Abstract
Schizophrenia is a complex neuropsychiatric disorder, influenced by a combined action of genes and environmental factors. The neurodevelopmental origin is one of the most widely recognized etiological models of this heterogeneous disorder. Environmental factors, especially infections during gestation, appear to be a major risk determinant of neurodevelopmental basis of schizophrenia. Prenatal infection may cause maternal immune activation (MIA) and enhance risk of schizophrenia in the offspring. However, the precise mechanistic basis through which MIA causes long-lasting schizophrenia-like behavioral deficits in offspring remains inadequately understood. Herein, we aimed to delineate whether prenatal infection-induced MIA causes schizophrenia-like behaviors through its long-lasting effects on immune-inflammatory and apoptotic pathways, oxidative stress toxicity, and antioxidant defenses in the brain of offspring. Sprague-Dawley rats were divided into three groups (n = 15/group) and were injected with poly (I:C), LPS, and saline at gestational day (GD)-12. Except IL-1β, plasma levels of IL-6, TNF-α, and IL-17A assessed after 24 h were significantly elevated in both the poly (I:C)- and LPS-treated pregnant rats, indicating MIA. The rats born to dams treated with poly (I:C) and LPS displayed increased anxiety-like behaviors and significant deficits in social behaviors. Furthermore, the hippocampus of the offspring rats of both the poly (I:C)- and LPS-treated groups showed increased signs of lipid peroxidation, diminished total antioxidant content, and differentially upregulated expression of inflammatory (TNFα, IL6, and IL1β), and apoptotic (Bax, Cas3, and Cas9) genes but decreased expression of neuroprotective (BDNF and Bcl2) genes. The results suggest long-standing effects of prenatal infections on schizophrenia-like behavioral deficits, which are mediated by immune-inflammatory and apoptotic pathways, increased oxidative stress toxicity, and lowered antioxidant and neuroprotective defenses. The findings suggest that prenatal infections may underpin neurodevelopmental aberrations and neuroprogression and subsequently schizophrenia-like symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is an etiologically complex and phenotypically heterogeneous disorder of the central nervous system (CNS). A combination of multiple genetic and environmental factors may increase risk and progression of schizophrenia [1]. Exposure to adverse environmental factors, especially during the prenatal and early postnatal periods, has consistently been shown to increase the risk of schizophrenia in adult offspring [2, 3]. Among the environmental factors, prenatal infection (viral, bacterial and protozoan) has emerged as a predominant risk determinant [4,5,6]. Compelling evidence from epidemiological, preclinical, and clinical studies indicates that prenatal infections may enhance the risk of schizophrenia in the offspring by inducing maternal inflammation or maternal immune activation (MIA). MIA may disturb the fetal microenvironment and interfere with the critical phases of fetal brain development, leading to neurodevelopmental aberrations. Early environmental insults, MIA, and subsequent structural and functional abnormalities in the brain have been proposed to cause a spectrum of neurodevelopmental disorders, including schizophrenia [7, 8]. To gain more insights into the underlying mechanism of the MIA-associated risk of schizophrenia, experimental animals, especially rodents, have been used by many studies. Synthetic viral analogs of double-stranded RNA (dsRNA), including polyinosinic:polycytidylic acid [poly (I:C)], and bacterial analogs, including lipopolysaccharides (LPS), are useful to decipher the mechanisms through which MIA affects neurodevelopment and enhances the risk of schizophrenia-like behaviors in rodent studies [9]. Data obtained from animal studies have delineated several mechanisms through which MIA disrupts neurodevelopment including microglia activation and developmental neuroinflammation, activation of oxidative stress pathways, and altered fetal dopaminergic development [10,11,12,13,14]. A substantial body of evidence from animal model studies also demonstrates that LPS and poly (I:C)-induced MIA may cause behavioral deficits reminiscent of the positive, negative, and neurocognitive symptoms of schizophrenia in the adult offspring [15, 16].
However, the precise central mechanism by which MIA exerts long-lasting effects on the brain and behavior of the offspring is not completely elucidated. Among the several hypothesized mechanisms, systemic inflammation and neuroinflammation are prominent. For example, poly (I:C)-induced MIA in mice increased the levels of inflammatory cytokines in the hippocampus and cerebellum of adult offspring [17, 18]. MIA may also shape the immunological phenotypes in the offspring whereby poly(I:C) is associated with a pro-inflammatory phenotype including a Th17 phenotype [19, 20]. Poly (I:C) injection in C57BL/6J mice on a gestational day (GD) 12.5 led to activation of inflammatory macrophages in the offspring, suggesting that MIA can exert lasting changes in macrophage function [21]. Poly (I:C) treatment on GD9 in mice led to enlargement of lateral ventricles in adult offspring and disruption of sensorimotor gating [22]. Moreover, MIA resulted in defective adult neurogenesis and neuronal architecture, nonspatial information processing, and cognitive functions [23,24,25]. In nonhuman primate, MIA caused increased striatal dopamine in the offspring in late adolescence [26]. These physiological, molecular, structural, and behavioral changes driven by MIA in animal models may serve as hallmark pathobiological indicators of schizophrenia. In addition, MIA caused alteration in the structure and function of the brain in adult offspring [27].
Data from clinical studies show activated immune-inflammatory and oxidative stress pathways in schizophrenia [28, 29]. Activated immune-inflammatory pathway including increased levels of the pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, increased oxidative stress toxicity, and lowered antioxidant defenses are associated with the positive, negative, and neurocognitive deficits and the deficit phenotype of schizophrenia [30,31,32]. The theory is that lowered antioxidant defenses and lowered neuroprotection coupled with increased immune and oxidative neurotoxicity underpin the neuronal dysfunctions in schizophrenia, herein conceptualized as neuroprogression, including impairments in synaptic plasticity, neurotransmission, neurogenesis, apoptosis, and altered receptor expression [33,34,35]. Already in 1995, schizophrenia was conceptualized as a neurodevelopmental disorder whereby MIA may induce long-lasting changes in neuronal functions associated with the symptoms and neurocognitive deficits of schizophrenia through neuro-immune and neuro-oxidative pathways [36]. However, in animal models, it is unknown whether MIA may cause schizophrenia-like behavioral deficits reminiscent of positive, negative, and cognitive symptoms by activating immune, oxidative, and apoptotic pathways and lowering antioxidant and neuroprotective resilience against the neurotoxicity associated with immune and oxidative pathways.
Hence, this study was conducted to delineate the effects of poly (I:C)- and LPS-induced MIA on inflammatory, oxidative stress and apoptotic signatures, as well as antioxidant contents and neuroprotective genes in the hippocampus of the offspring in association with schizophrenia-like behaviors during the peri-adolescent and adult periods. Moreover, we also assessed the impact of MIA on the levels of pro-inflammatory cytokines in the peripheral blood of offspring rats.
Materials and Methods
Animals
All experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and initiated after obtaining approval from the Institutional Animal Ethics Committee (AEC/65/389/HG).
All experiments were conducted on Sprague-Dawley rats (Rattus norvegicus), maintained in the central animal research facility (CARF) of National Institute of Mental Health and Neurosciences, Bangalore, India. Thirteen- to fifteen-week-old rats (both sex) weighing approximately 250–300 g were housed in standard cages (22.5 × 35.5 × 15 cm) with corncob bedding and maintained on a 12-h light/dark cycle (lights on between 7 am and 7 pm) with ad libitum access to food and water. Female and male rats were housed together overnight for mating. Following this, female rats were examined for the presence of a vaginal plug in the morning and the presence of which was marked as gestational day 0 (GD0). Additionally, body weights of putative pregnant female rats were checked periodically to confirm pregnancy. The pregnant rats were then subjected to MIA studies.
