Abstract
Cannabinoid CB1 receptors (CB1R) and the GPR55 receptor are expressed in striatum and are potential targets in the therapy of Parkinson’s disease (PD), one of the most prevalent neurodegenerative diseases in developed countries. The aim of this paper was to address the potential of ligands acting on those receptors to prevent the action of a neurotoxic agent, MPP+, that specifically affects neurons of the substantia nigra due to uptake via the dopamine DAT transporter. The SH-SY5Y cell line model was used as it expresses DAT and, therefore, is able to uptake MPP+ that inhibits complex I of the respiratory mitochondrial chain and leads to cell death. Cells were transfected with cDNAs coding for either or both receptors. Receptors in cotransfected cells formed heteromers as indicated by the in situ proximity ligation assays. Cell viability was assayed by oxygen rate consumption and by the bromide-based MTT method. Assays of neuroprotection using two concentrations of MPP+ showed that cells expressing receptor heteromers were more resistant to the toxic effect. After correction by effects on cell proliferation, the CB1R antagonist, SR141716, afforded an almost full neuroprotection in CB1R-expressing cells even when a selective agonist, ACEA, was present. In contrast, SR141716 was not effective in cells expressing CB1/GPR55 heteromeric complexes. In addition, an agonist of GPR55, CID1792197, did not enhance neuroprotection in GPR55-expressing cells. These results show that neurons expressing heteromers are more resistant to cell death but question the real usefulness of CB1R, GPR55, and their heteromers as targets to afford PD-related neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is an unrelenting neurodegenerative disorder caused by the progressive degeneration of dopamine-producing neurons of the substantia nigra pars compacta. Dopamine loss triggers dysfunction of basal ganglia circuits, ultimately leading to the cardinal symptoms of the disease (reviewed in [1]). Although levodopa is still viewed as the gold-standard pharmacological treatment for PD, affording an effective symptomatic alleviation, chronic levodopa administration leads to abnormal involuntary movements known as dyskinesia (LIDs; [2,3,4]). High-frequency deep brain stimulation (HFS-DBS) is a neurosurgical procedure that provides good clinical management of LIDs, particularly when HFS-DBS electrodes are placed in the internal segment of the globus pallidus (GPi; [5]). Despite symptomatic relief, it is worth noting that both dopamine-replacement strategies and functional neurosurgical interventions do not have any effect on disease progression and, therefore, in the long run, PD patients often end up with a cognitive decline that has trends in common with those observed in Alzheimer’s disease [6].
In the last two decades, extensive research has been carried out to elucidate the mechanisms underlying why dopaminergic neurons are particularly vulnerable to degenerate, as well as in pushing forward safe and efficacious approaches for neuroprotection, in an attempt to slow down (or ideally arrest) disease progression rates. Regarding neuroprotection, most studies performed so far have relied on neurotoxin-induced mice and non-human animal models of PD [7, 8]. A number of in vitro cellular models of PD-like neurodegeneration have been made available, such as those using neural-related cell lines exposed to neurotoxic compounds as MPP+ (C12H12N+) that inhibits complex I of the respiratory mitochondrial chain and leads to cell death. MPP+ by far is the most often used compound mimicking PD-related cell death in cellular models [9,10,11]. In what concerns suitable cell lines for MPP+ in vitro administration, SH-SY5Y human neuroblastoma cell line seems to be a convenient choice (reviewed in [12]).
When looking for novel drug candidates, cannabinoids rank among the compounds with a more potential as neuroprotective agents [13, 14]. They act via two specific receptors that belong to the superfamily of G protein-coupled receptors (GPCRs): cannabinoid CB1 and CB2. Within the central nervous system (CNS), CB1 receptors (CB1R) are mainly expressed in neurons, whereas CB2 receptors (CB2R) are mainly expressed in glial cells. Actually, CB1R are considered as the most abundant GPCR in the CNS. Although not directly engaged in PD pathophysiology, polymorphisms in the receptor gene were linked to PD-associated depression [15]. Pharmacology of CB1R is complex due to the hydrophobic nature of the natural ligands and the very particular positioning of the orthosteric site, which is hidden from the extracellular milieu in the 3D structures already reported [16,17,18]. The entrance of CB1R ligands seems thus possible via the lipid bilayer of the plasma membrane. These recent discoveries lead to the possibility of finding specific ligands that may fully activate, partially activate, partially deactivate, or fully block the receptor’s response. Acting on CB1R, endogenous agonists, 2-arachidonoylglycerol and anandamide, lack the psychotropic effects exerted by the phytocannabinoid, ∆9-tetrahydrocannabinol (THC) [19]. Moreover, it should be possible to develop structurally different antagonists showing less side effects than rimonabant, an anti-obesity drug that was withdrawn from the market due to suicide cases [20]. Cannabinoids are able to interact and affect the signaling of the orphan GPR55 receptor, which was considered as a third cannabinoid receptor. Despite the receptor may be activated by an endogenous molecule, L-α-lysophosphatidylinositol, IUPHAR (http://www.guidetopharmacology.org) did not consider GPR55 as de-orphaned because in vivo efficacy of L-α-lysophosphatidylinositol acting on GPR55 has not yet been reported [21,22,23,24,25,26]. GPR55 is expressed in different regions within the brain and has been proposed as a potential target for PD [27,28,29].
