Abstract
Systemic inflammation and ammonia (hyperammonemia) act synergistically in the pathogenesis of hepatic encephalopathy (HE), the neurobehavioral sequelae of advanced liver disease. In cirrhotic patients, we have recently observed elevated levels of circulating neuronal tight junction (TJ) protein, zonula occludens 1 (ZO-1), reflective of a change to blood–brain barrier (BBB) integrity. Moreover, ZO-1 levels positively correlated with hyperammonemia, although any potential relationship remains unclear. Using a carbon tetrachloride (CCl4)–induced mouse model of cirrhosis, we primarily looked to explore the relationship between neuronal TJ protein expression and hyperammonemia. Secondarily, we assessed the potential role of a natural antioxidant, resveratrol, on neuronal TJ protein expression and hyperammonemia. Over 12 weeks, male Swiss mice were randomized (n = 8/group) to either naïve controls or induced cirrhosis, using two doses of intraperitoneal CCl4 (0.5 ml/kg/week). After 12 weeks, naïve and cirrhotic mice were randomized to receive either 2 weeks of par-oral resveratrol (10 mg/kg). Plasma samples were analyzed for ammonia, liver biochemistry (ALT, AST, albumin, and bilirubin), and pro-inflammatory cytokines (TNF-α and IL-1β), and brain tissue for brain water content, TJ protein expression (e.g., ZO-1, claudin 5, and occludin), and tissue oxidative stress and inflammatory markers (NF-κB and iNOS) using western blotting. Compared to naïve mice, cirrhosis significantly increased circulating ammonia, brain water, ALT, AST, TNF-α, IL-1β, 4HNE, NF-κB, and iNOS levels, with a concomitant reduction in all TJ proteins (P < 0.05, respectively). In cirrhotic mice, resveratrol treatment ameliorated these changes significantly (P < 0.05, respectively). Our findings provide evidence for a causal association between hyperammonemia and inflammation in cirrhosis linked to TJ protein alterations, BBB disruption, and HE predilection. Moreover, this is the first report of a potential role for resveratrol as a novel therapeutic approach to managing neurological sequelae complicating cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a neuropsychiatric manifestation of patients with acute and/or chronic liver failure, invariably signaling a deterioration of health [1] and defining progression to formal liver failure. It is characterized by cognitive, psychiatric, and motor dysfunctions and severe HE in patients with decompensated cirrhosis is associated with > 50% mortality in the first years from diagnosis [2, 3]. The pathogenesis of HE is multifactorial and incompletely understood, but since HE was first described, disrupted ammonia metabolism has been considered central to the development of HE [4]. With progressive liver failure, concomitant alterations to multiorgan ammonia and amino acid metabolism are associated with elevated levels of circulating ammonia [5, 6], termed hyperammonemia. Moreover, ammonia can be neurotoxic to the brain if tissue levels rise significantly. For the body to function optimally, the brain is required to operate in a highly regulated homeostatic ionic environment, which may offer protection from neuroactive blood-borne solutes. Therefore, entry of circulating metabolites such as ammonia is tightly regulated by the blood–brain barrier (BBB) [7].
The BBB is made up of individual neurovascular units (NVUs) each composed of neurons, astrocytes and microglia (“astroglia”), pericytes, and brain microvascular endothelial cells (BMECs). The BBB is held together as a physical barrier by tight junctions (TJs) between BMECs [8]. TJs are composed of large multiprotein complexes that essentially seal the gaps between biological barriers [9] and provide cellular adhesion between adjacent BMECs through anchorage to the actin cytoskeleton. This creates a tight interendothelial seal that impedes paracellular diffusion of ions, macromolecules, and other polar solutes. Interlocked BMECs therefore form a selective transport interface between the blood circulation and the brain, with expression of transporters, metabolite-degrading enzymes, receptors, ion channels, and ion transporters, ensuring that nutrients such as glucose, amino acids, nucleosides, and electrolytes are delivered to the brain from the blood and that solutes and metabolite waste products are effluxed from the brain to the blood [8]. Crucially, this allows for cells of the NVU to act in concert to orchestrate activity-dependent regulation of vascular permeability, cerebral blood flow, and neuroimmune responses in health and disease, with BBB integrity central to the onset and progression of neurodegeneration and cognitive impairment [10].
In liver disease, ammonia neurotoxicity has been linked to changes in TJ protein expression [11, 12]. Among the proteins involved in the multiprotein complexes, zonula occludens (ZO-1, ZO-2, and ZO-3) are the major TJ-multidomain scaffolding proteins. ZO-1 directly interacts with transmembrane TJ proteins such as claudins and the TJ-associated MARVEL protein (TAMP) family, which includes occludin, providing the backbone of the TJ functional structure [13,14,15,16]. Importantly, oxidative stress and inflammation can cause decreased TJ protein expression leading to alterations in paracellular permeability. Since BBB is chiefly involved in strict regulation of paracellular permeability to maintain an optimal extracellular environment for brain homeostasis [17], when it is compromised, it may lead to irreversible injuries to the brain [18]. Despite the fact that BBB disruption is a frequent complication in chronic neurodegenerative and neuroinflammatory diseases, potential treatment approaches protecting against any direct loss of BBB integrity remain challenging.