Establishment of MIA Model
Three different groups of pregnant rats (n = 15 rats/group) received either poly (I:C) (20 mg/kg) or LPS (1.5 mg/kg) or an equivalent volume of saline (0.9%) injections intraperitoneally on GD12. After 24 h of injection, maternal blood was collected from each group of rats (n = 5/group) by cardiac puncture. Plasma was separated and stored at −80 °C. Plasma levels of pro-inflammatory cytokines, namely IL-1β, IL-6, TNF-α, and IL-17A, were analyzed by multiplex suspension assay in a Bio-plex 200 platform using commercially available rat Milliplex map kit (RECYTMAG-65K) following the manufacturer’s guidelines (Millipore, France).
The remaining pregnant rats from each group (n = 10/group) were allowed to have a normal delivery. Offspring were weaned at postnatal day 21 (PD21); male and female pups were housed separately, 3 pups per cage. The offspring obtained from poly (I:C)- (n = 12–14), LPS- (n = 12–14), and saline- (n = 12–14) treated dams were allowed to grow in normal conditions and subjected to various behavioral assessments such as open field test (OFT), social interaction test (SIT), and prepulse inhibition (PPI) during the peri-adolescent (39–44 days) and adult (60–70 days) periods. After the completion of behavioral assessments during the adult period, hippocampal tissues were collected from these offspring for the analysis of markers of inflammatory, oxidative stress, and apoptotic pathways, and peripheral blood samples were collected for the analysis of only inflammatory markers. The experimental design is depicted in Fig. 1.
Assessments in the Offspring Rats
We obtained 8–9 pups from each group; therefore, 80–90 pups were available each for saline-, poly (I:C)-, and LPS-treated groups, respectively. Approximately1–2 pups from each litter had a natural death. In addition, 1–2 pups from each litter were the victim of maternal cannibalism; however, this was not consistent across all the mothers. Two to three offspring rats from each group were used for the standardization of the behavioral tests. Three behavioral tests, such as open field test (OFT), social interaction test (SIT), and prepulse inhibition (PPI) test, were carried out on three different sets of offspring rats, each set comprising 12–14 rats. As the rats show considerable variations in behavioral performances in the test chambers, it is important to study behavioral performances with more numbers of rats (12–14 rats per group). Hence, in the present study, we have used 12–14 offspring rats for each behavioral test and this was calculated based on our previous study on similar behavioral assessments [37]. The sample size required for gene expression studies was 5, and this was based on a study that examined the expression of cerebellar cytokines in the offspring of poly (I:C)-treated mice [18]. We also performed post hoc power analysis on our data indicating that the power was greater than 0.85 when the data from 5 rats were used. The behavioral as well as molecular experiments were performed on the same number of both the male and female offspring. Remaining rats were euthanized as per the guidelines of CPCSEA, Government of India as well Institutional Animal Ethics Committee.
Behavioral Assessments in the Offspring Rats
Open Field Test (OFT)
Rats (n = 12–14/group) were subjected to anxiety-like and exploratory behaviors in an open field during peri-adolescent and adult periods in the Behavior Laboratory of the Department of Neurophysiology, NIMHANS. The floor of OFT (100 × 100 × 40 cm, L × W × H) is divided into equally sized squares to make two virtual zones viz, center zone (center) represented by inner 9 squares and outer zone (periphery) represented by remaining 16 squares covering border. The center of the field was illuminated, which was the only direct light in the testing room. Rats were habituated for at least 1 h before the testing. The test began with placing each rat individually in the center of the arena and the behavior was recorded for 5 min using a digital camera and data were analyzed with EthoVision™ XT 8 software (Noldus, Wageningen, Netherlands). Behavioral parameters like total distance moved, time spent in the center zone, number of entries to center zone, latency to move to the center zone first time, and zone transition were considered for data analyses.
Social Interaction Test (SIT)
The offspring rats were also tested for social behaviors during peri-adolescent and adult periods. A different set of rats were used for assessing the social behaviors (n = 12–14/group). Both sociability and social novelty tests were performed in a three-chambered container with dimensions (120 × 100 × 40 CM, L × W × H) using the method described in a previous study from the same Behavior Laboratory [37].
Rats were habituated to the lab for at least 1 h before the assessment. Stranger rats were normal Sprague-Dawley rats housed in groups of three per cage. Briefly, baseline adaptation was studied by placing the test rat in the central home chamber for a 10-min habituation period, followed by the partitions being raised and allowing the rat to explore freely among all three chambers (two test chambers and the central home chamber) for a 10-min period. Following baseline data collection, each rat was returned to the home chamber with partitions closed. During this time, an unfamiliar adult rat (stranger-1) was placed within one of the restrainer cages (in test chamber 1) and an object was placed in the restrainer cage of the second chamber (test chamber 2). Again, partitions were raised, allowing the test rat to move freely from the central home chamber to other chambers for a duration of 10 min test session. The preference for the adult rat to stranger-1 (in test chamber 1) and towards the novel object (in test chamber 2) was assessed by calculating the percentage of time spent with the adult rat (stranger-1) over the sum of time spent with both the stranger-1 and the novel object: [(Stranger 1TimeMakingContact/(ObjectTimeMakingContact + Stranger 1TimeMakingContact)] × 100.
Similarly, for assessing social novelty, a 10-min social interaction test session was performed immediately following the first session of the sociability test. The stranger-1 rat remained in the same restrainer cage (test chamber 1), while a second unfamiliar rat (novel stranger rat-2) was introduced to the second restrainer (in test chamber 2), which was previously kept with the object.
Both stranger-1 and stranger-2 rats were taken from different home cages and never came into physical contact with each other or with the test animal. Thus, in this experiment for assessing social novelty, we used a familiar rat (stranger-1) and a totally unfamiliar rat (stranger-2). The sociability test was repeated by allowing the test animal to move freely across all three chambers. After the partitions were again raised, we have assessed the time spent by the test rat with stranger-1 and stranger-2. The preference for the novel rat (stranger-2) towards the now familiar rat (stranger-1) was assessed by calculating the percentage of time spent making contact with the novel rat (novel) over the sum of time spent with both the novel and the familiar: [(NovelTimeMakingContact/(NovelTimeMakingContact + FamiliarTimeMakingContact)] × 100. The sessions were video-recorded, and the amount of time spent in each chamber was calculated by video tracking software (Ethovision XT, Noldus Information Technology, Netherlands). Entry into a chamber was defined when all four paws have reached the chamber. The restrainer cages and chambers were cleaned by 70% ethanol between different rats to avoid olfactory cues. Sociability and social novelty tests were conducted only once.
Prepulse Inhibition (PPI)
Prepulse inhibition (PPI) of acoustic startle was measured using the Coulbourn instruments startle apparatus (Holliston, Massachusetts) during the peri-adolescent (39–44 days) and adult (60–70 days) periods. Rats were habituated with the animal holder and the room having the Animal Acoustic Startle Response System before beginning the sessions followed by testing for PPI [method adapted from [38]]. Each test began with 10 min of apparatus acclimatization, at 65 dB white background noise, which continued throughout the session followed by ten pulse alone trials (120 dB for 20 ms), ten null trials with no stimulus, and twenty prepulse + pulse trials at each of two different prepulse intensities (80 and 85 dB), in a pseudo-random order and with an average intertrial interval of 17 s (range 9–29 s). The prepulse + pulse trials consisted of a 20-ms prepulse (at 80 and 85 dB), followed by a 100-ms delay, then a startle pulse (120 dB, 20 ms). The testing session lasted for 25 min. Prepulse inhibition is expressed as % PPI, defined as [Pulse alone − (startle amplitude on prepulse + pulse trial)/mean startle amplitude on pulse alone trials] × 100.