GPR55 may be coupled to diverse signaling pathways [30], and indeed the CB1R may modulate GPR55-mediated signaling [31]. These results led to the search for potential CB1R/GPR55 heteromeric complexes that were identified in in vitro models and also in the rat striatum [29, 31]. This paper aims to assess whether the expression of CB1 or GPR55 receptors or of CB1/GPR55 heteromeric receptor complexes may be engaged in response to the MPP+ toxic effects within neuroblastoma SH-SY5Y cells.
Materials and Methods
Drugs
The following drugs have been used here: N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA; ref. 1319, Tocris Bioscience) and 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide (SR141716; ref. 168273–06-1, Cayman Chemical). CID1792197 [21] was in-house synthesized (> 99% purity). ACEA was supplied as pale yellow oil that was diluted in ethanol in a 10 mM concentration stock solution. SR141617 (10 mM) and CID1792197 (10 mM) stock solutions were prepared in DMSO. Aliquots of these stock solutions were kept frozen at - 20 °C until use.
Fusion Proteins and Expression Vectors
The human cDNAs for the CB1R and GPR55 were cloned in pcDNA3.1 and amplified without their stop codons using sense and antisense primers harboring either unique EcoRI or BamH1 sites. The fragments were then subcloned to be in-frame with EYFP into the BamH1 and EcoRI restriction sites of an EYFP expressing vector (EYFP-N1; enhanced yellow variant of GFP; Clontech, Heidelberg, Germany), to generate the plasmids expressing CB1R and GPR55 fused to YFP on the C-terminal end of the receptor (CB1R-YFP or GPR55-YFP). Expression of receptors was tested by confocal microscopy and the receptor functionality was tested performing ERK1/2 activation assays.
Cell Line Culture and Transfection
Human neuroblastoma SH-SY5Y cell line was obtained from Sigma (ref 94030304; Sigma-Aldrich, St. Louis, MO, USA). Cells were grown in DMEM supplemented with 2 mM L-glutamine, 100 units/ml penicillin/streptomycin, 1% nonessential amino acids, and 5% (v/v) heat inactivated fetal bovine serum (FBS) (all supplements were from Invitrogen, Paisley, Scotland, UK). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and were passaged when they were 80–90% confluent, i.e., approximately twice a week for no more than 20 passages.
SH-SY5Y cells growing in 10 cm dishes were transiently transfected with the corresponding fusion proteins cDNAs (see figure legend) using Lipofectamine 2000 as described by the manufacturer (ref 11668027; Invitrogen, Paisley, Scotland, UK) and were used 48 h post-transfection.
Cell Treatments
Cell treatments were performed 48 h post-transfection using 3000 cells per well in 96-well plates for MTT reduction assay (MTT is a water-soluble tetrazolium reagent in REDOX reactions involving mitochondrial components), 40,000 cells per well in 96-well plates for oxygen consumption assay and 30,000 cell/well in 8-well chambered cover glasses (Nunc® Lab-Tek® II, Sigma-Aldrich, St. Louis, MO, USA) for in situ proximity ligation assay (PLA). After 24 h, the culture medium was replaced by fresh serum-free DMEM and cells were cultured for another 24 h. In each experiment, cells were treated or not with the CB1R agonist, ACEA (100 nM), and with the GPR55 agonist, CID1792197 (1 μM), for 30 min before freshly added MPP+ (ref D048, Sigma-Aldrich, St. Louis, MO, USA), or pre-treated for 20 min with CB1R antagonist, SR141716 (rimonabant; 250 nM), prior to agonist treatment. If more than one compound was used, they were added simultaneously to the culture media. The drug concentrations and times of treatments used in these experiments were based on the bibliography related to pharmacology of the receptors and on our previous experience [21, 29].
Oxygen Consumption Rate (OCR) Assay
Oxygen consumption was monitored in oxygen-sensing microplates (Oxoprobics Biosciences S. L., Madrid, Spain). The probe is quenched in the presence of oxygen; as oxygen is consumed by cellular respiration, the fluorescence signal increases being directly related to cell metabolism [32]. Briefly, cells were seeded in oxygen-sensing plates and incubated for 24 h, the different treatments were added to the cells at the indicated final concentrations, and the wells were sealed from ambient oxygen by the addition of 100 μl/well of mineral oil. Plates were placed in a plate reader (Envision, Perkin-Elmer, Waltham, MA, USA) previously equilibrated at 37 °C and monitored using 340/665 nm excitation/emission filters, with a delay time of 70 μs for 24–48 h.
MTT Reduction Assay
Cell viability was also studied by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. Once the treatments were completed, 10 μl of MTT [5 mg/ml in phosphate buffered saline (PBS)] (ref M5655; Sigma-Aldrich, St. Louis, MO, USA) were added to each well. Four hours later, 100 μl of lysis solution [20% sodium dodecyl sulfate (SDS); 50% dimethylformamide; pH 4] were added to the culture and incubated overnight at 37 °C. Absorbance at 570 nm was measured using a Multiskan EX Microplate Reader (ThermoFisher Scientific, Waltham, MA, USA). Values from blank dishes, containing only medium, were subtracted from the values of the samples. Viability was expressed as the percentage of the controls.
