Abstract
The stomach-derived hormone ghrelin mainly acts in the brain. Studies in mice have shown that the accessibility of ghrelin into the brain is limited and that it mainly takes place in some circumventricular organs, such as the median eminence. Notably, some known brain targets of ghrelin are distantly located from the circumventricular organs. Thus, we hypothesized that ghrelin could also access the brain via the blood-cerebrospinal fluid (CSF) barrier, which consists of the choroid plexus and the hypothalamic tanycytes. Using systemic injection of ghrelin or fluorescent-ghrelin in mice, we found that cells of the blood-CSF barrier internalize these molecules. In time-response studies, we found that peripherally injected fluorescent-ghrelin quickly reaches hypothalamic regions located in apposition to the median eminence and more slowly reaches the periventricular hypothalamic parenchyma, adjacent to the dorsal part of the third ventricle. Additionally, we found that CSF ghrelin levels increase after the systemic administration of ghrelin, and that central infusions of either an anti-ghrelin antibody, which immuno-neutralizes CSF ghrelin, or a scrambled version of ghrelin, which is also internalized by cells of the blood-CSF barrier, partially impair the orexigenic effect of peripherally injected ghrelin. Thus, current evidence suggests that the blood-CSF barrier can transport circulating ghrelin into the brain, and that the access of ghrelin into the CSF is required for its full orexigenic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ghrelin is a 28-residue octanoylated peptide hormone secreted from endocrine cells of the stomach [1, 2]. Ghrelin acts via the growth hormone secretagogue receptor (GHSR), a G protein-coupled receptor highly expressed in the brain [2, 3]. The main target of ghrelin signaling is the central nervous system, where ghrelin plays a variety of actions including the modulation of growth hormone secretion, blood glucose homeostasis, stress responses, and gastrointestinal tract motility, among others [2]. Notably, ghrelin signaling engages a network of neuronal circuitries that modulate feeding-linked behaviors, and it is recognized as the only mammalian peptide hormone able to increase appetite [2, 4]. Ghrelin orexigenic effects are rapid since food intake increases within 5–10 min after its systemic administration [5, 6]. Interestingly, several brain targets of ghrelin have been shown to elicit food intake when the hormone is locally administered [7, 8]. However, the blood-to-brain influx of mouse ghrelin into the mouse brain is very low and only some circumventricular organs, such as the median eminence (ME) and the area postrema (AP), seem to be able to sense acute changes of circulating ghrelin [9,10,11,12]. In this context, the hypothalamic arcuate nucleus (ARC) has emerged as the key target mediating the acute orexigenic effects of ghrelin. The ARC is not only enriched in GHSR-expressing neurons [13, 14] but is also located in close apposition to the ME, where the fenestrated capillaries allow ghrelin to quickly and passively diffuse towards neighboring brain areas [15]. In addition, the ventromedial ARC represents an exceptional case of privileged permeability to circulating molecules due to the presence of a fenestrated vasculature branching from the ME that would also allow circulating ghrelin to diffuse to the ARC [16]. In support of the key role of the ARC mediating the orexigenic effects of circulating ghrelin, we have shown that ARC-ablated mice fail to increase food intake in response to systemically administered ghrelin but show a full response to centrally administered ghrelin [10].
Besides the passive diffusion of ghrelin to the ARC/ME or the AP regions, plasma ghrelin could also reach some of its brain targets via the cerebrospinal fluid (CSF). In support of this possibility, it has been shown that CSF ghrelin levels in sheep are pulsatile and that around half of the CSF ghrelin peaks are detected after plasma ghrelin peaks [17]. Moreover, the systemic injection of ghrelin to sheep induces an increase of CSF ghrelin levels at around 40–50 min after treatment [17]. CSF ghrelin is able to, in turn, reach most of its GHSR-expressing brain areas, even those that are distant from the circumventricular organs [14]. In order to reach the CSF, ghrelin needs to be transported through the blood-CSF barrier, which consists of both the choroid plexus and the hypothalamic tanycytes [18,19,20]. The choroid plexus is a layer of cuboidal ependymal cells that surrounds a core of fenestrated capillaries in the ventricles of the brain and produces CSF. The hypothalamic tanycytes are a specialized layer of bipolar ependymal cells that line the floor of the third ventricle (3V) and bridge the CSF and the fenestrated capillaries of the ME. Recent evidence has shown that hypothalamic tanycytes are able to take up systemically injected ghrelin in mice, suggesting that this cell type may be important to control ghrelin signaling in the brain [21]. Here, we provide a variety of neuroanatomical and functional studies in order to gain some insights for the role of the blood-CSF barrier as a transporter of ghrelin into the brain.