Quantification of Expression of Immune-Inflammatory and Apoptotic Pathway-Related Genes in the Brain Tissue of Offspring Rats
About 100 mg hippocampal tissue from offspring rats (n = 5) during the adult period were collected and stored in RNAlater solution to quantify the expression of pro-inflammatory (IL1β, IL6, and TNF-α) and apoptotic (BCl2, Cas3, Cas9, Bax, and BDNF) pathway-related genes. Total RNA was extracted from homogenized tissue using commercially available RNeasy Lipid Tissue Mini Kits (Qiagen, Germantown, Maryland), followed by conversion of mRNA to cDNA using high-capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems, USA). cDNA samples were diluted to a final concentration of 10 ng/μl. The quantitative PCR (qPCR) was performed in a QuantStudio 6 platform (Applied Biosystems, USA) using TaqMan gene expression assays. The corresponding TaqMan assay IDs are listed in Table 1. PCR cycling conditions for all the genes were as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles consisting of 95 °C for 15 s and 60 °C for 1 min. Samples were run in triplicate for each target gene. The qPCR reaction was performed with a commercially available TaqMan Universal PCR Master Mix (Applied Biosystems, USA) containing AmpliTaq Gold® DNA polymerase. Reactions were performed in a final volume of 10 μl containing TaqMan Universal PCR Master Mix, probes and primers of each gene as well as 1 μl of cDNA. All amplification batches included no template control (NTC) and cDNA of a control rat (not from the treatment groups) was used as a calibrator (reference sample) and was run in each plate to assess intra- and inter-assay variability, which was <0.5 Ct. The 2−ΔΔCt method of relative quantification was used. The final result is presented as the fold change of target gene expression relative to a reference sample, normalized to the endogenous control (GAPDH).
Biochemical Analysis of Oxidative Stress Markers in the Brain Tissue of the Offspring Rats
Hippocampal tissues (approximately 100 mg) were collected, homogenized in 1 ml phosphate-buffered saline using a homogenizer (Polytron PT 2500 E, Kinamatica, Switzerland). Protease inhibitor was added just before homogenization to prevent protein degradation. The homogenate was centrifuged at 12,000 rpm for 10 min and the supernatant was collected and stored at −80 °C until thawed for analyzing lipid peroxidation and total antioxidant contents. The total protein concentration in each sample was normalized to correct intra-assay variation. The total protein concentration was calculated according to the Bradford [39] method.
Determination of Lipid Peroxidation
Lipid peroxidation was measured using a commercially available TBARS (Thiobarbituric Acid Reactive Substances) kit (Cayman Chemicals, USA). Samples from tissue homogenates were treated with sodium dodecyl sulfate (SDS) solution followed by the addition of a color reagent (supplied with the kit). The malondialdehyde-thiobarbituric acid reactive substance (MDA-TBA) adduct formed by the reaction of MDA and TBA under high temperature (90–100 °C) and acidic conditions were measured colorimetrically at 535–540 nm using a plate reader (Multiskan GO, Thermo Scientific, USA).
Determination of Total Antioxidant Content
The total antioxidant content in hippocampal tissue homogenates was measured using a commercially available antioxidant assay kit (Cayman Chemicals, USA). The assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS (2,2′-Azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS+ by metmyoglobin. The amount of ABTS+ produced was measured by reading absorbance at 750 nm in a plate reader (Multiskan GO, Thermo Scientific, USA).
Estimation of Plasma Levels of the Pro-inflammatory Cytokines in Offspring Rats
Five milliliters of blood was collected by cardiac puncture from offspring rats during the adult period. Plasma was separated by centrifuging the blood at 4000 rpm and stored at −80 °C. The pro-inflammatory cytokines, IL-1β, IL-6, IL-17A, and TNF-α were analyzed using the MILLIPLEX map kit (RECYTMAG-65K) specific for rat (Millipore, France) in a multiplex suspension array system (Bio-Plex 200, Bio-Rad, USA) following the manufacturer’s guidelines.
Statistical Analyses
GraphPad Prism 8.0.1 (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses. For analyzing SIT and PPI data, two-way ANOVA was used to test the effect of treatment vs chambers and treatment vs trials across the whole session followed by Dunnett’s multiple comparison tests. For the analysis of the remaining behavioral tests and biomarker data, a one-way ANOVA followed by Dunnett’s multiple comparison tests were performed. Pearson’s correlation matrix was used to assess the association of behavioral parameters with biochemical and gene expression data.
Results
Poly (I:C) and LPS Treatment Induce MIA
MIA was assessed by examining the plasma levels of IL-1β, IL-6, IL-17A, and TNF-α 24 h after injecting the pregnant rats at GD12 with poly (I:C) and LPS. Significantly higher levels of IL-6 were observed in poly (I:C)- [mean difference (MD) of 107 with 95% CI 62.94 to 151.0; P < 0.0001] and LPS- (MD of 218.3 with 95% CI 174.3 to 262.3; P < 0.0001) treated pregnant rats as compared to control group. Similar results were also observed for IL-17A [poly (I:C) (MD of 73.10 with 95% CI 42.28 to 103.9; P = 0.0001), and LPS (MD of 48.12 with 95% CI 17.30 to 78.93; P = 0.0039)] as well as TNF-α [poly(I:C) (MD of 27.81 with 95% CI 22.62 to 32.99; P < 0.0001) and LPS (MD of 30.87 with 95% CI 25.69 to 36.06; P < 0.0001)] in the treated pregnant rats when compared to the control rats. IL-1β was greater only in LPS- (MD of 77.73 with 95% CI 0.83 to 154.6; P = 0.04) treated rats. However, no significant difference was observed for IL-1β in the poly (I:C)-treated pregnant rats as compared to the control group (MD of −52.46 with 95% CI −129.44 to 24.44; P = 0.19) (Fig. 2a–d).
Differences in maternal plasma levels of pro-inflammatory cytokines between treated and control rats. Shown are the concentrations of maternal plasma IL-6 (a), TNF-α (b), IL-17A (c), and IL-1β (d) in poly (I:C)- and LPS-treated and control rats. Data are shown as mean ± SD (n = 5). Poly (I:C) vs saline: ***P < 0.001, ****P < 0.0001, LPS vs saline: #P < 0.05, ##P < 0.01, ####P < 0.0001
Behavioral Abnormalities in the MIA Offspring
Poly (I:C)- and LPS-Treated Offspring Exhibit Anxiety-like Behaviors
Rats born to poly (I:C)- and LPS-treated dams showed a significant decrease in number of entries to the center zone [poly (I:C) group: MD of 20.50 with 95% CI 14.12 to 26.88; P < 0.0001 and LPS group: MD of 19.67 with 95% CI 13.29 to 26.04; P < 0.0001)] (Fig. 3a), time spent in the center zone [poly (I:C) group: MD of 7.47 with 95% CI 2.881 to 12.07; P = 0.001 and LPS group: MD of 9.76 with 95% CI 5.164 to 14.35; P < 0.0001)] (Fig. 3b), and zone transition [poly (I:C) group: MD of 41.00 with 95% CI 28.25 to 53.75; P < 0.0001 and LPS group: MD of 39.33 with 95% CI 26.58 to 52.08; P < 0.0001)] (Fig. 3c) when tested in the open field as compared to the control rats during the peri-adolescent period. Additionally, latency to move to the center zone first time (Fig. 3d) [(poly (I:C) group: MD of 38.27 with 95% CI 16.23 to 60.32; P = 0.0006) and LPS group: MD of 71.28 with 95% CI 49.23 to 93.32; P < 0.0001)] and time spent in the periphery (Fig. 3e) [poly (I:C) group: MD of 7.47 with 95% CI 2.9 to 12.07; P = 0.001 and LPS group: MD of 9.8 with 95% CI 5.16 to 14.35; P < 0.0001)] were significantly greater in both the poly (I:C)- and LPS-treated rats as compared to the control rats. However, the total distance moved (Fig. 3f) did not differ significantly among the offspring of the treated groups [poly (I:C) group: MD of −178.8 with 95% CI −697.9 to 340.3; P = 0.65 and LPS group: MD of 204.8 with 95% CI −314.3 to 723.8; P = 0.57)].