Polyphenols, including flavonoids and stilbenes, are widely present in plant-based foods and beverages and are part of the average human diet [19]. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is one of the polyphenols and a known antioxidant abundant in grapes and red wine [20]. Resveratrol is a lipophilic compound that can also cross the BBB and affect various cellular signaling molecules; these characteristics may be beneficial especially in the treatment of neuroinflammatory disorders. Many recent studies have evidenced a wide range of health-promoting benefits of resveratrol for chronic ailment including neurological disorders [21]. Resveratrol treatment has also been shown to inhibit inflammation, viral infection, and oxidative stress and prevent bacterial translocation (BT) in cirrhosis [22,23,24]. Furthermore, resveratrol treatment has been shown to protect against TJ disruption and thus maintain BBB integrity both in vivo and in vitro [9, 25, 26].
The aim of this study was to explore the hypothesis that targeting hyperammonemia and inflammation with natural antioxidants like resveratrol may improve TJ integrity by restoration of TJ protein in a CCl4-induced mice model of decompensated cirrhosis.
Materials and Methods
Animals
All the experimental procedures were carried out in accordance with the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA) guidelines. Sixty male Swiss strain (CD-1) mice weighing 25 ± 2 g were used for the study and housed in polypropylene cages. All animals were maintained at room temperature (~ 30 ± 2 °C) with 12-h light/dark cycle and had ad libitum access to standard rodent chow and water.
Animal Model and Experimental Design
All animals were allowed to acclimatize to laboratory conditions for 1 week. Animals were randomly allocated to different experimental groups. To induce liver cirrhosis, the mice were intoxicated chronically with carbon tetrachloride (CCl4—0.5 ml/kg b.w.) intraperitoneally in corn oil twice a week over the period of 12 weeks. Following the induction of ascites (indicating decompensated cirrhosis), animals were randomized into disease control and treatment groups. The treatment group received resveratrol (10 mg/kg; Sigma-Aldrich Inc., USA) through oral gavage daily for 2 weeks. Control mice received corn oil only.
The final study groups were as follows:
- (a)
Naïve mice administered vehicle–corn oil (naïve group)
- (b)
Naïve mice administered resveratrol (naïve + treatment group)
- (c)
CCl4-induced mice (cirrhosis group)
- (d)
CCl4-induced mice administered resveratrol (cirrhosis + treatment group)
At the end of the experimental period, all animals in different study groups were sacrificed by cervical dislocation. Ketamine hydrochloride was used as anesthesia. Then, ~ 1 ml of blood was collected using cardiac puncture method. Brain tissue collected was stored in − 80 °C for molecular studies.
Histological Evaluation
Four percent paraformaldehyde-fixed mouse brain specimen was removed, dehydrated in alcohols, incubated in xylene, and embedded in paraffin. Then, 5-μm-thick tissue sections were cut and stained with hematoxylin and eosin (H&E) using standard procedures.
Plasma Biochemistry and Quantification of Cytokines and Ammonia
Biochemical parameters were analyzed using 400 μl of respective plasma samples using the Beckman Coulter autoanalyzer (USA). TNF-α and IL-1β cytokine levels were measured using ELISA kit (R&D Systems, USA). Ammonia was estimated using Ammonia ultra-kit (Proton Biological Pvt. Ltd., India).
Brain Water Measurement
The whole brain tissue was rapidly removed and 50-mm2 samples were dissected from the frontal cortex (gray matter) immediately after surgery. Percentage of brain tissue water content was determined using a previously described dry weight technique [27, 28]. Tissue water content was then calculated as % water = (1 − dry weight/wet weight) × 100%.
Western Blot Analysis
For western blot analysis, freshly harvested brain tissue was snap frozen immediately in liquid nitrogen. For each sample, 150 mg of tissue was weighed and homogenized in 300 μl ice-cold TRIS–EDTA buffer (pH 7.4) with protease inhibitor cocktail (Sigma-Aldrich, USA) and protein methyl sulfonyl fluoride (PMSF in ethanol). Protein was estimated by Bradford method using Pierce BCA protein assay kit (Thermo Fisher Scientific, USA).
The individual samples were resolved using SDS-PAGE electrophoresis and blotted onto PVDF membranes (Invitrogen, UK). The non-specific binding sites were blocked using 5% non-fat skimmed milk (Himedia) in TBS–Tween 20 (TBST) buffer, followed by incubation with primary antibodies overnight at 4 °C. The primary antibodies used were anti-rabbit polyclonal 4HNE (1:1000, BS-6313R; Bioss, USA), rabbit polyclonal anti-iNOS (1:1000, BS-2072R; Bioss, USA), rabbit monoclonal anti-NFKBp65 and anti-total NFKBp65 (1:1000, 8242S; CST), rabbit polyclonal anti-ZO-1 (1:1000, 61-7300; Thermo Fisher, USA), mouse monoclonal anti-occludin (1:1000, sc-133256; Santa Cruz, USA), and mouse monoclonal anti-claudin-5 (1:1000, 35-2500; Thermo Fisher, USA). Peroxidase-conjugated goat anti-rabbit (1:10,000 dilution, catalog 074-1506; KPL) and goat anti-mouse (1:10,000 dilution, catalog 074-1806; KPL) were used appropriately as secondary antibodies. The bands were visualized in Chemidoc system (Bio-Rad) using chemiluminescent substrate (West Pico detection kit from Thermo Scientific). Quantification of bands was performed using image J software.
