuged for 15 min at 15,000×g at 4°C, and the supernatants were collected for the next steps (the protein extracts were mainly the soluble tau [24]). The concentration of protein was measured via a bicinchoninic acid (BCA) assay (Boster, China).
Equal amounts of protein (20 μg) samples were separated in 10% Bis-Tris SDS-polyacrylamide gels and then transferred to the PVDF membranes at 200 mA. The membranes were blocked in blocking buffer for 1 h at room temperature, and then were incubated overnight with primary antibody at 4°C. The primary antibodies included tau5 (1:5000, Abcam, Cat # ab80579), p-Ser199 (1:5000, Abcam, Cat # ab81268), p-Ser396 (1:5000, Abcam, Cat # ab109390), p-Thr231 (1:5000, Abcam, Cat # ab151559), and β-actin (1:10000, Proteintech, Cat # 66009-1-Ig). After washing for several times, the membranes were incubated with secondary antibodies (1:7500, Proteintech, Cat # SA00001-1 and SA00001-2) for 2 h. The immunoreactive bands were visualized by enhanced chemiluminescence (ECL) detection (Biosharp, China), and were scanned using GelView 6000 Pro (Antpedia, China). Each band density was measured using the ImageJ software (V1.8.0.112).
Immunohistochemical Analysis
The immunohistochemistry analysis was detected as the previous study [25]. The immunoreactivities of the CA1 hippocampal regions were measured with specific primary antibodies against anti-beta amyloid 1-42 antibody (1:200, Abcam, ab201061). We used the streptavidin–horseradish peroxidase method and the reaction was visualized with the diaminobenzidine (DAB) detection process. The Aβ 1-42 immunoreactivities were calculated by counting blindly the numbers of Aβ plaques in CA1 regions under ×10, ×20, and ×40 magnification. Data was collected from four random fields in every section (n = 3-5) for statistical analysis.
Statistical Analysis
All statistical analyses were assessed by Student’s t test and one-way and two-way ANOVAs using SPSS (version 24.0 for Windows). Figures were plotted with GraphPad Prism 8. The ImageJ software (V1.8.0.112) was used to evaluate the band density of tau protein. We used post hoc with Bonferroni’s correction when appropriate. Results were regarded as significance at P < 0.05.
Results
Altered Light Cycles Contributed to Disrupted Circadian Rhythms in the Hippocampus in db/db Mice
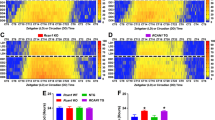
Light from environment is the important zeitgeber to coordinate the oscillation between outside and body rhythms. In contrast to human, the rodents are always active at night and take a rest during the day. We prolonged the dark duration of mice to simulate the overexposed to artificial light in this industrial society. The diurnal expression of key clock genes in the hippocampus were measured to confirm the effects of light on hippocampal rhythmicity. Our results found that the rhythms of Bmal1 and Rev-erbα expression were significantly disrupted in the 6:18 LD cycle (Fig. 1A and 1C), which indicated that the hippocampus was susceptible to light-induced rhythm disturbance, while no change was observed in gene Per2 expression compared to the 12:12 LD cycle (Fig. 1B).
Altered light/dark cycles induced hippocampal rhythm disruption. Bmal1 (A) and Rev-erbα (C) expression were significantly disrupted in the 6:18 LD cycle (n = 3-4), while gene Per2 expression showed no difference compared to controls (B, n = 4). ZT, zeitgeber time; data were presented as mean ± SEM. *P < 0.05, **P< 0.01, ***P < 0.001
Circadian Disruption Contributed to Weight Gain
Mice in the both groups showed a gradually weight gain since the first week (Fig. 2A). On the 2nd week, the increase in body weight of the mice fed in the 6:18 LD cycle was significant. On the 8th week, the db/db mice displayed a decrease in weight gain probably because their pancreas function was in failure [26]. We also tested their blood glucose. Given that the blood glucose levels ranged from 5.6 to 11.1 mmol/L in wild-type mice [27], the db/db mice exhibited continuous hyperglycemia during experiment (Fig. 2B).