In situ Proximity Ligation Assay (PLA)
For PLA, SH-SY5Y transfected cells (SH-SY5Y-CB1R, SH-SY5Y-GPR55, or SH-SY5Y-CB1R/GPR55) were fixed in 4% paraformaldehyde for 15 min, washed with PBS containing 20 mM glycine to quench the aldehyde groups, and permeabilized with the same buffer containing 0.05% Triton X-100 (5 min treatment). To create our PLA probes we conjugated a rabbit anti-CB1R (ref: PA1-745, Thermo Scientific, Rockford, USA) with a PLUS oligonucleotide (Duolink® In Situ Probemaker PLUS DUO92009, Sigma-Aldrich, St. Louis, MO, USA) and a rabbit anti-GPR55 (ref 10224, Cayman Chemical, Michigan, USA) with a MINUS oligonucleotide (Duolink® In situ Probemaker MINUS DUO92010, Sigma-Aldrich, St. Louis, MO, USA) following manufacturer’s instructions. Fixed cells were incubated for 1 h at 37 °C with blocking solution (from PLA kit, see below), followed by overnight incubation (4 °C) with the PLA probe-linked antibodies described above. Specificity of antibodies was tested in non-transfected SH-SY5Y cells (data not shown).
Duolink II in situ PLA detection kit (Duolink® In situ Detection Reagents Red, DUO92008, developed by Olink Bioscience, Uppsala, Sweden; now distributed by Sigma-Aldrich as Duolink® using PLA® Technology) was used to detect the presence/absence of receptor clusters in the samples, which were incubated with the ligation solution for 1 h, washed and subsequently incubated with the amplification solution for 100 min (both steps at 37 °C in a humid chamber). Nuclei were stained with Hoechst (1/100; ref. 33258, Sigma-Aldrich, St. Louis, MO, USA). Mounting was performed using 30% MOWIOL™ (ref 9002-89-5; EMD Millipore Calbiochem™). Negative controls were performed by omitting the primary anti-GPR55 antibody.
Samples were observed in a Leica SP2 confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with an apochromatic 63X oil-immersion objective (N.A. 1.4), and 405 nm and 561 nm laser lines. For each field of view, a stack of two channels (one per staining) and five Z stacks with a step size of 1 μm were acquired. The number of cells containing one or more red spots versus total cells (blue nucleus) and, in cells containing spots, the ratio r (number of red spots/cell), were determined by means of the Duolink Image tool software.
Labeling of Mitochondria
SH-SY5Y cells transfected whit CB1R and GPR55, subjected or not to treatments, were labeled with MitoTracker® Red CMXRos probe (ref M7512; Invitrogen, Paisley, Scotland, UK), according to the manufacturer’s instructions. CB1R or GPR55 fused to YFP protein was detected by its fluorescence properties. Samples were observed in a Zeiss 510 Meta confocal laser-scanning microscope.
Data Analysis
Data result from at least five independent experiments. The data in the graphs are presented as the mean ± SEM. Statistical analysis was performed with SPSS 18.0 software. The test of Kolmogorov-Smirnov with the correction of Lilliefors was used to evaluate the fit of the data to a normal distribution and the test of Levene to evaluate the homogeneity of variance. Significance was analyzed by one- or two-way ANOVA test followed by Tukey’s test for multiple comparisons. Significant differences were considered when p < 0.05.
Results
CB1 and GPR55 Receptor Cellular Localization
The heterologous expression of the human version of CB1R-YFP and GPR55-YFP fusion proteins was analyzed in single-transfected SH-SY5Y neuroblastoma cells following the protocol described in the “Materials and Methods” section and with the MitoTracker® Red CMXRos probe, which labels mitochondria. As shown in Fig. 1, the two GPCRs were expressed in different cell locations of SH-SY5Y-CB1R-YFP and SH-SY5Y-GPR55-YFP cells; interestingly, there were receptors expressed in intracellular organelles (or “structures”) that mainly correspond to mitochondria. The presence of the CB1R in mitochondria has been reported elsewhere [33, 34].
Cellular localization of CB1R and GPR55. SH-SY5Y cells, transfected with CB1R (top left image) and GPR55 (bottom left image) fused to YFP protein and identified by its fluorescence properties (green signal), were labeled with MitoTracker® Red CMXRos probe (top and bottom middle images) (red signal). Colocalization is shown in yellow in the merge images (top and bottom right images), where CB1R and GPR55 are present in the surface of mitochondria, as it is shown in the inserts. Confocal microscopy images are shown (× 100). Scale bars 5 μm
Neuroprotective Effect of Cannabinoid Receptor Expression in a MPP+ Cellular Model of Parkinson’s Disease
In order to evaluate the potential neuroprotective effect of cannabinoid receptors in cells treated with MPP+, the toxic metabolite of MPTP, we firstly performed a dose-response curve to elucidate the proper concentration of MPP+ to be used in subsequent experiments. A wide range of MPP+ concentrations, from 0.005 to 5 mM, was hence used to induce cytotoxicity in SH-SY5Y cells, and cell viability was assessed by two different approaches. As shown in Fig. 2a, b, cell viability assessed by the degree of MTT reduction decreased in a concentration-dependent manner 24 and 48 h after treatment with MPP+. Data analysis revealed a statistically significant decrease of circa 45% in cell viability upon 24 h treatment with 2 mM MPP+. In cells treated for 48 h under similar increasing concentrations, the effect of 2 mM MPP+ was too strong, within a 70–80% decreased viability range, and doses of 5 mM led to a virtually complete cell death. Based in these dose-response results, 1 mM and 2 mM MPP+ were the concentrations chosen for further assays. These findings were confirmed by measuring OCR (Fig. 3a, b). In fact, our data demonstrated a dose-response decrease in cellular respiration caused by MPP+ in SH-SY5Y cells at both 24 and 48 h of incubation. It should be noted that toxicity at low MPP+ concentrations may be better detected using the OCR than the MTT assay. However, the effect on OCR measurements has a narrow dynamic range and, and for this reason, the MTT assay was preferred.