Immunohistochemistry
Brain tissues were fixed in 10% normal buffered formalin and embedded in paraffin. Three- to five-micrometer-thick sections were cut using automated microtome (Leica). Silane-coated tissue slides were used for immunohistochemistry. Deparaffinized sections were blocked for endogenous peroxidase activity with 10% H2O2 in phosphate buffer for 10 min. Antigen retrieval was performed using citrate buffer in Decloaking system at 110 °C for 10 min. 4HNE, occludin, and claudin-5 were detected by immunohistochemistry using anti-rabbit polyclonal 4HNE (1:1000, BS-6313R; Bioss, USA), mouse monoclonal anti-occludin (1:1000, sc-133256; Santa Cruz), and anti-claudin-5 (1:1000, 35-2500; Thermo Fisher), respectively. Primary antibodies were used in dilution of 1:100 and incubated at room temperature for 1 h. Appropriate secondary antibody system (Vector lab) was used. Sections were mounted with Histomount solution and the immunostaining was examined using EVOS FLc imaging system (Invitrogen, UK).
Data Sharing Statement
No additional data are available. All data generated or analyzed during this study are included in this published article.
Statistics
Data were exported using Microsoft Excel 2014 and analyzed using GraphPad Prism 6.0 software. All data were recorded as mean ± SEM. One-way ANOVA followed by Tukey’s multiple comparison tests was performed and P values less than 0.05 was considered statistically significant.
Results
All mice showed clear signs of decompensated cirrhosis as evidenced by the pronounced nodular appearance of the liver and ascites that was confirmed during sacrifice (data not shown). Body weight of all animals was noted from the commencement of experiment. Animals treated with CCl4 had a tendency to lose body weight and presented with a significantly lower body weight (P < 0.05) compared to naïve controls at the end of experimental period, prior to sacrifice. In CCl4 mice that were treated with resveratrol, a slight increase in the body weight was observed; however, the change was not statistically significant (data not shown). Furthermore, brain pathological changes observed by H&E are shown in Fig. 1a–d. Normal mouse brain (a) and resveratrol-treated naïve mouse brain (b) show similar histology with normal glia. CCl4-induced cirrhotic mouse brain (c) and CCl4 + resveratrol-treated mouse brain (d) show reactive gliosis with evidence of prominent cytoplasmic process and minimal nuclear atypia.
Representative images show hematoxylin and eosin (H&E) of brain sections from the indicated naïve and experimental mice (× 20 magnification). Sections from naïve mouse brain (a) and resveratrol-treated naïve mouse brain (b) show similar histology with normal glia. CCl4-treated mouse brain (c) and CCl4 + resveratrol-treated mouse brain (d) show reactive gliosis with evidence of prominent cytoplasmic process and minimal nuclear atypia
Effect of Resveratrol on Plasma Biochemical Parameters in CCl4-Induced Cirrhosis
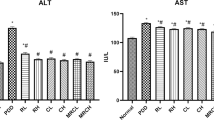
When compared to naïve mice, CCl4-treated mice showed a significant increase in liver function parameters such as ALT, AST, and bilirubin, while albumin levels reduced significantly (P < 0.05). CCl4 animals that received resveratrol showed a significant (P < 0.05) decrease in ALT and AST whereas albumin level was increased significantly (Fig. 2a–c). Resveratrol treatment in CCl4 mice had no significant effect on bilirubin concentrations (Fig. 2d).
a–d Serum biochemical parameters in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. *P < 0.05 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. ALT, alanine aminotransferase; AST, aspartate aminotransferase
Effect of Resveratrol on Arterial Ammonia Concentration and Brain Water Content in CCl4-Induced Cirrhosis
Figure 3a shows arterial ammonia concentration, which was significantly (P < 0.01) increased in CCl4-induced cirrhosis compared to naïve mice. Resveratrol treatment to cirrhotic mice show significantly (P < 0.05) decreased arterial ammonia concentration. Furthermore, the observed frontal cortex brain water content (3B) was significantly (P < 0.01) increased in CCl4-induced cirrhosis when compared to naïve mice. Cirrhotic mice that received resveratrol show significantly decreased percent brain water compared to CCl4 alone treated mice.
Effect of Resveratrol on Plasma Cytokines in CCl4-Induced Cirrhosis
Plasma pro-inflammatory cytokines such as TNF-α and IL-1β concentrations were significantly increased (P < 0.05, respectively) in CCl4-induced cirrhosis. Resveratrol treatment to CCl4 mice resulted in a significant (P < 0.05) downregulation of both TNF-α and IL-1β levels (Fig. 4a, b).