Profile of anxiety-like behaviors during the peri-adolescent period. The figures show entries to center zone (a), time spent in center zone (b), time spent in periphery (c), zone transition (d), latency to move to center zone (e), total distance moved (f) in poly (I:C)- and LPS-treated and control rats. Data are shown as mean ± SD, (n = 12–14). Poly (I:C) vs saline: **P < 0.01, ***P < 0.001, ****P < 0.0001, LPS vs saline: ###P < 0.001, ####P < 0.0001
Notably, similar observations were repeated even when the rats were tested for the second time during the adulthood. Total number of entries to the center zone [poly (I:C) group: MD of 26.23 with 95% CI 20.96 to 31.50; P < 0.0001 and LPS group: MD of 12.58 with 95% CI 7.206 to 17.96; P < 0.0001)] (Fig. 4a), time spent in the center zone [poly (I:C) group: MD of 2.70 with 95% CI 0.4591 to 4.938; P = 0.02 and LPS group: MD of 3.3 with 95% CI 1.050 to 5.528; P = 0.004)] (Fig. 4b), and zone transition [poly (I:C) group: MD of 52.46 with 95% CI 41.98 to 62.94; P < 0.0001 and LPS group: MD of 25.33 with 95% CI 14.65 to 36.02; P < 0.0001)] (Fig. 4c) were significantly decreased in offspring born to dams treated with poly (I:C) and LPS as compared to controls. Latency to move to the center zone first time (Fig. 4d) [poly (I:C) group: MD of 59.64 with 95% CI 29.89 to 89.39; P < 0.0001 and LPS group: MD of 40.27 with 95% CI 11.14 to 69.40; P = 0.006)] and time spent in the periphery (Fig. 4e) [poly (I:C) group: MD of 2.7 with 95% CI 0.46 to 4.93; P = 0.02 and LPS group: MD of 3.3 with 95% CI 1.1 to 5.5; P = 0.004)] were significantly greater in treated groups as compared to controls. However, the total distance moved by the offspring rats of the treated as well as control groups did not differ significantly [poly (I:C) group: MD of 33.78 with 95% CI −688.8 to 756.3; P = 0.99 and LPS group: MD of −223.9 with 95% CI −946.5 to 498.7; P < 0.70)] (Fig. 4f).
Anxiety-like behaviors during the adult period. The figures show entries to center zone (a), time spent in the center zone (b), time spent in the periphery (c), number of zone transition (d), latency to move to center (e), and total distance moved (f) in poly (I:C)- and LPS-treated and control rats. Data are shown as mean ± SD, (n = 12–14). Poly (I:C) vs saline: *P < 0.05, ****P < 0.0001, LPS vs saline: ##P < 0.01, ####P < 0.0001.
In summary, the rats born to the poly (I:C)- and LPS-treated dams showed significant anxiety-like behaviors in the OFT as they spent more time in the periphery and not in the center of the chamber compared to rats born to saline-treated dams. The ambulatory behaviors especially the rate of ambulation was comparable across all three groups indicating normal locomotor behaviors.
Poly (I:C)- and LPS-Treated Offspring Rats Display Deficits in Social Behaviors
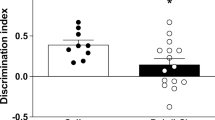
Rats born to the poly (I:C)- and LPS-treated dams showed social deficits in the peri-adolescent period. Two-way ANOVA showed significant effect of treatment X chamber on time spent with animals or objects. There was a significant interaction between treatment X chamber on time spent [F (4, 102) = 40.73, P < 0.0001] and main effect of chamber [F (2, 102) = 742.3, P < 0.0001]. Offspring born to the poly (I:C)-treated rats spent significantly lesser time in making contact with animals than objects (MD of 159.8 with 95% CI 120.0 to 199.5; P < 0.0001) (Fig. 5a), whereas offspring born to the control rats spent more time in making contact with animals than objects (MD of 61.15 with 95% CI 22.93 to 99.36; P = 0.001)]. No difference in time spent was observed in the offspring born to the LPS-treated rats (MD of 27.28 with 95% CI -12.49 to 67.06; P = 0.22). We further assessed the sociability index by one-way ANOVA, which was found to be significantly decreased in offspring of both the poly (I:C)- (MD of 67.22 with 95% CI 55.96 to 78.49; P < 0.0001) and LPS- (MD of 36.43 with 95% CI 25.16 to 47.69; P < 0.0001) treated rats as compared to the controls (Fig. 5b).
Outcome measures of the social interaction test during the peri-adolescent period. This figure shows time spent investigating animal (a), home or object, sociability preference for animal over the object (b), time spent investigating novel animal, home or familiar animals (c), social novelty index (d) in poly (I:C)- and LPS- and control rats. Data are shown as mean ± SD, (n = 12–14). Saline: object vs animal/familiar vs novel ++ P < 0.01, poly (I:C): object vs animal/ familiar vs novel ****P < 0.0001, LPS: object vs animal/familiar vs novel ####P < 0.0001, poly (I:C) vs saline: ****P < 0.0001, LPS vs saline: ####P < 0.0001
Similarly, there was a significant effect of treatment and chamber on time spent with familiar or novel rats. There was a significant interaction between the effects of the treatment X chamber on time spent [F (4, 111) = 13.94, P < 0.0001], and the main effect of the chamber [F (2, 111) =1329, P < 0.0001]. Offspring born to both the treated groups spent significantly lesser time making contact with a novel than the familiar rats; poly (I:C) group (MD of 75.08 with 95% CI 39.55 to 110.6; P < 0.0001) and LPS group (MD of 84.83 with 95% CI 49.30 to 120.4; P < 0.0001) (Fig. 5c), whereas offspring from the saline-treated rats spent significantly greater time making contact with a novel than familiar rats (MD of 42.17 with 95% CI 11.40 to 72.93; P = 0.005). A one-way ANOVA showed a significantly reduced social novelty index in offspring of both the poly (I:C)- (MD of 34.28 with 95% CI 23.89 to 44.68; P < 0.0001) and LPS- (MD of 44.87 with 95% CI 34.47 to 55.26; P < 0.0001) treated rats as compared to controls (Fig. 5d).
The rats consistently showed deficits in social behavior in the adult period. A two-way ANOVA showed significant effect of treatment and chamber on time spent with animals or objects. There was a significant interaction between the effects of treatment X chamber on time spent, [F (4, 102) = 42.37, P < 0.0001] and a main effect of chamber [F (2, 102) = 2307, P < 0.0001]. Offspring born to both the poly (I:C)- (MD of 63.21 with 95% CI 38.78 to 87.64; P < 0.0001) and LPS- (MD of 91.61 with 95% CI 67.18 to 116.0; P < 0.0001)] treated groups spent less time making contact with animals than the objects (Fig. 6a), whereas offspring born to the control (MD of 83.08 with 95% CI 59.61 to 106.6; P < 0.0001) rats spent more time making contact with animals than the objects. A one-way ANOVA demonstrated a significantly decreased sociability index in offspring of both the poly (I:C)- (MD of 50.29 with 95% CI 42.50 to 58.08; P < 0.0001) and LPS- (MD of 56.40 with 95% CI 48.45 to 64.35; P < 0.0001) treated rats as compared to the control rats (Fig. 6b).