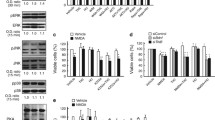
MTT reduction assay in SH-SY5Y cells treated with increasing concentrations of MPP+ (0.005–5 mM) for 24 (a) and 48 h (b). Cell damage is represented as the p percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. *p < 0.05, ***p < 0.001 compared with control
OCR assay in SH-SY5Y cells treated with increasing concentrations of MPP+ (0.005–5 mM) for 24 (a) and 48 h (b). Oxygen consumption was monitored in real-time using 96-well plate oxygen-sensing plates during 24 or 48 h. Data are the mean ± SEM of five independent experiments. RFU relative fluorescence units
The next step was to assess whether the expression of the cannabinoid receptor could be neuroprotective by itself. For this purpose, we tested cell viability of SH-SY5Y cells transiently expressing CB1R and incubated with MPP+ for 24 h. We found that SH-SY5Y-CB1R cells treated with 1 mM MPP+ did not show any reduction in the MTT signal while the toxic effect was noticeable in non-transfected cells (Fig. 4a, b). Interestingly, similar results were found in SH-SY5Y-GPR55 cells treated with 1 or 2 mM MPP+ (Fig. 4c). Due to the higher degree of toxicity over time, all these seemingly neuroprotective effects were not found at 48 h of treatment (Supplementary Fig. 1). Accordingly, we selected 24 h incubation for the majority of subsequent experiments.
MTT reduction assay in SH-SY5Y (a), SH-SY5Y-CB1R (b), and SH-SY5Y-GPR55 (c) cells treated with MPP+ (1–2 mM) for 24 h. Cell damage is represented as the percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. **p < 0.01, ***p < 0.001 compared with control
Neuroprotection in SH-SY5Y Cells Expressing CB1/GPR55 Heteroreceptor Complexes
Cannabinoids, which may bind to both cognate receptors and non-cognate GPR55 receptors, are considered as neuroprotective agents. To better assess the underlying mechanism of neuroprotection, CB1R and GPR55 were transiently coexpressed in SH-SY5Y cells. Of the two types of cannabinoid receptors, the CB1R were selected because they are the GPCRs with the highest presence in CNS neurons [35,36,37]. In contrast, the CB2R is expressed in fewer neuronal populations, at restricted CNS sites, and in glial cells [38, 39]. It is known that GPR55 is also expressed in neurons at different brain regions [27, 40,41,42].
CB1R/GPR55 heteromers have been demonstrated in vitro and in vivo [29, 31]; in the CNS, the heteromers have been identified in the striatum [29]. Accordingly, we performed in situ PLA to identify direct receptor-receptor interactions in SH-SY5Y-CB1R/GPR55 cells. This approach showed that CB1R and GPR55 readily form receptor heteromeric complexes in cotransfected cells (Fig. 5). We found that cells expressing those receptors, i.e., which express receptor heteromers, are less sensitive to the MPP+ insult. In fact, SH-SY5Y cells transfected with cDNAs for CB1R and GPR55 were resistant to a 24 h treatment with 1 mM MPP+ (Fig. 6a, b). However, the protection was not afforded when cells were treated with 2 mM MPP+ or for 48 h with the toxic molecule. Interestingly, incubation with MPP+ for 48 h in cotransfected cells led to similar results that those encountered in cells expressing only one receptor (Supplementary Fig. 2), i.e., coexpression of receptors leads to a decrease in the neuroprotective potential of cells against the MPP+ insult.