Plasma TNF-α (a) and IL-1β (b) concentrations in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. *P < 0.05 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. TNF-α, tumor necrosis factor alpha; IL-1β, interleukin 1 beta
Brain tissue inflammatory markers NF-κB p65 (a) and iNOS (b) protein expression in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using the Tukey’s multiple comparison test. *P < 0.05 and **P < 0.01 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. NF-κB, nuclear factor kappa B; iNOS, inducible nitric oxide synthase
Resveratrol Treatment Attenuates the Neuronal Expression of NF-κB and iNOS in CCl4-Induced Cirrhosis
Figure 5a and b shows neuronal protein expression of phosphorylated NF-κB (p65) and iNOS in control and experimental mice. Protein expression of both NF-κB (a) and iNOS (b) were significantly (P < 0.01 and P < 0.05, respectively) increased in the brain of CCl4-induced cirrhotic mice when compared to normal control mice. Resveratrol treatment to cirrhotic mice results in decreased brain expression of NF-κB and iNOS significantly (P < 0.05) when compared to lone CCl4 mice brain.
Resveratrol Treatment Attenuates Brain Oxidative Stress in CCl4-Induced Cirrhosis
Figure 6A and B shows the neuronal 4HNE protein expression by western blotting and cellular expression by IHC in the control and experimental mice. Neuronal 4HNE protein expression was significantly (P < 0.05) elevated in CCl4-induced cirrhotic mice compared to naïve mice. Resveratrol treatment to cirrhotic mice shows significantly (P < 0.05) decreased neuronal 4HNE protein expression compared to cirrhotic mice alone. Furthermore, immunostaining of normal brain sections shows negative reactivity of 4HNE expression (6B-a), whereas strong nuclear positivity appeared in CCl4-treated mouse brain (6B-b). CCl4 + resveratrol mouse brain (6B-c) shows reduced 4HNE positivity compared to CCl4 alone.
A Neuronal 4HNE protein expression by Western blotting in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. *P < 0.05 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. β-Actin was used as loading control. B Representative images show 4HNE immunostaining of brain sections from the indicated mice groups (× 20 magnification). Naïve mouse brain section (a) shows negative reactivity of 4HNE expression, whereas strong nuclear positivity was found in a CCl4-treated mouse brain (b); CCl4 + resveratrol mouse brain (c) shows reduced positivity compared to CCl4 alone. Resveratrol treatment to naïve mice had no effect on 4HNE expression (data not shown)
Resveratrol Treatment Improves Brain Tight Junction Proteins Expression in CCl4-Induced Cirrhosis
Figure 7 shows the neuronal ZO-1 protein expression in control and experimental mice. When compared to naïve, ZO-1 protein expression was significantly (P < 0.05) decreased in cirrhotic mice brain. Resveratrol treatment to cirrhotic mice show significantly (P < 0.05) increased neuronal ZO-1 protein expression compared to CCl4 alone.
Neuronal ZO-1 protein expression by Western blotting in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. *P < 0.05 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. β-Actin was used as loading control
Figure 8 shows the neuronal occludin protein expression by western blotting (A) and cellular expression by IHC (B) in control and experimental mice. When compared to naïve, occludin protein expression was significantly (P < 0.01) decreased in cirrhotic mice brain. Resveratrol treatment to cirrhotic mice show significantly (P < 0.05) increased neuronal occludin protein expression compared to lone CCl4. Furthermore, immunostaining of normal brain sections shows occludin positivity in endothelial cells (8B-a), whereas loss of expression of occludin was found in CCl4-treated cirrhotic mouse brain (8B-b). CCl4 + resveratrol mouse brain (8B-c) shows increased positivity of occludin in endothelial cells compared to CCl4 alone.
A Neuronal occludin protein expression by Western blotting in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. **P < 0.01 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. β-Actin was used as loading control. B Representative images show occludin immunostaining of brain sections from the indicated mice groups (× 20 magnification). Naïve mouse brain section (a) shows occludin positivity in endothelial cells, whereas loss of expression of occludin was found in CCl4-treated cirrhotic mouse brain (b); CCl4 + resveratrol mouse brain (c) shows increased positivity of occludin in endothelial cells compared to CCl4 alone. Resveratrol treatment to naïve mice had no effect on altering occludin expression (data not shown)
Figure 9 shows the neuronal claudin-5 protein expression by western blotting (A) and cellular expression by IHC (B) in control and experimental mice. When compared to naïve, claudin-5 protein expression was significantly (P < 0.05) decreased in cirrhotic mice brain. Resveratrol treatment to cirrhotic mice shows significantly (P < 0.05) increased neuronal claudin-5 protein expression compared to lone CCl4. Furthermore, immunostaining of normal brain sections shows claudin-5 positivity in endothelial cells (9B-a), whereas loss of expression of claudin-5 was found in CCl4-treated cirrhotic mouse brain (9B-b). CCl4 + resveratrol mouse brain (9B-c) shows patchy positivity seen in scattered glia compared to CCl4 alone.