Circadian disruption resulted in weight gain. The body weight gain of the mice fed in the 6:18 LD cycle was significantly higher than the controls (A, n = 15 in each group). The blood glucose between the two groups showed no difference (B, n = 15 in each group). Data were presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Circadian-Disrupted Animals Showed Decreased Cognitive Ability
We further examined the effects of circadian disruption on locomotor behaviors. We observed no significant difference between the 12:12 LD cycle group and the 6:18 LD cycle group for total distance traveled or average velocity (Fig. 3A and 3B). In the next day, we measured the novel object recognition in the same arena. There was no significant difference in the total time of mice spent exploring both objects in the two groups (Supplementary Figure). The circadian-disrupted mice showed significantly more poor performance than the control mice in novel object exploration frequency (Fig. 3C) and duration (Fig. 3D).
Circadian disruption led to decreased cognitive ability. No significant difference between the 12:12 LD cycle group and the 6:18 LD cycle group for total distance traveled (A) or average velocity (B) were observed. The novel object recognition was performed in the same open field in the next day. Circadian-disrupted mice showed significantly poor performance than the control mice in novel object exploration frequency (C) and duration (D). We subsequently assessed spatial memory of mice using a Barnes maze. The circadian-disrupted mice made more errors in Acquisition trials 3 and 5 compared with the controls (E). After 24 h of rest, the mice of the 6:18 LD cycle committed more errors than the 12:12 LD cycle in the Probe trial (F), indicating reduced spatial memory in disrupted mice. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, n = 15 in each group
We subsequently assessed spatial memory of mice using a Barnes maze. The circadian-disrupted mice made more errors in Acquisition trials 3 and 5 compared with controls (Fig. 3E). After 24 h of rest, the mice of the 6:18 LD cycle committed more errors than the 12:12 LD cycle in the Probe trial (Fig. 3F), indicating reduced spatial memory in mice with disrupted circadian rhythms.
Circadian Disruption Increased the Hyperphosphorylation of Tau Protein in the Hippocampus
Accumulation of tau protein was one of the typical pathophysiological characteristics of AD. To investigate the influence of circadian rhythms on hippocampal tauopathy, we further detected the phosphorylation levels of tau protein and the AD-related tau protein sites (Ser199, Ser396, and Thr231) in the hippocampus. The levels of tau protein were significantly hyperphosphorylated at the Ser199, Ser396, and Thr231 sites in the 6:18 LD cycle group (Fig. 4).
Circadian disruption increased the hyperphosphorylation of tau protein in the hippocampus. The phosphorylation levels of tau protein were detected in the two groups (n = 15 in each group). The AD-related tau protein sites (Ser199, Ser396, and Thr231) in the hippocampus were examined (A). The levels of tau protein were significantly hyperphosphorylated at the Ser199 (B), Ser396 (C), and Thr231 (D) sites in the 6:18 LD cycle group. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
We also observed the deposition of Aβ plaques in CA1 regions under ×10, ×20, and ×40 magnification (Fig. 5). However, no significance between the two groups was found.
The Hyperphosphorylation of Tau Protein Exhibited More Disruptive Daily Oscillations in Animals with Disrupted Circadian Rhythms
In order to explore the effects of circadian disruption on tau phosphorylation, we compared the phosphorylation levels of tau protein at different time points between the two groups. The results indicated that phosphorylation of tau protein showed daily oscillations. At ZT0 and ZT12 time points, no difference of tau phosphorylation was discovered between the 6:18 LD cycle and the 12:12 LD cycle group (Fig. 6). At ZT6 and ZT18 time points, the phosphorylation levels were significantly increased in the disrupted group at Ser199, Ser396, and Thr231 sites (Fig. 6). The loss of central circadian rhythms led to disruption of daily hippocampal phosphorylation of tau protein oscillations.
The phosphorylation of tau protein was regulated by circadian rhythms. The phosphorylation levels of tau protein at different time points (ZT0, ZT6, ZT12, and ZT18, n = 3-4 in each group at different time points) between the two groups were compared. At ZT0 and ZT12 time points, no difference of tau phosphorylation was discovered between the 6:18 LD cycle group and the 12:12 LD cycle group (A). At ZT6 and ZT18 time points, the phosphorylation levels were significantly increased in the disrupted group at Ser199 (B), Ser396 (C), and Thr231 (D) sites. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In the present work, we demonstrated a number of findings: (1) Altered light/dark cycles disrupted circadian rhythms in the hippocampus of db/db mice. (2) Circadian disturbance led to body weight gain. (3) Decreased cognitive ability was more obvious in circadian-disrupted mice. (4) Circadian disruption increased the hyperphosphorylation of tau protein in the hippocampus. (5) The hyperphosphorylation of tau protein exhibited more disruptive daily oscillations in animals with disrupted circadian rhythms. These findings indicated that disruption of circadian rhythms could influence weight and result in cognitive changes, demonstrating the role of circadian rhythms mattered in both mental and physical health.