In situ PLA assay performed as described in the “Materials and Methods” section in SH-SY5Y and SH-SY5Y-CB1R/GPR55 cells using specific primary antibodies against these receptors. Panel a: representative PLA confocal images showing CB1R/GPR55 complexes as red dots in cells with Hoechst-stained nuclei. Scale bars 20 μm. Panel b: percentage of positive cells (containing one or more red dots; from cells in four–six different fields), and number (r) or red dots/cell-containing dots in cotransfected SH-SY5Y cells. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. ***p < 0.001 compared with control
MTT reduction assay in SH-SY5Y (a) and SH-SY5Y-CB1R/GPR55 (b) cells treated with MPP+ (1–2 mM) for 24 h. Cell damage is represented as the percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. **p < 0.01, ***p < 0.001 compared with control
Neuroprotective Effect of Ligands of CB1 and GPCR55 Receptors in the MPP+-Based Cellular Model of Parkinson’s Disease
A final aim was to assess the neuroprotective potential of ligands interacting with CB1R or GPR55. As a control, the effect of selective ligands on the growth of transiently single-transfected SH-SY5Y cells was tested (Fig. 7). Virtually, all assayed compounds affected cell growth after 24 h when compared with the results obtained in control. Differences in growth rate after transfection are expected and the effect of ACEA, the CB1R agonist, and of CID1792197, the GPR55 agonist was significant. ACEA was able to reduce growth in CB1R and GPR55-expressing cells but not in cotransfected cells. The GPR55 agonist was able to significantly affect growth but increasing it in untransfected cells, in cells expressing CB1R or GPR55 but not in cotransfected cells (Fig. 7). These results indicate that cell growth is affected by ACEA and by CID1792197, which would not be exerting its effect via its cognate receptor but via unknown targets. The effect of the selective CB1R antagonist, SR141617, increased growth only in cells expressing the cognate receptor (Fig. 7). This effect was remarkable as the antagonist not only reversed the effect of the agonist thus suggesting that CB1Rs are already activated even in the absence of ACEA, i.e., they display a constitutive activity when heterologously expressed in HEK-293T cells. It should be noted that no validated GPR55 antagonist is available.
MTT reduction assay in SH-SY5Y transiently transfected with cDNA coding for CB1R, GPR55 or both receptors treated with the CB1R agonist, ACEA (100 nM), with the GPR55 agonist, CID1792197 (1 μM), and with the CB1R antagonist, SR141716 (250 nM). Cell damage is represented as the percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a two-way ANOVA followed by post-hoc Tukey’s test. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the respective controls (vehicles); #p < 0.05, ###p < 0.001 compared with ACEA treatment
As above indicated, neuroprotection may be afforded by heterologous expression of receptors. We were subsequently interested in the neuroprotective potential of agonists acting on these receptors. For this purpose, SH-SY5Y cells were treated for 24 h with 1 mM MPP+ in the presence of agonists, ACEA (100 nM) for CB1R and CID1792197 (1 μM) for GPR55, or with the CB1R antagonist, SR141716 (250 nM), in the absence or presence of receptor agonists. Compounds were added 30 min before the toxic. The assays were performed in non-transfected cells and in cells expressing either CB1R, GPR55, or both. The growth of each cell type in supplemented medium in the absence of the neurotoxic and of any receptor ligand was used as reference (100% viability) in Fig. 8. On the one hand, results confirmed that the simple presence of CB1R or GPR55 was protective (Fig. 8). However, activation of the CB1R by ACEA was not protective neither in CB1R nor in CB1R/GPR55-expressing cells. Actually, the compound blocked the protection due to the presence of the receptors. On the other hand, the agonist of GPR55, CID1792197, did not enhance neuroprotection in GPR55-expressing cells, and the result was, again, similar to that obtained in CB1R-expressing cells. This is consistent with off-target effects of this compound. Agonists were neither effective in cotransfected cells. As it would be expected, the CB1R antagonist, SR141716, did not affect the results obtained using the GPR55 agonists. However, this antagonist afforded an almost full neuroprotection in CB1R-expressing cells even when ACEA was present (p < 0.05). These results suggest that both the presence of CB1R and of CB1R antagonists results in further neuroprotection. Finally, the beneficial effect of the CB1R antagonist was lost when cells expressed CB1R/GPR55 heteromers (Fig. 8).
MTT reduction assay in SH-SY5Y transiently transfected with cDNA coding for CB1R, GPR55 or both receptors treated with the CB1R agonist, ACEA (100 nM), and with the GPR55 agonist, CID1792197 (1 μM), in the presence or in the absence of the CB1R antagonist, SR141716 (250 nM), before MPP+ (1 mM) addition for 24 h. Cell damage is represented as the percentage of MTT reduction versus control (represented by each cell type as 100%, i.e., the growth in the absence of MPP+ and of receptor ligand). Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a two-way ANOVA followed by post-hoc Tukey’s test. ***p < 0.001 compared with their respective control (vehicle). #p < 0.05 compared with ACEA treatment
Discussion
Neuroprotection by cannabinoid compounds may be afforded by targeting either CB1R or CB2R. PD is usually diagnosed when a significant number of nigral dopaminergic neurons are already lost. Therefore, targeting neurons to prevent further death appears as the most promising choice for disease-modifying approaches. In that sense, CB1R have been considered as a relevant target and receptor ligands as potentially neuroprotective candidates. On the one hand, success has been partial and the underlying mechanisms not fully elucidated until now. On the other hand, CB1R may be targeted by compounds acting in the orthosteric center (agonists, antagonists or inverse agonists) or by cannabidiol, which has been reported as an allosteric modulator of CB1R function [43]. Further work is needed to properly ascertain whether CB1R ligands are neuroprotective for dopaminergic neurons and whether the neuroprotection requires agonists, antagonists, inverse agonists or allosteric modulators [44,45,46,47,48,49].