Outcome measures of Social interaction test during the adult period. a Time spent investigating animal (A), home or object (O) by poly (I:C), LPS, and control rats. b Sociability preference for the animal over the object by poly (I:C), LPS, and control rats. c Time spent investigating novel animal (N), home or familiar animal by poly (I:C), LPS, and control rats. d Social novelty by poly (I:C), LPS, and control rats. Data are shown as Mean ± SD, (n = 12–14). Saline: object vs animal/familiar vs novel ++++P < 0.0001, poly (I:C): object vs animal/ familiar vs novel ***P < 0.001,****P < 0.0001, LPS: object vs animal/ familiar vs novel ####P < 0.0001; poly (I:C) vs saline: ****P < 0.0001, LPS vs saline: ####P < 0.0001
There was a significant effect of treatment and chamber on time spent with familiar or novel rat, and a significant interaction between the effects of treatment and chamber on time spent, [F (4, 102) = 9.94, P < 0.0001] and main effects of the chamber [F (2, 102) = 736.7, P < 0.0001]. Offspring born to poly (I:C)- (MD of 53.17 with 95% CI 20.98 to 85.35; P = 0.0007) treated rats spent less time making contact with a novel rat than familiar (Fig. 6c), whereas control (MD of 57.28 with 95% CI 23.78 to 90.78; P = 0.0004) offspring spent more time making contact with a novel rat than familiar. One-way ANOVA showed significantly reduced social novelty index in offspring of both poly (I:C)- (MD of 26.49 with 95% CI 21.79 to 31.18; P < 0.0001) and LPS- (MD of 20.01 with 95% CI 15.22 to 24.80; P < 0.0001) treated rats as compared to controls (Fig. 6d).
In summary, the rats born to poly (I:C)- and LPS-treated dams displayed significant social behavioral deficits in terms of both sociability and social novelty aspects of social cognition.
MIA Offspring Exhibits Altered Prepulse Inhibition
Percentage PPI was analyzed using two prepulse-pulse trial types during the peri-adolescent and adult periods. There was no significant difference of percentage PPI in offspring of both the treated and control groups during peri-adolescent period (Fig. 7a). However, a two-way ANOVA showed main effects of treatment [F (2, 75) = 7.85, P = 0.0008] and trial types [F (1, 75) =11.00, P = 0.001] during the adult period with no interaction effect [F (2,75) = 0.057, P = 0.94]. LPS offspring had a decreased percentage PPI as compared to the controls at both 80 dB (MD of 13.19 with 95% CI 2.08 to 24.30; P = 0.02) (Fig. 7b) and 85 dB (MD of 12.27 with 95% CI 1.17 to 23.38; P = 0.03), but poly (I:C) offspring showed diminished PPI only at 85 dB (MD of 11.94 with 95% CI 0.83 to 23.04; P = 0.03). Although reduced PPI was observed in poly (I:C) offspring at 80 dB (MD of 10.54 with 95% CI −0.78 to 21.85; P = 0.07), it was not statistically significant.
Impaired sensorimotor gating in offspring of poly (I:C)- and LPS-treated rats. a Although PPI was diminished during the peri-adolescent period, the difference was not significant. b Reduced PPI at 80 dB and 85 dB in LPS-treated offspring, whereas poly (I:C) offspring showed reduced PPI at 85 dB during the adult period. Data are shown as mean ± SD, (n = 12–14). Poly (I:C) vs saline: *P < 0.05, LPS vs saline: #P < 0.05
Assessments of Markers of Inflammation, Oxidant-Antioxidant Balance, and Apoptosis in MIA Offspring
Elevated Peripheral Inflammatory Cytokines in MIA Offspring
To assess the inflammatory status in the offspring of poly (I:C)- and LPS-treated rats, pro-inflammatory cytokines viz, IL-6, TNF-α, IL-1β, and IL-17A were analyzed in the peripheral blood. The plasma levels of TNF-α in poly (I:C) group (MD of 21.64 with 95% CI 4.2 to 39.08; P = 0.02) and LPS group (MD of 23.31 with 95% CI 5.87 to 40.75; P = 0.01) (Fig. 8a) and IL-17A in poly (I:C) group (MD of 54.50 with 95% CI 23.36 to 85.64; P = 0.002) as well as LPS group (MD of 36.97 with 95% CI 5.83 to 68.11; P = 0.02) (Fig. 8b) were significantly increased. However, IL-6 levels were significantly elevated only in offspring of poly (I:C)-treated rats (MD of 68.91 with 95% CI 12.30 to 125.5; P = 0.01)] (Fig. 8c). The difference in the levels of IL-1β was not significant between the offspring of treated [poly (I:C) group: MD of 3.32 with 95% CI −28.76 to 22.12; P = 0.92 and LPS group: MD of 8.21 with 95% CI −35.69 to 19.26; P = 0.66] and control groups (Fig. 8d).
Differences in peripheral blood cytokine levels in offspring of poly (I:C)- and LPS-treated and control rats. This figure shows differences of plasma TNF-α (a), IL-17A (b), IL-6 (c), and IL-1β (d). Data are shown as mean ± SD, (n = 5). Poly (I:C) vs saline: *P < 0.05, **P < 0.01, LPS vs saline: #P < 0.05
Upregulated Inflammatory Cytokine Gene Expression in the Brain Tissue of MIA Offspring
Significantly upregulated expression of TNF-α was observed in offspring of both the poly (I:C)- (MD of 0.77 with 95% CI 0.14 to 1.4; P = 0.02) and LPS- (MD of 0.75 with 95% CI 0.12 to 1.38; P = 0.02) treated rats (Fig. 9a); IL6 only in offspring born to poly (I:C)- (MD of 0.50 with 95% CI 0.07 to 0.93; P = 0.02) treated rats (Fig. 9b), while IL1β only in offspring of the LPS- (MD of 0.77 with CI 0.03 to 1.51; P = 0.04) treated rats (Fig. 9c). TNF-α gene expression levels and anxiety-like behaviors [(r = 0.93), (95% CI = 0.25 to 0.99)] in the offspring of poly (I:C)-treated rats were positively correlated. The percentage PPI at 85 dB was negatively correlated with IL1β gene expression in the offspring of LPS-treated rats [(r = −0.94) (95% CI, −0.99 to −0.35)].
Expression of pro-inflammatory genes in hippocampal tissue in the offspring of poly (I:C)- and LPS-treated and control rats. Gene expression levels of TNF-α (a), IL6 (b), and IL-1β (c) in the offspring of poly (I:C)- and LPS-treated and control rats. Data are shown as mean ± SD (n = 5). Poly (I:C) vs saline: *P < 0.05, LPS vs saline: #P < 0.05
Altered Oxidant-Antioxidant Balance in Brain Tissue of MIA Offspring
Lipid peroxidation, expressed as the level of thiobarbituric acid reactive substances (TBARS), was significantly higher in the hippocampal tissue of the offspring of both the poly (I:C)- (MD of 2.68 with 95% CI 0.65 to 4.72; P = 0.009) and LPS- (MD of 4.76 with 95% CI 2.72 to 6.80; P < 0.0001) treated rats as compared to the controls (Fig. 10). Total antioxidant contents were significantly lower in hippocampal tissue of the offspring of both the poly (I:C)- (MD of 0.21 with 95% CI 0.07 to 0.34; P = 0.002) and LPS- (MD of 0.28 with 95% CI 0.15 to 0.42; P < 0.0001) treated rats as compared to the controls (Fig. 11). In addition, a positive correlation was found between TBARS and sociability [(r = 0.90), (95% CI = 0.06–0.99)].