Materials and Methods
Animals
Male adult (8–10 weeks old) C57BL/6 wild-type mice were housed in a 12-h light/dark cycle with regular chow and water available ad libitum. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and all efforts were made to minimize suffering. All experimentation received approval from the Institutional Animal Care and Use Committee of the Multidisciplinary Institute of Cell Biology (approval ID 10-0112).
Reagents
Ghrelin (GSS(octanoyl)FLSPEHQKAQQRKESKKPPAKLQPRSEQ) was purchased from Global Peptide (cat# PI-G-03). F-ghrelin (GSDpr(octanoyl) FLSPEHQRVQQRKESK(fluorescein) amide, 2741 Da) is a 19-amino acid analog of ghrelin conjugated to fluorescein isothiocyanate through the side-chain amine of the C-terminal lysine. Scrambled F-ghrelin, hereafter named Scr-F-ghrelin, consists of the same amino acid residues as F-ghrelin but in a different order (FRVESKESPQGRDpr(octanoyl)QHSQLK(fluorescein)). F-ghrelin and Scr-F-ghrelin were synthesized as previously detailed [22] in a purity of > 95% and characterized by MS (ESI+) as follows: F-ghrelin, calculated [M + 2H]2+ = 1372.17 [M + 3H]3+ = 915.11, found [M + 2H]2+ = 1372.88 [M + 3H]3+ = 915.81; Scr-F-ghrelin, calculated [M + 2H]2+ = 1372.17 [M + 3H]3+ = 915.11, found [M + 2H]2+ = 1371.65 [M + 3H]3+ = 914.40. Free sodium fluorescein was purchased from Poen (Argentina, 10% solution). The rest of the reagents were purchased from Biopack (Argentina), unless otherwise stated.
Central Administration
For central injections, mice were stereotaxically implanted with a single indwelling intracerebroventricular guide cannula (4 mm long, 22 gauge, Plastics One) into the lateral ventricle. The placement coordinates for the lateral ventricle were as follows: AP: − 0.34 mm; L: + 1 mm; and V: − 2.3 mm. A 28-gauge obturator was inserted into each cannula. After surgery, animals were individually housed and allowed to recover for at least 5 days. On the morning of the experimental day, animals were centrally injected with 2 μL of vehicle alone or containing 60 pmol/mouse of ghrelin, F-ghrelin, Scr-F-ghrelin, or free fluorescein. Here, vehicle was artificial CSF (hereafter named as aCSF), whose composition was 140 mM NaCl, 3.35 mM KCl, 1.15 mM MgCl2, 1.26 mM CaCl2, 1.2 mM Na2HPO4, and 0.3 mM NaH2PO4, pH 7.4 in sterile water. The dose of ghrelin chosen induces a maximal food intake response in our experimental conditions and, at the same time, represents the minimum amount of fluorescent tracer that allows the direct observation and quantification of fluorescent labeling (unpublished observations). All central injections were made over 2 min through a 30-gauge needle that extends 0.5 mm below the guide cannula and that was connected by polyethylene tubing to a Hamilton syringe. The needle was left in place for 2 min, following the injection, to prevent backflow of the injected solution. After 7 or 15 min, mice were anesthetized and perfused with formalin as previously described [23]. Brains were removed, post-fixed, immersed in 20% sucrose, and cut coronally at 40 μm into four equal series on a cryostat. The correct placement of the cannula was confirmed by histological observation at the end of the experiment. Each experimental group contained 3–6 mice.