A Neuronal claudin-5 protein expression by Western blotting in naïve and experimental mice. Values are expressed as mean ± SEM. Statistical analysis was determined using Tukey’s multiple comparison test. *P < 0.05 compared to naïve mice. $P < 0.05 compared to CCl4-induced cirrhotic mice. β-Actin was used as loading control. B Representative images show claudin-5 immunostaining of brain sections from the indicated mice groups (× 20 magnification). Naïve mouse brain section (a) shows claudin-5 positivity in endothelial cells, whereas loss of expression of claudin-5 was found in CCl4-treated mouse brain (b); CCl4 + resveratrol mouse brain (c) shows patchy positivity seen in scattered glia compared to CCl4 alone. Resveratrol treatment to naïve mice had no effect on altering claudin-5 expression (data not shown)
Proposed hypothesis of how resveratrol prevents brain endothelial tight junction disruption and ammonia toxicity in cirrhosis. Chronic carbon tetrachloride (CCl4) treatment induces hepatic cirrhosis, which leads to increased systemic inflammation and ammonia accumulation. Hyperammonemia and inflammation may act synergistically to disrupt tight junction integrity made by tight junction proteins such as ZO-1, occludin, and claudin-5 and sensitizes the astroglial cells to inflammatory mediators. Treatment with resveratrol reduces the diffusion of ammonia and other inflammatory mediators through the cerebral endothelial cells, by preventing the disruption of tight junction and thereby protecting from ammonia-induced neurotoxicity
Discussion
This study demonstrates for the first time that resveratrol treatment exhibits protective effects in the cirrhotic brain through antioxidant and anti-inflammatory properties, associated with attenuation of hyperammonemia and restoration of TJ protein expression. Resveratrol treatment reversed the pathognomonic hepatic dysfunction of induced cirrhosis, with improved biochemistry, body weight, and behavior indicative of liver recovery. Moreover, brain water and the hyperammonemic state of cirrhosis were significantly attenuated by resveratrol treatment. Although this was in a mouse model, CCl4-induced cirrhosis in mice share many characteristics with CCl4 rat models [27] and human cirrhosis [29].
Elevated arterial and brain ammonia levels are central to the clinical expression of HE. Ammonia is a weak base that exists in biological solutions in two molecular forms, NH3 and NH4+, that can easily diffuse across the BBB and accumulate in the brain to act as a neurotoxin on astroglial cells of the NVU [30, 31]. Resveratrol supplementation has previously been shown to prevent ammonia-induced mitochondrial dysfunction, glutamatergic communication, and cellular redox imbalance in astroglial cells [32, 33]. Our findings would indicate that resveratrol can suppress pathological increases in ammonia levels and brain water content, and have the potential to reverse neurological sequelae associated with cirrhosis. The neuroprotective effect of resveratrol may be due to the improved liver function and thus ammonia metabolism and/or a direct effect on neuroinflammatory pathways as it is known to cross the BBB.
One of the key findings of this study was that resveratrol treatment improved brain TJ protein expression in cirrhotic mice. Endothelial BBB breach as a critical event in the pathogenesis of several cerebral disorders including HE. Recent evidence suggests that activated astrocytes induce BBB permeability by disrupting TJs [34]. Moreover, elevated cerebral and arterial ammonia may disrupt BBB integrity by downregulating TJ proteins [12]. In the current study, significantly decreased neuronal expression of ZO-1, claudin-5, and occludin were found in CCl4-induced cirrhosis, which were significantly improved by resveratrol treatment. These results suggest that resveratrol supplementation may improve BBB breach via alteration of TJ protein expression against CCl4-induced hyperammonemia (Fig. 10), with other recent studies already showing that the beneficial effect of resveratrol on BMECs of the BBB is related to changes to TJ protein expression [25].
Recently, in a bile-duct ligated (BDL) rat model of HE, they demonstrated decreased ZO-1 in areas of increased BBB permeability within the cortex, hippocampus, cerebellum, and striatum, associated with activated MMP-9 [35]. Furthermore, they showed decreased expressions of claudin-5 and occludin in all brain regions except the cerebellum [35]. Claudin-5 is predominantly expressed by CNS endothelial cells and acts as a main determinant of TJ properties [36, 37]. Claudin-5 knock-out mice die perinatally following selective BBB opening, whereas claudin-5 gene transfection improve barrier properties [38]. In a neoplastic human CNS disorder, claudin-5 disruption is linked to endothelial cell resistance and barrier breakdown [39]. The relative importance of occludin in the TJ complex is more complicated. Occludin is a component of TJ strands [40], but the strands are also evident in occludin-deficient cells [41] and occludin knock-out mice do not develop TJ abnormalities [42]. However, in models of acute liver failure (associated with hyperammonemia), expression of brain occludin and claudin-5 is significantly degraded [43].