TBARS levels in the offspring of poly (I:C)- and LPS-treated and control rats in the adult period. Poly (I:C) and LPS offspring showed increased lipid peroxidation (measured as the level of TBARS-MDA adduct) as compared to controls. Data are shown as mean ± SD, (n = 12–14). Poly (I:C) vs saline: **P < 0.01, LPS vs saline: ####P < 0.0001
Total antioxidant levels in the offspring of poly (I:C)- and LPS-treated and control rats in the adult period. Poly (I:C) and LPS offspring showed decreased levels of total antioxidant capacity as compared to saline. Data are shown as mean ± SD, (n = 12–14). Poly (I:C) vs saline: **P < 0.01, LPS vs saline: ####P < 0.0001
Altered Expression of Apoptotic and Neuroprotective Genes in Brain Tissue of MIA Offspring
The expressions of Bax (MD of 0.41 with 95% CI 0.09 to 0.73; P = 0.01), Cas3 (MD of 0.66 with 95% CI 0.15 to 1.18; P = 0.01), and Cas9 (MD of 0.47 with 95% CI 0.06 to 0.90; P = 0.03) genes were significantly increased in the hippocampus of offspring of the LPS-treated rats. However, a significantly decreased expressions of BDNF [poly (I:C) group: MD of 0.68 with 95% CI 0.13 to 1.23; P = 0.02, and LPS group: MD of 0.73 with 95% CI 0.18 to 1.3; P = 0.01] as well as BCl2 [poly (I:C):MD of 0.54 with 95% CI 0.11 to 0.97; P = 0.02, and LPS group: MD of 0.53 with 95% CI 0.10 to 0.96; P = 0.02) were observed in the offspring of both the treated groups (Fig. 12a–d). Besides this, there was a negative correlation between sociability and Bax gene expression in LPS offspring [(r = −0.95), (95% CI = −0.99 to −0.38)]. The expression of the cas9 gene was also negatively correlated with the novelty index in poly (I:C) offspring [(r = −0.94), (95% CI = −0.99 to −0.32).
Expression of apoptotic genes in the offspring of poly (I:C)- and LPS-treated and control rats. This figure shows the gene expression of Bax (a), Cas3 (b), Cas9 (c), BDNF (d), and BCl2 (e) in the offspring of poly (I:C)- and LPS-treated and control rats. Data are shown as mean ± SD, (n = 5). Poly (I:C) vs saline: *P < 0.05, LPS vs saline: #P < 0.05
Discussion
Preclinical studies, particularly MIA models in rodents, have made significant advances in establishing the causal link between prenatal infection and risk of schizophrenia-like behaviors in the adult offspring [9, 40]. These studies have provided important insights into the neurodevelopmental and immune origin of schizophrenia with immense translational potential. Herein, we aimed to discern a common central mechanism through which MIA induces schizophrenia-like behavioral deficits in the offspring during the peri-adolescent and adult periods.
Effect of Prenatal Infection on Maternal Immune System
In the present study, exposure to viral [poly (I:C)] and bacterial (LPS) analogs on GD12 was shown to significantly elevate levels of inflammatory cytokines, such as IL-1β, IL-6, IL-17A, and TNF-α in the maternal plasma, indicating MIA status in the pregnant rats. Furthermore, similar changes in the expression profile of the inflammatory cytokines in both the poly (I:C)- and LPS-treated groups suggest that bacterial and viral infections in pregnant rats may cause similar immune-inflammatory responses.
Effect of MIA on Schizophrenia-like Behavioral Deficits in the Offspring
MIA in rodents resulted in significant behavioral changes in the offspring, which are reminiscent of schizophrenia phenotypes in humans [18, 41]. In the present study, the offspring of poly (I:C)- and LPS-treated dams were assessed for anxiety-like behaviors, social interactions, and sensorimotor gating (as assessed using prepulse inhibition) during peri-adolescent and adult periods. Importantly, the outcome measurements of these behavior tests in rodents may reflect the behavioral deficits observed in schizophrenia in humans [42]. For example, anxiety-like and exploratory behaviors and locomotor activity examined in the OFT test in rodents reflect impairments in social behaviors in humans including positive and negative symptoms. The measurement of social interaction in the SIT test in rodents reflects deficits in social interaction attributes representing the negative symptoms of schizophrenia. In addition, PPI is commonly used as a measure of sensorimotor gating, a neurocognitive function, which is frequently impaired in schizophrenia [43].
In the current study, anxiety-like behaviors and socio-cognitive deficits were observed in the offspring of rats with MIA. Interestingly, offspring of poly (I:C)- and LPS-treated rats exhibited comparable anxiety-like features in the open field during peri-adolescent and adult periods. Moreover, the offspring of poly (I:C)- and LPS-treated rats spent significantly less time with animals compared to an inanimate object. In addition to this, the offspring of both the poly (I:C)- and LPS-treated rats spent less time with novel conspecifics as compared to the familiar rat during adult period. The measures of social deficits were also comparable among the offspring of poly I:C- and LPS-treated rats, except the situation where offspring born to LPS-treated rats spent comparably equal time in making contact with the animal and the inanimate object during the peri-adolescent period. These behavioral changes point towards impaired social cognition including sociability (tendency to initiate social contact) and social novelty (tendency to initiate social contact with a new individual) in the offspring. These observations coincide with previous findings, and our data further reinforce the notion that MIA leads to sustained abnormalities in social behaviors in the offspring [44,45,46].
Besides altered social behaviors, impaired sensory gating represents one of the fundamental clinical features of schizophrenia [47]. In this study, offspring born to both the poly (I:C)- and LPS-treated rats exhibited reduced PPI during the adult period. This finding is also in line with the previous studies showing that prenatal infection may induce a disruption of sensory gating in the offspring [48, 49]. However, the most notable and unique finding observed in the current study was the comparable pattern of behavioral deficits among the offspring of both the poly (I:C)- and LPS-treated dams during peri-adolescent and adult periods. This suggests that viral and bacterial infections might result in similar impairments in schizophrenia-like behaviors. We performed a stratified analysis based on the sex of the offspring rats; however, sex was not found to have any significant effect on the studied behavioral parameters.
Impact of MIA on Inflammatory, Oxidant, Antioxidant, Neuroprotective, and Apoptotic Biomarkers in the Offspring
In this study, we observed elevated levels of inflammatory cytokines both in the peripheral blood and hippocampus of the adult offspring of poly (I:C)- and LPS-treated rats, of which the levels of IL-6 and TNF-α correlated between brain and blood. To the best of our knowledge, this is a first study to demonstrate the ability of MIA to induce cytokine-mediated inflammation both in the periphery and brain of the same offspring rats. Although a previous study examined the levels of cytokines in the brain and serum of MIA offspring, the changes in brain cytokines did not correlate with the levels of serum cytokines [17]. In the current study, the pattern of cytokine (TNF-α and IL-17A in the blood and TNF α in the brain) expression was observed to be same in the offspring of both the poly (I:C)- and LPS-treated rats indicating that viral and bacterial infections may elicit similar cytokine-mediated inflammatory responses. The elevated levels of TNF-α were positively correlated with anxiety-like behaviors in the offspring of poly (I:C)-treated rats.
Another major finding of this study is that lipid peroxidation is increased and total antioxidant content decreased in the hippocampus of offspring rats following administration of poly (I:C) and LPS to the mothers. Importantly, the contents of oxidants and antioxidants in the hippocampus did not differ significantly among the offspring of LPS- and poly (I:C)-treated rats. There is a considerable lack of data from animal model studies on the effects of MIA on oxidative stress pathways in adult offspring. The long-term effect of MIA on oxidative stress was examined only in one study in C57BL/6 mice reporting that poly (I:C) injection at embryonic day 9.5 induced an oxidative imbalance due to alterations in gene expression of SOD1 and NOX2 mainly in the cerebral cortex and hippocampus of male offspring at adulthood [46]. Taken together, these findings suggest long-lasting deleterious effects of MIA on oxidative stress pathway in brain tissue. The increased neurotoxicity by neuro-immune and neuro-oxidative pathways coupled with lowered antioxidant defenses may contribute to schizophrenia-like behaviors including impaired sociability in the offspring.