First, we disrupted the circadian clock of db/db mice by changing the light duration, with the control group exposed to a normal 24-h day (a 12:12 LD cycle), and the disrupted group exposed to a longer dark (a 6:18 LD cycle) to imitate the stay-up in human. It is of note that, circadian rhythm disturbance that 1 day of 24 h is shortened into 20 h has been confirmed to have adverse effects on cardiac function [28] and impair memory [16]. Our method of changing light duration was applied in rodents to investigate the association between chronic disruption in light/dark cycles and brain trace element concentrations [29]. In Tahsili-Fahadan’s study, the mice were maintained on the 12:12 and 6:18 LD cycle respectively to evaluate the impact of chronic light/dark cycle changes on morphine-related conditioned place preference [30]. Here, we explored the progression of AD in db/db mice under similar circadian rhythm disturbance.
The studies linking sleep deficiency and obesity are increasing [31]. Our findings suggested that altered light/dark cycles resulted in the weight gain in db/db mice. The rodents are usually active during the night and sleep at daytime. When we did not restrict their access to food, the mice exposed to longer dark duration had longer feeding time. In human, it is like eating midnight snack when we stay up late in the evening. Some reports have also observed similar changes of weight in Clock mutant mice [10]. A longitudinal study has found that long-term exposure to night-shift work can promote weight gain and obesity in a nurse cohort [32]. We confirmed that the circadian disruption might accelerate weight gain in patients with T2DM.
In addition to body weight, we demonstrated behavior changes in the disrupted animals. Our behavioral results indicated that there was no difference between the two groups in open field test, suggesting that they performed similar locomotor activity in this arena. The mice of the 6:18 LD cycle showed a decreased ability in exploring the novel object, indicating contextual learning was impaired under disrupted circadian clocks. In a spatial memory task, the animals of disruptive circadian rhythms made more errors in finding targeting zone, which confirmed that circadian disruption might be harmful for longer-term learning in patients with T2DM. The sleep deprivation was confirmed to increase interstitial fluid (ISF) tau levels [33] and cognitive impairment [34] in wild-type mice. Previous work in humans also indicated that flight crews of short recovery had decreased performance on hippocampal memory and spatial cognitive deficits [35]. Our finding further confirmed that circadian disruption could accelerate the cognitive impairment in patients with T2DM.
Tau hyperphosphorylation, especially soluble tau, which leads to NFT accumulation, results in the destruction of cytoskeleton structural and microtubule dysfunction to promote cognitive disturbance [36, 37]. One research showed that phosphorylated tau levels were significantly increased in sleep-deprived mice [38]. Similarly, we found that the circadian rhythm disruption aggravated the hyperphosphorylation levels of soluble tau protein in db/db mice. Interestingly, we observed that the difference between the two groups varied at different time points. At ZT0 and ZT12, no significance was found in the two groups, while the 6:18 LD cycle had more increased hyperphosphorylation of tau protein than the controls at ZT6 and ZT18. The loss of central circadian rhythms led to more disruptive daily oscillations in phosphorylation of tau protein in the 6:18 LD cycle. These findings may provide an effective view to target tau-related therapy in AD. However, there are few reports on the rhythmicity of tau pathology, and further researches are needed to investigate this issue. We did not observe the significant difference between the two groups in brain Aβ plaques. Significant Aβ plaques were more likely to be observed in the AD mouse model [39]. In the young db/db mouse model, typical Aβ plaques were seldom found in many reports [40, 41]. It is quite possible that 14-week-old db/db mice show only early changes in AD (increase in oligomeric Aβ and phosphorylated tau) [42, 43], but do not show overt AD neuropathology (Aβ plaque deposition and tau tangle formation).
In the present work, we have some advantages. First, we found weight gain and decreased cognitions after circadian disruption in the diabetic model. Second, the phosphorylation of tau protein had rhythmicity, which will promote the new target of AD treatment. This research also has limitations. We have not explored the potential mechanism how circadian disruption promotes AD in diabetic mice. The Aβ deposition should be detected in older db/db mice.
In conclusion, our study suggested that the disruption of circadian rhythms could promote the progression of AD in db/db mice. Prevention from circadian disruption may slow down the progression of AD in patients with T2DM.
Data Availability
Data will be made available on reasonable quest.