Inflammation is central to the pathogenesis of many human neurological disorders including HE. Systemic inflammation is a key player in precipitating and exacerbating HE, possibly by rendering the brain more susceptible to concurrent hyperammonemia [44]. Activated microglia also release proinflammatory mediators (e.g., cytokines TNF-α, IL-6, and IL-1β) that have also been directly linked to brain dysfunction and altered BBB permeability [45, 46]. Furthermore, these proinflammatory cytokines are known to be elevated in the brain of experimental models of chronic hyperammonemia [47]. However, whether this neuroinflammation, potentially enacted through microglial activation, is initially triggered by systemic inflammation (or other distant mediators) and/or more “de novo” brain effects remains unclear. TNF-α in particular is thought to play an important role in BBB dysfunction [48, 49]. In this context, inhibition of TNF-α by injection of TNF-α antibodies has been shown to restore BBB integrity through increased occludin-1 expression [48]. Antibodies against IL-1β have also been reported to suppress the loss of BBB integrity in human BMECs [50]. In this study, systemically derived TNF-α and IL-1β concentrations were significantly downregulated following resveratrol treatment in CCl4-induced cirrhosis.
Our previous studies in acute liver failure (ALF) patients, and models of ALF and cirrhosis (e.g., BDL) that are characterized by hyperammonemia and HE, show significantly higher brain cytokines and inflammatory proteins such as iNOS and NF-κB [27, 51, 52]. Ammonia intoxication of animals increases the brain superoxide production and iNOS-driven nitric oxide (NO)–mediated protein tyrosine nitration that may further alter cerebral vascular hemodynamics. Thus, hyperammonemia and inflammation may synergistically play a role in disrupting TJ integrity and the neurological sequelae of cirrhosis. In this study, CCl4-induced cirrhotic mice brains show elevated iNOS and NF-κB protein expression that was significantly attenuated by resveratrol treatment. This is in line with previous observations in which resveratrol treatment influenced NF-κB-mediated inflammatory signaling, cyclooxygenase (COX) pathways, cellular response to stimuli, and immune responses to infection [53].
Oxidative stress not only induces cellular injury but critically regulates several fundamental cellular processes [54]. This includes BBB integrity, as oxidation of cytoskeleton protein modulates the TJ protein complex (e.g., ZO-1) [55]. Hyperammonemia induces oxidative stress with generation of reactive oxygen and nitrogen species (ROS and RNS) and plays a vital role in HE pathogenesis [56, 57]. Moreover, 4-hydroxy-2-nonenal (4HNE), a major product of lipid peroxidation, is highly toxic to cells and elevated in several brain disorders [58]. In this study, cellular 4HNE protein expression was found to be increased in the brains of our CCl4-induced cirrhotic mice and reversed by resveratrol treatment. This supports our previous report of significantly elevated 4HNE in hyperammonemic BDL rats with HE [27]. Pharmacological agents with potent suppressive effects on inflammation and oxidative stress appear to preserve BBB integrity and prevent neuroinflammation [59]. More specifically, in an environment of heightened oxidative stress, resveratrol has been shown to suppress endothelial ROS production [25, 26] through activation of MAPK pathways [60] while suppressing NADPH oxidase and NADPH activity in neuroinflammatory conditions [9].
In conclusion, the results of the present study suggest that in CCl4-induced cirrhotic mice, resveratrol treatment restores changes to tight junction protein expression, which may have an impact upon BBB permeability associated with advanced liver disease. Moreover, the beneficial effects of resveratrol treatment were enacted through its antioxidant and anti-inflammatory properties associated with reduction on ammonia levels. Therefore, this study supports the hypothesis that correcting hyperammonemia and inflammation with natural antioxidants that cross the BBB may provide a novel therapeutic intervention for the neurological sequelae of advanced liver disease.