Despite the fact that GPR55 has been by far less studied in CNS than the CB1R, its activation leads to increased neurotransmitter release in CNS synapses [41], and a recent report shows that hippocampal GPR55 mediates an increase of neural stem cell proliferation and of adult neurogenesis [50]. A further aspect that may be relevant is the finding of GPR55 in mitochondria. There are few reports showing GPCRs located in the mitochondrial membrane but, interestingly, it was described that CB1R in this location may regulate the energy status of neurons [33, 51]. We also found that AT1 and AT2 angiotensin receptors could be expressed in the mitochondria of dopaminergic neurons; the data suggested that altered expression of these receptors impacts on aging-related neurodegeneration [52]. At present, the exact mechanisms by which GPCRs located in mitochondria regulate the activity of this organelle are unknown.
As previously described, our results confirmed that MPP+ is toxic for SH-SY5Y cells [12]. Interestingly, the expression of CB1R was enough to almost completely prevent the toxic effect exerted by a moderate concentration of the neurotoxin. At higher concentrations, the MPP+-induced cell death is very high and virtually unsurmountable. Also noteworthy was the finding that the expression of GPR55 was also protective against the insult exerted by MPP+. Coexpression of the two receptors led to the formation of CB1/GPR55 heteroreceptor complexes (as shown in Fig. 5) as it was reported in a heterologous expression system and in rat striatum [29].
Our initial hypothesis considering a neuroprotection enhancement in the presence of agonists was not confirmed. This study was designed using another reportedly GRP55 agonist, CID1792197, but the results indicate that it may be a biased agonist that uncouples GPR55 from any neuroprotective mechanisms and/or that it is acting in unknown targets. Activation of GPR55 by CID1792197 did not lead to any significant modification in the protective effect against MPP+. Furthermore, some of the results were similar in cells other than those expressing GPR55. We do not favor the idea that our findings might be due to a constitutive activation of the receptor (a fact that to our knowledge has not yet been reported), although a CID1792197-mediated production of a significant amount of the putative endogenous agonist of the receptor (L-α-lysophosphatidylinositol) cannot be completely ruled out. The results found when using a well-characterized synthetic CB1R agonist, ACEA, are difficult to interpret. On the one hand, ACEA by itself decreases the growth rate of cells (e.g., in the absence of MPP+). As ACEA reverses neuroprotection in CB1R-expressing cells, it may be producing off-target effects leading to a decrease in the growth of cells that shades the neuroprotective effect due to CB1R. In addition, it has been reported that ACEA may act via GPR55 receptors and that other presumably cannabinoid receptors ligands, such as AM251, may act as antagonist of CB1R but agonist of GPR55 [53]. The results obtained using a very selective CB1R antagonist, SR141716, were interesting as this compound afforded neuroprotection against the MPP+ insult. This effect may be due either to the reversal of CB1R constitutive activity that decreases growth as when ACEA is used, or to the blockade of the action of endocannabinoids having a detrimental role in this cellular MPP+ model. Although technically challenging, we think that it would be very relevant to know whether CB1R or GPR55 display any constitutive activity in their own (both in SH-SY5Y cells and in primary neuronal cultures), as well as whether SH-SY5Y cells are able to produce endogenous agonists of CB1R, GPR55, or of both receptors. Also relevant was the finding of negative cross-talk when the two receptors were coexpressed and forming heteromers. The pharmacology and signaling of GPR55 are still under scrutiny in the CNS, and our previous results [29] indicate that functional interactions between these two receptors are complex and may depend on the nature of the compounds and on their potential to skew signal transduction events (biased agonism). In what concerns the resistance to the effects of MPP+, a neurotoxic used to produce models of parkinsonism, our results show benefits due to individual receptors but not to receptor heteromers. In summary, neuroprotection against MPP+ is likely prevalent in neurons expressing one of the receptors. In neurons expressing the two receptors, disrupting the heteromer may be a likely intervention to afford neuroprotection.