Another novel finding of our study is that regulators of apoptotic and neuroprotective pathways are significantly altered in the hippocampus of the offspring rats during adult period. We observed a significantly upregulated expression of Bax, Cas3, and Cas9 genes in the offspring of LPS-treated rats, and a significant downregulation of protective genes including BDNF and BCl2 in the offspring of both poly (I:C)- and LPS-treated rats. Moreover, we have stratified our analysis based on the sex of the offspring rats and we were unable to detect a significant effect of sex on the inflammatory, oxidative stress, and apoptotic markers.
The dysregulated inflammatory, oxidative stress, and apoptotic pathways driven by MIA might lead to manifestation of schizophrenia-like symptoms by disrupting multiple neurobiological processes. Support towards this notion can be derived from multiple lines of studies. For example, MIA in mice may result in maturation-dependent dopaminergic hyperfunction and cognitive impairment in the offspring [50]. MIA may inhibit cortical neurogenesis thereby causing behavioral disturbances in adult offspring [51]. MIA was also proposed to affect the ability of cerebellar neurons to form synapses, resulting in synaptic impairments and contributing to behavioral impairments [18]. MIA in mice may result in altered brain morphometry (smaller brain regions like corpus callosum and disruption in myelin/fiber structure), resting-state functional connectivity, and overall lower neural activity, which may possibly correlate with the behavioral deficits [27]. A recent study revealed that poly (I:C)-induced MIA triggers behavioral abnormalities in association with exacerbated inflammation and oxidative stress [46]. Taken together, these data suggest an important role of activated immune-inflammatory and oxidative stress pathways in causing schizophrenia-like behaviors through their detrimental effects on apoptotic pathways and brain structures and functions. The immune-inflammatory response and oxidative stress driven by lowered antioxidant and neuroprotective defenses in the MIA offspring may activate apoptotic pathway in the brain of offspring rats, and the cumulative effects of all dysfunctions may underpin the pathophysiology of schizophrenia-like behaviors in rodents.
One of the core changes that accompanies schizophrenia is increased neurotoxicity due to inflammatory products (including IL-1β, IL-6, and TNF-α), oxidative stress (including lipid peroxidation), and apoptotic changes, which coupled to lowered neuroprotective and antioxidant defenses may induce neuroprogression [30, 32, 35]. It is plausible that maternal immune activation, developmental immune changes, and concurrent neurodevelopmental aberrations might be involved in early priming of neuroprogressive changes, and onset of these changes later in life could be a secondary phenomenon of already and early primed events. Based on the findings of the current study, we may propose that prenatal infections are associated with schizophrenia-like behavioral deficits through effects of activated immune-inflammatory and apoptotic pathways, increased oxidative stress toxicity, and lowered antioxidant and neuroprotective defenses. Our findings suggest that those long-lasting detrimental effects of prenatal infections may underpin neurodevelopmental aberrations and neuroprogression and, subsequently, schizophrenia-like symptoms.
Conclusion
The MIA model received more attention because studies using MIA models provided crucial insights into the neurobiological consequences of MIA, which are relevant to the pathophysiology of schizophrenia, including altered cortical neurogenesis, dopaminergic activity, and kynurenine pathway activation. We focused on immune-inflammatory, oxidative, and apoptotic pathways in the hippocampus of the offspring rats, as this region of the brain in humans plays a pivotal role in the pathobiology of schizophrenia by disrupting several processes like neurotransmission, adult neurogenesis, learning, memory, and neurocognition. The current study reported novel data including (i) poly (I:C)- and LPS-induced MIA exert sustained and similar changes in immune-inflammatory biomarkers in peripheral blood and oxidative stress and apoptotic markers in the hippocampus of offspring rats, (ii) MIA-induced changes in the immune-inflammatory and oxidative stress markers in the hippocampus of the adult offspring may influence cell death pathways in the brain, and (iii) MIA-induced immune-inflammatory, oxidative stress, and apoptotic pathways correlate with the schizophrenia-like behavioral deficits in the offspring rats. Our findings corroborate the theory that MIA may play a crucial role in causing schizophrenia-like symptoms through long-lasting effects on immune-inflammatory, oxidative, antioxidant, neuroprotective, and apoptotic pathways thereby causing neuroprogression. The aberrations in those five pathways may drive positive and negative symptoms and neurocognitive impairments by altering neuronal functions in the hippocampus.
References
van Os J, Rutten BP, Poulton R (2008) Gene-environment interactions in schizophrenia: Review of epidemiological findings and future directions. Schizophr Bull 34:1066–1082. https://doi.org/10.1093/schbul/sbn117
Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S (1999) Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: Case-control study. BMJ 318:421–426. https://doi.org/10.1136/bmj.318.7181.421
Dean K, Murray RM (2005) Environmental risk factors for psychosis. Dialogues Clin Neurosci 7:69–80
Westergaard T, Mortensen PB, Pedersen CB, Wohlfahrt J, Melbye M (1999) Exposure to prenatal and childhood infections and the risk of schizophrenia: Suggestions from a study of sibship characteristics and influenza prevalence. Arch Gen Psychiatry 56:993–998. https://doi.org/10.1001/archpsyc.56.11.993
Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES (2005) Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 162:767–773. https://doi.org/10.1176/appi.ajp.162.4.767
Brown AS, Derkits EJ (2010) Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. https://doi.org/10.1176/appi.ajp.2009.09030361
Debnath M, Venkatasubramanian G, Berk M (2015) Fetal programming of schizophrenia: Select mechanisms. Neurosci Biobehav Rev 49:90–104. https://doi.org/10.1016/j.neubiorev.2014.12.003
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702. https://doi.org/10.1523/JNEUROSCI.2178-07.2007
Bergdolt L, Dunaevsky A (2019) Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol 175:1–19. https://doi.org/10.1016/j.pneurobio.2018.12.002
Meyer U (2013) Developmental neuroinflammation and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:20–34. https://doi.org/10.1016/j.pnpbp.2011.11.003
Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M (2012) Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun 26:623–634. https://doi.org/10.1016/j.bbi.2012.01.015
Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH (2001) Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 47:27–36. https://doi.org/10.1016/s0920-9964(00)00032-3
Pratt L, Ni L, Ponzio NM, Jonakait GM (2013) Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: Role of interleukin-6. Pediatr Res 74:393–401. https://doi.org/10.1038/pr.2013.126
Meyer U, Engler A, Weber L, Schedlowski M, Feldon J (2008) Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience 154:701–709. https://doi.org/10.1016/j.neuroscience.2008.04.031
Wolff AR, Cheyne KR, Bilkey DK (2011) Behavioural deficits associated with maternal immune activation in the rat model of schizophrenia. Behav Brain Res 225:382–387. https://doi.org/10.1016/j.bbr.2011.07.033
Missault S, Van den Eynde K, Vanden Berghe W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S, Dedeurwaerdere S (2014) The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun 42:138–146. https://doi.org/10.1016/j.bbi.2014.06.013