References
Wijdicks EF (2016) Hepatic encephalopathy. N Engl J Med 375(17):1660–1670. https://doi.org/10.1056/NEJMra1600561
Weiss N, Jalan R, Thabut D (2018) Understanding hepatic encephalopathy. Intensive Care Med 44(2):231–234. https://doi.org/10.1007/s00134-017-4845-6
Fichet J, Mercier E, Genee O, Garot D, Legras A, Dequin PF, Perrotin D (2009) Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care 24(3):364–370. https://doi.org/10.1016/j.jcrc.2009.01.008
Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD (2010) Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 51(3):1062–1069. https://doi.org/10.1002/hep.23367
Sawhney R, Holland-Fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R (2016) Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl 22(6):732–742. https://doi.org/10.1002/lt.24443
Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB (2002) Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 36(5):1163–1171. https://doi.org/10.1053/jhep.2002.36497
Skowronska M, Albrecht J (2012) Alterations of blood brain barrier function in hyperammonemia: an overview. Neurotox Res 21(2):236–244. https://doi.org/10.1007/s12640-011-9269-4
Stamatovic SM, Keep RF, Andjelkovic AV (2008) Brain endothelial cell–cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol 6(3):179–192. https://doi.org/10.2174/157015908785777210
Wang D, Li SP, Fu JS, Zhang S, Bai L, Guo L (2016) Resveratrol defends blood–brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol 116(5):2173–2179. https://doi.org/10.1152/jn.00510.2016
Takechi R, Lam V, Brook E, Giles C, Fimognari N, Mooranian A, Al-Salami H, Coulson SH et al (2017) Blood–brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front Aging Neurosci 9:399. https://doi.org/10.3389/fnagi.2017.00399
Hadjihambi A, De Chiara F, Hosford PS, Habtetion A, Karagiannis A, Davies N, Gourine AV, Jalan R (2017) Ammonia mediates cortical hemichannel dysfunction in rodent models of chronic liver disease. Hepatology 65(4):1306–1318. https://doi.org/10.1002/hep.29031
Belanger M, Asashima T, Ohtsuki S, Yamaguchi H, Ito S, Terasaki T (2007) Hyperammonemia induces transport of taurine and creatine and suppresses claudin-12 gene expression in brain capillary endothelial cells in vitro. Neurochem Int 50(1):95–101. https://doi.org/10.1016/j.neuint.2006.07.005
Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR (2010) Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21(7):1200–1213. https://doi.org/10.1091/mbc.E09-08-0734
Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1994) Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127(6 Pt 1):1617–1626
Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D et al (2013) In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci 126(Pt 2):554–564. https://doi.org/10.1242/jcs.114306
Chow BW, Gu C (2015) The molecular constituents of the blood–brain barrier. Trends Neurosci 38(10):598–608. https://doi.org/10.1016/j.tins.2015.08.003
Rochfort KD, Cummins PM (2015) The blood–brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans 43(4):702–706. https://doi.org/10.1042/BST20140319
McLoughlin A, Rochfort KD, McDonnell CJ, Kerrigan SW, Cummins PM (2017) Staphylococcus aureus-mediated blood–brain barrier injury: an in vitro human brain microvascular endothelial cell model. Cell Microbiol 19 (3). doi:https://doi.org/10.1111/cmi.12664
Amararathna M, Johnston MR, Rupasinghe HP (2016) Plant polyphenols as chemopreventive agents for lung cancer. Int J Mol Sci 17(8):1352. https://doi.org/10.3390/ijms17081352
Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, Maciag A, Puca AA et al (2013) Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol 61:215–226. https://doi.org/10.1016/j.fct.2013.07.021
Hu J, Han H, Cao P, Yu W, Yang C, Gao Y, Yuan W (2017) Resveratrol improves neuron protection and functional recovery through enhancement of autophagy after spinal cord injury in mice. Am J Transl Res 9(10):4607–4616
Ling KH, Wan ML, El-Nezami H, Wang M (2016) Protective capacity of resveratrol, a natural polyphenolic compound, against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation. Chem Res Toxicol 29(5):823–833. https://doi.org/10.1021/acs.chemrestox.6b00001
Lee S, Yoon KD, Lee M, Cho Y, Choi G, Jang H, Kim B, Jung DH et al (2016) Identification of a resveratrol tetramer as a potent inhibitor of hepatitis C virus helicase. Br J Pharmacol 173(1):191–211. https://doi.org/10.1111/bph.13358
Kessoku T, Imajo K, Honda Y, Kato T, Ogawa Y, Tomeno W, Kato S, Mawatari H et al (2016) Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci Rep 6:22251. https://doi.org/10.1038/srep22251
Lin YL, Chang HC, Chen TL, Chang JH, Chiu WT, Lin JW, Chen RM (2010) Resveratrol protects against oxidized LDL-induced breakage of the blood–brain barrier by lessening disruption of tight junctions and apoptotic insults to mouse cerebrovascular endothelial cells. J Nutr 140(12):2187–2192. https://doi.org/10.3945/jn.110.123505
Hu M, Liu B (2016) Resveratrol attenuates lipopolysaccharide-induced dysfunction of blood–brain barrier in endothelial cells via AMPK activation. Korean J Physiol Pharmacol 20(4):325–332. https://doi.org/10.4196/kjpp.2016.20.4.325
Balasubramaniyan V, Wright G, Sharma V, Davies NA, Sharifi Y, Habtesion A, Mookerjee RP, Jalan R (2012) Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am J Physiol Gastrointest Liver Physiol 302(1):G145–G152. https://doi.org/10.1152/ajpgi.00097.2011
Wright G, Jalan R (2007) Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora's box? Hepatology 46(2):291–294. https://doi.org/10.1002/hep.21843
Perez Tamayo R (1983) Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology 3(1):112–120
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67(4):259–279
Bosoi CR, Rose CF (2009) Identifying the direct effects of ammonia on the brain. Metab Brain Dis 24(1):95–102. https://doi.org/10.1007/s11011-008-9112-7