References
Lanciego JL, Luquin N, Obeso JA (2012) Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2:a009621. https://doi.org/10.1101/cshperspect.a009621
Winkler C, Kirik D, Björklund A, Cenci MA (2002) L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 10:165–186. https://doi.org/10.1006/nbdi.2002.0499
Hornykiewicz O (2006) The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl 70:9–15. https://doi.org/10.1007/978-3-211-45295-0_3
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonucelli U, Damier P, de Yebenes J, Gershanik O et al (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005. https://doi.org/10.1002/mds.20243
Guridi J, Rodriguez-Rojas R, Carmona-Abellan M et al (2018) History and the future challenges of the subthalamic nucleus as surgical target. Mov Disord In the Pre 33:1540–1550
Goldman JG, Vernaleo BA, Camicioli R, Dahodwala N, Dobkin RD, Ellis T, Galvin JE, Marras C et al (2018) Cognitive impairment in Parkinson’s disease: A report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Park Dis 4:1–11. https://doi.org/10.1038/s41531-018-0055-3
Le W, Sayana P, Jankovic J (2014) Animal models of Parkinson’s disease: a gateway to therapeutics? Neurotherapeutics 11:92–110. https://doi.org/10.1007/s13311-013-0234-1
Gubellini P, Kachidian P (2015) Animal models of Parkinson’s disease: an updated overview. Rev Neurol (Paris) 171:750–761. https://doi.org/10.1016/j.neurol.2015.07.011
Cardoso SM, Esteves AR, Arduíno DM (2012) Mitochondrial metabolic control of microtubule dynamics impairs the autophagic pathway in Parkinson’s disease. Neurodegener Dis 10:38–40. https://doi.org/10.1159/000332601
Chaturvedi RK, Flint Beal M (2013) Mitochondrial diseases of the brain. Free Radic Biol Med 63:1–29. https://doi.org/10.1016/j.freeradbiomed.2013.03.018
Do JH (2014) Neurotoxin-induced pathway perturbation in human neuroblastoma SH-EP cells. Mol Cells 37:672–684. https://doi.org/10.14348/molcells.2014.0173
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J 123:1086–1092. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.08.021
Fernández-Ruiz J, Romero J, Ramos JA (2015) Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. Handb Exp Pharmacol 231:233–259. https://doi.org/10.1007/978-3-319-20825-1_8
Fernández-Ruiz J, Gómez-Ruiz M, García C, et al (2017) Modeling neurodegenerative disorders for developing cannabinoid-based neuroprotective therapies. Methods Enzymol 175–198. https://doi.org/10.1016/bs.mie.2017.06.021
Barrero FJ, Ampuero I, Morales B, Vives F, de Dios Luna del Castillo J, Hoenicka J, García Yébenes J (2005) Depression in Parkinson’s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J 5:135–141. https://doi.org/10.1038/sj.tpj.6500301
Shao Z, Yin J, Chapman K, Grzemska M, Clark L, Wang J, Rosenbaum DM (2016) High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 540:602–606. https://doi.org/10.1038/nature20613
Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, Pu M, Korde A et al (2017) Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 547:468–471. https://doi.org/10.1038/nature23272
Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, Zhao S, Shui W et al (2016) Crystal structure of the human cannabinoid receptor CB1. Cell 167:750–762.e14. https://doi.org/10.1016/j.cell.2016.10.004
Pertwee RG (2008) The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215. https://doi.org/10.1038/sj.bjp.0707442
Sam AH, Salem V, Ghatei MA (2011) Rimonabant: from RIO to ban. J Obes 2011:432607. https://doi.org/10.1155/2011/432607
Kotsikorou E, Madrigal KE, Hurst DP, Sharir H, Lynch DL, Heynen-Genel S, Milan LB, Chung TDY et al (2011) Identification of the GPR55 agonist binding site using a novel set of high-potency GPR55 selective ligands. Biochemistry 50:5633–5647. https://doi.org/10.1021/bi200010k
Andradas C, Caffarel MM, Pérez-Gómez E, Salazar M, Lorente M, Velasco G, Guzmán M, Sánchez C (2011) The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30:245–252. https://doi.org/10.1038/onc.2010.402
Drzazga A, Sowinska A, Krzeminska A, Rytczak P, Koziolkiewicz M, Gendaszewska-Darmach E (2017) Lysophosphatidylcholine elicits intracellular calcium signaling in a GPR55-dependent manner. Biochem Biophys Res Commun 489:242–247. https://doi.org/10.1016/j.bbrc.2017.05.145
Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, Ross RA (2012) Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J Biol Chem 287:91–104. https://doi.org/10.1074/jbc.M111.296020
Falasca M, Ferro R (2016) Role of the lysophosphatidylinositol/GPR55 axis in cancer. Adv Biol Regul 60:88–93. https://doi.org/10.1016/j.jbior.2015.10.003
Gómez-Cañas M, Morales P, García-Toscano L, Navarrete C, Muñoz E, Jagerovic N, Fernández-Ruiz J, García-Arencibia M et al (2016) Biological characterization of PM226, a chromenoisoxazole, as a selective CB2 receptor agonist with neuroprotective profile. Pharmacol Res 110:205–215. https://doi.org/10.1016/j.phrs.2016.03.021
Celorrio M, Rojo-Bustamante E, Fernández-Suárez D, Sáez E, Estella-Hermoso de Mendoza A, Müller CE, Ramírez MJ, Oyarzábal J et al (2017) GPR55: a therapeutic target for Parkinson’s disease? Neuropharmacology 125:319–332. https://doi.org/10.1016/j.neuropharm.2017.08.017
García-Gutiérrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, Giner S, Manzanares J (2018) Alterations in gene and protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics 15:796–806. https://doi.org/10.1007/s13311-018-0610-y
Martínez-Pinilla E, Reyes-Resina I, Oñatibia-Astibia A, Zamarbide M, Ricobaraza A, Navarro G, Moreno E, Dopeso-Reyes IG et al (2014) CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp Neurol 261:44–52. https://doi.org/10.1016/j.expneurol.2014.06.017