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013) Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68. https://doi.org/10.1016/j.bbi.2012.07.008
Pendyala G, Chou S, Jung Y, Coiro P, Spartz E, Padmashri R, Li M, Dunaevsky A (2017) Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology 42:1435–1446. https://doi.org/10.1038/npp.2017.7
Mandal M, Donnelly R, Elkabes S, Zhang P, Davini D, David BT, Ponzio NM (2013) Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun 33:33–45. https://doi.org/10.1016/j.bbi.2013.04.012
Mandal M, Marzouk AC, Donnelly R, Ponzio NM (2011) Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun 25:863–871. https://doi.org/10.1016/j.bbi.2010.09.011
Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P (2014) Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun 38:220–226. https://doi.org/10.1016/j.bbi.2014.02.007
Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, Chung S, Chua SE et al (2009) Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: Evidence from MRI in a mouse model. PLoS One 4:e6354. https://doi.org/10.1371/journal.pone.0006354
Liu YH, Lai WS, Tsay HJ, Wang TW, Yu JY (2013) Effects of maternal immune activation on adult neurogenesis in the subventricular zone-olfactory bulb pathway and olfactory discrimination. Schizophr Res 151:1–11. https://doi.org/10.1016/j.schres.2013.09.007
Li WY, Chang YC, Lee LJ (2014) Prenatal infection affects the neuronal architecture and cognitive function in adult mice. Dev Neurosci 36:359–370. https://doi.org/10.1159/000362383
Ito HT, Smith SE, Hsiao E, Patterson PH (2010) Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun 24:930–941. https://doi.org/10.1016/j.bbi.2010.03.004
Bauman MD, Lesh TA, Rowland DJ, Schumann CM, Smucny J, Kukis DL, Cherry SR, McAllister AK et al (2019) Preliminary evidence of increased striatal dopamine in a nonhuman primate model of maternal immune activation. Transl Psychiatry 9:135. https://doi.org/10.1038/s41398-019-0449-y
Kreitz S, Zambon A, Ronovsky M, Budinsky L, Helbich TH, Sideromenos S, Ivan C, Konerth L et al (2020) Maternal immune activation during pregnancy impacts on brain structure and function in the adult offspring. Brain Behav Immun 83:56–67. https://doi.org/10.1016/j.bbi.2019.09.011
Fraguas D, Diaz-Caneja CM, Ayora M, Hernandez-Alvarez F, Rodriguez-Quiroga A, Recio S, Leza JC, Arango C (2019) Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr Bull 45:742–751. https://doi.org/10.1093/schbul/sby125
Upthegrove R, Khandaker GM (2020) Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci 44:49–66. https://doi.org/10.1007/7854_2018_88
Maes M, Sirivichayakul S, Matsumoto AK, Maes A, Michelin AP, de Oliveira SL, de Lima Pedrao JV, Moreira EG et al (2020) Increased levels of plasma tumor necrosis factor-alpha mediate schizophrenia symptom dimensions and neurocognitive impairments and are inversely associated with natural IgM directed to malondialdehyde and paraoxonase 1 activity. Mol Neurobiol 57:2333–2345. https://doi.org/10.1007/s12035-020-01882-w
Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA Jr, Cordeiro Q, Belangero SI et al (2019) Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol 29:416–431. https://doi.org/10.1016/j.euroneuro.2018.12.008
Maes M, Sirivichayakul S, Matsumoto AK, Michelin AP, Semeão LDO, de Lima Pedrão JV, Moreira EG, Barbosa DS, Carvalho AF, Solmi M, Kanchanatawan B. Lowered Antioxidant Defenses and Increased oxidative toxicity are hallmarks of deficit schizophrenia: neurocognitive and symptom correlates. Preprints 2020, 2020050145 (https://doi.org/10.20944/preprints202005.0145.v1).
Davis J, Moylan S, Harvey BH, Maes M, Berk M (2014) Neuroprogression in schizophrenia: Pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry 48:512–529. https://doi.org/10.1177/0004867414533012
Muller N (2017) Neuroprogression in schizophrenia and psychotic disorders: The possible role of inflammation. Mod Trends Pharmacopsychiatry 31:1–9. https://doi.org/10.1159/000470802
Anderson G, Berk M, Dodd S, Bechter K, Altamura AC, Dell'osso B, Kanba S, Monji A et al (2013) Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:1–4. https://doi.org/10.1016/j.pnpbp.2012.10.008
Smith RS, Maes M (1995) The macrophage-T-lymphocyte theory of schizophrenia: Additional evidence. Med Hypotheses 45:135–141. https://doi.org/10.1016/0306-9877(95)90062-4
Subhadeep D, Srikumar BN, Shankaranarayana Rao BS, Kutty BM (2020) Short photoperiod restores ventral subicular lesion-induced deficits in affective and socio-cognitive behavior in male Wistar rats. J Neurosci Res 98:1114–1136. https://doi.org/10.1002/jnr.24601
Valsamis B, Schmid S (2011) Habituation and Prepulse Inhibition of Acoustic Startle in Rodents. In: Habituation and prepulse inhibition of acoustic startle in rodents. Journal of visualized experiments, JoVE, pp. e3446–e3446. https://doi.org/10.3791/3446
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Meyer U, Feldon J (2010) Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 90:285–326. https://doi.org/10.1016/j.pneurobio.2009.10.018
Zuckerman L, Weiner I (2005) Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res 39:311–323. https://doi.org/10.1016/j.jpsychires.2004.08.008
Powell CM, Miyakawa T (2006) Schizophrenia-relevant behavioral testing in rodent models: A uniquely human disorder? Biol Psychiatry 59:1198–1207. https://doi.org/10.1016/j.biopsych.2006.05.008
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 47:181–188. https://doi.org/10.1001/archpsyc.1990.01810140081011
Tellez-Merlo G, Morales-Medina JC, Camacho-Ábrego I, Juárez-Díaz I, Aguilar-Alonso P, de la Cruz F, Iannitti T, Flores G (2019) Prenatal immune challenge induces behavioral deficits, neuronal remodeling, and increases brain nitric oxide and zinc levels in the male rat offspring. Neuroscience 406:594–605. https://doi.org/10.1016/j.neuroscience.2019.02.018
Ratnayake U, Quinn T, LaRosa DA, Dickinson H, Walker DW (2014) Prenatal exposure to the viral mimetic poly I:C alters fetal brain cytokine expression and postnatal behaviour. Dev Neurosci 36:83–94. https://doi.org/10.1159/000362205
Hui CW, St-Pierre A, El Hajj H, Remy Y, Hébert SS, Luheshi GN, Srivastava LK, Tremblay M (2018) Prenatal immune challenge in mice leads to partly sex-dependent behavioral, microglial, and molecular abnormalities associated with schizophrenia. Front Mol Neurosci 11:13. https://doi.org/10.3389/fnmol.2018.00013
Braff DL, Geyer MA, Swerdlow NR (2001) Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156:234–258. https://doi.org/10.1007/s002130100810
Romero E, Guaza C, Castellano B, Borrell J (2010) Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: Implications for the etiopathology of schizophrenia. Mol Psychiatry 15:372–383. https://doi.org/10.1038/mp.2008.44
Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C (2002) Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia Neuropsychopharmacology 26:204–215. https://doi.org/10.1016/S0893-133X(01)00360-8
Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M (2006) Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biol Psychiatry 59:546–554. https://doi.org/10.1016/j.biopsych.2005.07.031
De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI (2010) Induction of toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio 1. https://doi.org/10.1128/mBio.00176-10
Acknowledgements
This study was partially supported by the Women Scientist-A grant from the Department of Science and Technology (SR/WOS-A/LS-1219/2014(G), Government of India to PMT. The financial support received from the Department of Science and Technology is acknowledged. The authors are grateful to Prof. B.S. Shankarnarayana Rao and Prof. Laxmi T. Rao, Department of Neurophysiology, NIMHANS, Bangalore, for their kind support in conducting the behavioral tests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talukdar, P.M., Abdul, F., Maes, M. et al. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol Neurobiol 57, 4345–4361 (2020). https://doi.org/10.1007/s12035-020-02028-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02028-8