Bobermin LD, Souza DO, Goncalves CA, Quincozes-Santos A (2017) Resveratrol prevents ammonia-induced mitochondrial dysfunction and cellular redox imbalance in C6 astroglial cells. Nutr Neurosci 21:1–10. https://doi.org/10.1080/1028415X.2017.1284375
Bobermin LD, Hansel G, Scherer EB, Wyse AT, Souza DO, Quincozes-Santos A, Goncalves CA (2015) Ammonia impairs glutamatergic communication in astroglial cells: protective role of resveratrol. Toxicol in Vitro 29(8):2022–2029. https://doi.org/10.1016/j.tiv.2015.08.008
Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN et al (2017) Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest 127(8):3136–3151. https://doi.org/10.1172/JCI91301
Dhanda S, Sandhir R (2017) Blood–brain barrier permeability is exacerbated in experimental model of hepatic encephalopathy via MMP-9 activation and downregulation of tight junction proteins. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0521-7
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc Natl Acad Sci U S A 106(6):1977–1982. https://doi.org/10.1073/pnas.0808698106
Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T (2007) Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol 210(1):81–86. https://doi.org/10.1002/jcp.20823
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. https://doi.org/10.1083/jcb.200302070
Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H (2000) Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol 100(3):323–331
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123(6 Pt 2):1777–1788
Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T et al (1998) Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141(2):397–408
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142
Chen F, Ohashi N, Li W, Eckman C, Nguyen JH (2009) Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 50(6):1914–1923. https://doi.org/10.1002/hep.23203
Aldridge DR, Tranah EJ, Shawcross DL (2015) Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol 5(Suppl 1):S7–S20. https://doi.org/10.1016/j.jceh.2014.06.004
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934. https://doi.org/10.1016/j.cell.2010.02.016
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45. https://doi.org/10.1186/1742-2094-5-45
Pozdeev VI, Lang E, Gorg B, Bidmon HJ, Shinde PV, Kircheis G, Herebian D, Pfeffer K et al (2017) TNFalpha induced up-regulation of Na(+),K(+),2Cl(−) cotransporter NKCC1 in hepatic ammonia clearance and cerebral ammonia toxicity. Sci Rep 7(1):7938. https://doi.org/10.1038/s41598-017-07640-8
Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P (2010) Tumour necrosis factor-alpha affects blood–brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int 30(8):1198–1210. https://doi.org/10.1111/j.1478-3231.2010.02211.x
Rochfort KD, Collins LE, McLoughlin A, Cummins PM (2016) Tumour necrosis factor-alpha-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J Neurochem 136(3):564–572. https://doi.org/10.1111/jnc.13408
Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A (2003) Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86(1):246–254
Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D et al (2007) Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45(6):1517–1526. https://doi.org/10.1002/hep.21599
Wright G, Shawcross D, Olde Damink SW, Jalan R (2007) Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis 22(3–4):375–388. https://doi.org/10.1007/s11011-007-9071-4
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 1:35. https://doi.org/10.1038/s41698-017-0038-6
Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46(5):531–542. https://doi.org/10.1016/j.freeradbiomed.2008.11.008
Gonzalez-Mariscal L, Quiros M, Diaz-Coranguez M (2011) ZO proteins and redox-dependent processes. Antioxid Redox Signal 15(5):1235–1253. https://doi.org/10.1089/ars.2011.3913
Kosenko E, Venediktova N, Kaminsky Y, Montoliu C, Felipo V (2003) Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res 981(1–2):193–200
Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS (2007) New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 22(3–4):219–234. https://doi.org/10.1007/s11011-007-9062-5
Schaur RJ (2003) Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Asp Med 24(4–5):149–159
Pallebage-Gamarallage M, Takechi R, Lam V, Elahy M, Mamo J (2016) Pharmacological modulation of dietary lipid-induced cerebral capillary dysfunction: considerations for reducing risk for Alzheimer's disease. Crit Rev Clin Lab Sci 53(3):166–183. https://doi.org/10.3109/10408363.2015.1115820
Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q, Tan J, Ma Q (2018) AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol-induced neurodegeneration by resveratrol. Mol Med Rep. https://doi.org/10.3892/mmr.2018.8482
Acknowledgements
This work was supported by the 5-year Ramalingaswami Re-entry Fellowship grant (102/IFD/SAN/22/2013-14) awarded to V.B. from the Department of Biotechnology (DBT), Government of India.
Author information
Authors and Affiliations
Contributions
V.B. designed the study; V.B. and M.S. conducted the study; V.B. and M.S. analyzed the data statistically; V.B. wrote and critically reviewed the manuscript; B.H.S. interpreted the histology and immunohistochemical findings.
Corresponding author
Ethics declarations
Conflict of Interest Statement
All the authors declare that there is no conflict of interest.
Language Certificate
The manuscript underwent proof read and plagiarism check prior submission to the Journal.
Institutional Review Board Statement
The study was reviewed and approved by the JIPMER Scientific Advisory Committee and Institutional Animal Ethics Committee.
Rights and permissions
About this article
Cite this article
Vairappan, B., Sundhar, M. & Srinivas, B.H. Resveratrol Restores Neuronal Tight Junction Proteins Through Correction of Ammonia and Inflammation in CCl4-Induced Cirrhotic Mice. Mol Neurobiol 56, 4718–4729 (2019). https://doi.org/10.1007/s12035-018-1389-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1389-x