Henstridge CM, Balenga NA, Schröder R et al (2010) GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol 160:604–614. https://doi.org/10.1111/j.1476-5381.2009.00625.x
Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M (2012) The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J Biol Chem 287:44234–44248. https://doi.org/10.1074/jbc.M112.364109
Hynes J, Floyd S, Soini AE, O'Connor R, Papkovsky DB (2003) Fluorescence-based cell viability screening assays using water-soluble oxygen probes. J Biomol Screen 8:264–272. https://doi.org/10.1177/1087057103008003004
Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, Piazza PV, Benard G et al (2014) Cannabinoid control of brain bioenergetics: exploring the subcellular localization of the CB1 receptor. Mol Metab 3:495–504. https://doi.org/10.1016/j.molmet.2014.03.007
Gutiérrez-Rodríguez A, Bonilla-Del Río I, Puente N et al (2018) Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 66:1417–1431. https://doi.org/10.1002/glia.23314
Katona I, Freund TF (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14:923–930. https://doi.org/10.1038/nm.f.1869
H-CC L, Mackie K (2016) An introduction to the endogenous cannabinoid system. Biol Psychiatry 79:516–525. https://doi.org/10.1016/j.biopsych.2015.07.028
Mackie K (2005) Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 299–325. https://doi.org/10.1007/978-3-319-20825-1_3
Sierra S, Luquin N, Rico AJ, Gómez-Bautista V, Roda E, Dopeso-Reyes IG, Vázquez A, Martínez-Pinilla E et al (2015) Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct 220:2721–2738. https://doi.org/10.1007/s00429-014-0823-8
Navarro G, Morales P, Rodríguez-Cueto C, Fernández-Ruiz J, Jagerovic N, Franco R (2016) Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with focus on neurodegenerative disorders. Front Neurosci 10:406. https://doi.org/10.3389/fnins.2016.00406
Hurst K, Badgley C, Ellsworth T, Bell S, Friend L, Prince B, Welch J, Cowan Z et al (2017) A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus 27:985–998. https://doi.org/10.1002/hipo.22747
Sylantyev S, Jensen TP, Ross RA, Rusakov DA (2013) Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A 110:5193–5198. https://doi.org/10.1073/pnas.1211204110
Marichal-Cancino B, Fajardo-Valdéz A, Ruiz-Contreras A et al (2016) Advances in the physiology of GPR55 in the central nervous system. Curr Neuropharmacol 14:1–1. https://doi.org/10.2174/1570159X14666160729155441
Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805. https://doi.org/10.1111/bph.13250
van der Stelt M, Veldhuis WB, Maccarrone M, Bär PR, Nicolay K, Veldink GA, di Marzo V, Vliegenthart JFG (2002) Acute neuronal injury, excitotoxicity, and the endocannabinoid system. Mol Neurobiol 26:317–346. https://doi.org/10.1385/MN:26:2-3:317
Molina-Holgado E, Vela JM, Arévalo-Martín A et al (2002) Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci 22:9742–9753
Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS (2013) Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci 33:6350–6366. https://doi.org/10.1523/JNEUROSCI.3786-12.2013
Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S (2017) Endocannabinoid system in neurodegenerative disorders. J Neurochem 142:624–648. https://doi.org/10.1111/jnc.14098
Solimini R, Rotolo MC, Pichini S, Pacifici R (2017) Neurological disorders in medical use of cannabis: an update. CNS Neurol Disord - Drug Targets 16:527–533. https://doi.org/10.2174/1871527316666170413105421
Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9:482. https://doi.org/10.3389/fphar.2018.00482
Hill JD, Zuluaga-Ramirez V, Gajghate S, Winfield M, Persidsky Y (2018) Activation of GPR55 increases neural stem cell proliferation and promotes early adult hippocampal neurogenesis. Br J Pharmacol 175:3407–3421. https://doi.org/10.1111/bph.14387
Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, Delamarre A et al (2012) Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci 15:558–564. https://doi.org/10.1038/nn.3053
Valenzuela R, Costa-Besada MAMA, Iglesias-Gonzalez J et al (2016) Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis 7:e2427. https://doi.org/10.1038/cddis.2016.327
Walsh SK, Hepburn CY, Keown O, Åstrand A, Lindblom A, Ryberg E, Hjorth S, Leslie SJ et al (2015) Pharmacological profiling of the hemodynamic effects of cannabinoid ligands: a combined in vitro and in vivo approach. Pharmacol Res Perspect 3:e00143. https://doi.org/10.1002/prp2.143
Funding
This study was funded by Fundació La Marató de TV3 (Grant Numbers 20141330 and 20141331).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
MTT reduction assay in SH-SY5Y (a), SH-SY5Y-CB1R (b) and SH-SY5Y-GPR55 (c) cells treated with MPP+ (1-2 mM) for 48 h. Cell damage is represented as the percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. ***p < 0.001 compared with control. (PNG 332 kb)
Supplementary Fig. 2
MTT reduction assay in SH-SY5Y (a) and SH-SY5Y-CB1R/GPR55 (b) cells treated with MPP+ (1-2 mM) for 48 h. Cell damage is represented as the percentage of MTT reduction versus control. Data are the mean ± SEM of five independent experiments. Significant differences were analyzed by a one-way ANOVA followed by post-hoc Tukey’s test. ***p < 0.001 compared with control. (PNG 243 kb)
Rights and permissions
About this article
Cite this article
Martínez-Pinilla, E., Aguinaga, D., Navarro, G. et al. Targeting CB1 and GPR55 Endocannabinoid Receptors as a Potential Neuroprotective Approach for Parkinson’s Disease. Mol Neurobiol 56, 5900–5910 (2019). https://doi.org/10.1007/s12035-019-1495-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1495-4