References
Roenneberg T, Merrow M (2005) Circadian clocks-the fall and rise of physiology. Nat Rev Mol Cell Biol 6(12):965–971. https://doi.org/10.1038/nrm1766
Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM (1999) A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96(1):57–68. https://doi.org/10.1016/s0092-8674(00)80959-9
Woodie LN, Johnson RM, Ahmed B, Fowler S, Haynes W, Carmona B, Reed M, Suppiramaniam V et al (2020) Western diet-induced obesity disrupts the diurnal rhythmicity of hippocampal core clock gene expression in a mouse model. Brain Behav Immun 88:815–825. https://doi.org/10.1016/j.bbi.2020.05.053
Boles A, Kandimalla R, Reddy PH (2017) Dynamics of diabetes and obesity: epidemiological perspective. Biochim Biophys Acta Mol basis Dis 1863(5):1026–1036. https://doi.org/10.1016/j.bbadis.2017.01.016
WHO (2014) Global status report on noncommunicable diseases 2014.
Mason IC, Qian J, Adler GK, Scheer F (2020) Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63(3):462–472. https://doi.org/10.1007/s00125-019-05059-6
Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A (2019) Circadian clocks and insulin resistance. Nat Rev Endocrinol 15(2):75–89. https://doi.org/10.1038/s41574-018-0122-1
Buckley TM, Schatzberg AF (2005) On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90(5):3106–3114. https://doi.org/10.1210/jc.2004-1056
van Raalte DH, Diamant M (2014) Steroid diabetes: from mechanism to treatment? Neth J Med 72(2):62–72
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308(5724):1043–1045. https://doi.org/10.1126/science.1108750
Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, Ma K, Yechoor VK (2011) Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets 3(6):381–388. https://doi.org/10.4161/isl.3.6.18157
Rakshit K, Hsu TW, Matveyenko AV (2016) Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia 59(4):734–743. https://doi.org/10.1007/s00125-015-3859-2
Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol basis Dis 1863(5):1078–1089. https://doi.org/10.1016/j.bbadis.2016.08.018
Joe E, Ringman JM (2019) Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. Bmj 367:l6217. https://doi.org/10.1136/bmj.l6217
Musiek ES, Xiong DD, Holtzman DM (2015) Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 47:e148. https://doi.org/10.1038/emm.2014.121
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108(4):1657–1662. https://doi.org/10.1073/pnas.1018375108
Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES (2018) Regulation of amyloid-β dynamics and pathology by the circadian clock. J Exp Med 215(4):1059–1068. https://doi.org/10.1084/jem.20172347
Stevanovic K, Yunus A, Joly-Amado A, Gordon M, Morgan D, Gulick D, Gamsby J (2017) Disruption of normal circadian clock function in a mouse model of tauopathy. Exp Neurol 294:58–67. https://doi.org/10.1016/j.expneurol.2017.04.015
Musiek ES (2015) Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol 6:29. https://doi.org/10.3389/fphar.2015.00029
Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M (2004) Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology 63(10):1902–1907. https://doi.org/10.1212/01.wnl.0000144278.79488.dd
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S et al (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326(5955):1005–1007. https://doi.org/10.1126/science.1180962
Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M et al (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 27(12):3090–3097. https://doi.org/10.1523/jneurosci.4854-06.2007
Volmar CH, Salah-Uddin H, Janczura KJ, Halley P, Lambert G, Wodrich A, Manoah S, Patel NH et al (2017) M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer’s disease genes and improving memory. Proc Natl Acad Sci U S A 114(43):E9135–e9144. https://doi.org/10.1073/pnas.1707544114
Hou TY, Zhou Y, Zhu LS, Wang X, Pang P, Wang DQ, Liuyang ZY, Man H et al (2020) Correcting abnormalities in miR-124/PTPN1 signaling rescues tau pathology in Alzheimer’s disease. J Neurochem 154(4):441–457. https://doi.org/10.1111/jnc.14961
Chen JL, Zhang DL, Sun Y, Zhao YX, Zhao KX, Pu D, Xiao Q (2017) Angiotensin-(1-7) administration attenuates Alzheimer’s disease-like neuropathology in rats with streptozotocin-induced diabetes via Mas receptor activation. Neuroscience 346:267–277. https://doi.org/10.1016/j.neuroscience.2017.01.027
Burke SJ, Batdorf HM, Burk DH, Noland RC, Eder AE, Boulos MS, Karlstad MD (2017) Collier JJ (2017) db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a Western diet. J Diabetes Res 8503754:1–17. https://doi.org/10.1155/2017/8503754
Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, Kaur M, Kreutzer D (2006) Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther 8(3):402–412. https://doi.org/10.1089/dia.2006.8.402
Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N et al (2007) Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 49(5):1104–1113. https://doi.org/10.1161/hypertensionaha.106.083568
Karakoc Y, Buruk MS, Aktan B, Kirvar R, Erdogan S, Sahbaz MA, Aksoy S, Gulyasar T (2011) Effects of chronic light/dark cycle on iron zinc and copper levels in different brain regions of rats. Biol Trace Elem Res 144(1-3):1003–1007. https://doi.org/10.1007/s12011-011-9081-2
Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Ghahremani MH, Dehpour AR (2005) Effect of light/dark cycle alteration on morphine-induced conditioned place preference. Neuroreport 16(18):2051–2056. https://doi.org/10.1097/00001756-200512190-00017
Li Y, Ma J, Yao K, Su W, Tan B, Wu X, Huang X, Li T et al (2020) Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res 69(3):e12682. https://doi.org/10.1111/jpi.12682
Niedhammer I, Lert F, Marne MJ (1996) Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. Int J Obes Relat Metab Disord 20(7):625–633
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M et al (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363(6429):880–884. https://doi.org/10.1126/science.aav2546
Zhao HY, Wu HJ, He JL, Zhuang JH, Liu ZY, Huang LQ, Zhao ZX (2017) Chronic sleep restriction induces cognitive deficits and cortical beta-amyloid deposition in mice via BACE1-antisense activation. CNS Neurosci Ther 23(3):233–240. https://doi.org/10.1111/cns.12667
Cho K (2001) Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci 4(6):567–568. https://doi.org/10.1038/88384
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121(2):171–181. https://doi.org/10.1007/s00401-010-0789-4
Guha S, Johnson GVW, Nehrke K (2020) The crosstalk between pathological tau phosphorylation and mitochondrial dysfunction as a key to understanding and treating Alzheimer’s disease. Mol Neurobiol 57(12):5103–5120. https://doi.org/10.1007/s12035-020-02084-0
Qiu H, Zhong R, Liu H, Zhang F, Li S, Le W (2016) Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer’s disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J Alzheim Disease 50(3):669–685. https://doi.org/10.3233/jad-150774
Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, Caccamo A, Oddo S (2019) Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 18(1):e12873. https://doi.org/10.1111/acel.12873
Pu D, Zhao Y, Chen J, Sun Y, Lv A, Zhu S, Luo C, Zhao K et al (2018) Protective effects of sulforaphane on cognitive impairments and AD-like lesions in diabetic mice are associated with the upregulation of Nrf2 transcription activity. Neuroscience 381:35–45. https://doi.org/10.1016/j.neuroscience.2018.04.017
Wu Y, Yuan Y, Wu C, Jiang T, Wang B, Xiong J, Zheng P, Li Y et al (2020) The reciprocal causation of the ASK1-JNK1/2 pathway and endoplasmic reticulum stress in diabetes-induced cognitive decline. Front Cell Dev Biol 8:602. https://doi.org/10.3389/fcell.2020.00602
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G et al (2020) Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci (London, England : 1979) 134(16):2161–2175. https://doi.org/10.1042/cs20200530
Kalani A, Chaturvedi P, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2017) Dementia-like pathology in type-2 diabetes: a novel microRNA mechanism. Mol Cell Neurosci 80:58–65. https://doi.org/10.1016/j.mcn.2017.02.005
Acknowledgements
We thank Danpei Li and Li Huang from Huazhong University of Science and Technology for the technical advice and assistance with this study.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81670754, 81800686, and 81974114) and funds of Jie Chu Jing Ying Foundation (Grants 2018076).
Author information
Authors and Affiliations
Contributions
YY and JH conceived and designed the study and wrote the manuscript. YY, XS, KD, and XY secured the study’s funding. JH, XP, RF, KD, XS, SZ, and XY acquired and analyzed the data. All authors revised the article and approved its final version.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PNG 28 kb)
Rights and permissions
About this article
Cite this article
Huang, J., Peng, X., Fan, R. et al. Disruption of Circadian Clocks Promotes Progression of Alzheimer’s Disease in Diabetic Mice. Mol Neurobiol 58, 4404–4412 (2021). https://doi.org/10.1007/s12035-021-02425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02425-7