Peripheral Administration
Individually housed mice received a subcutaneous injection of vehicle (saline solution 0.9%) alone or containing 300 pmol/g BW of ghrelin, F-ghrelin, Scr-F-ghrelin, or free fluorescein. This dose of ghrelin and fluorescent tracers is the minimal dose that allows their visualization in the brain after immunolabeling (unpublished observations). This dose of ghrelin induces a transient increase of plasma ghrelin levels, which is ~ 10-fold higher than basal levels at 30 min post-treatment and resembles concentrations observed in mice under energy deficit conditions [24]. After 7, 15, or 30 min, mice were anesthetized and perfused with formalin as previously described [23]. Each experimental group contained 4–6 mice.
Detection of Ghrelin and Fluorescent Analogs in Histological Samples
Brain sections of vehicle- and F-ghrelin-injected mice were used for direct visualization in a fluorescence microscope as well as for fluorescent and chromogenic immunostaining using an anti-fluorescein antibody. For fluorescent immunohistochemistry, brain sections were treated with blocking solution (3% normal donkey serum and 0.25% Triton X-100 in PBS) and incubated with a goat anti-fluorescein antibody (Life Technologies, cat# A11095, 1:1000) and a chicken anti-vimentin antibody (Millipore, cat# AB-5733, 1:2000) for 2 days at 4 °C. Then, sections were washed and incubated with a donkey anti-goat Alexa Fluor 488 antibody (Life Technologies, cat# A11055, 1:1000) and a goat anti-chicken Alexa Fluor 594 antibody (Abcam, cat# ab150176, 1:2000) for 2 h. For chromogenic immunohistochemistry, brain sections were pretreated with 0.5% H2O2, incubated with blocking solution, and then incubated with a goat anti-fluorescein antibody (1:7000) at 4 °C. After 2 days, sections were incubated with a biotinylated donkey anti-goat antibody (Vector Laboratories, cat# BA-5000, 1:2000) for 1 h and with Vectastain Elite ABC kit (Vector Laboratories, cat# PK-6200) for 1 h, according to manufacturer’s protocols. Then, visible signal was developed with 3-3′-diaminobenzidine (DAB)/nickel solution, giving a black/purple precipitate. Brain sections of fluorescein- and Scr-F-ghrelin-injected mice were used for direct visualization in a fluorescence microscope as well as for fluorescent immunostaining using an anti-fluorescein antibody, as described above. Brain sections of vehicle- and ghrelin-injected mice were used for fluorescent immunostaining using an anti-ghrelin antibody, as described above. Here, brain sections were incubated with goat or chicken anti-ghrelin antibodies (Santa Cruz, cat# sc-10368, 1:500 or GeneTex, cat# GTX-78202, 1:500, respectively) for 2 days at 4 °C, and then incubated with the anti-goat Alexa Fluor 488 or anti-chicken Alexa Fluor 594 antibodies, indicated above, depending on the primary antibody, for 2 h. Negative controls for immunostaining, which showed no immunolabeling, included elimination of either the primary or secondary antibody from the staining procedure. Sections were sequentially mounted on glass slides and coverslipped with mounting medium. Fluorescence microscopy images were acquired using a Zeiss AxioObserver D1 equipped with an Apotome.2 structured illumination module and an AxioCam 506 monochrome camera. Bright-field color images were acquired with a Nikon Eclipse 50i and a Nikon DS-Ri1 digital camera. All image processing and analysis were performed in Fiji [25].
Quantification of F-Ghrelin Diffusion Profiles
The diffusion of F-ghrelin into the periventricular parenchyma was quantified in mouse brain slices using direct fluorescence or immunohistochemistry, depending on the administration route. Slices from bregma − 1.58 to − 1.94 were included. For centrally injected F-ghrelin, brain slices were imaged using wide-field epifluorescence. Images were corrected for illumination inhomogeneities. Contribution of tissue autofluorescence to the green channel was estimated using the red channel and subtracted pixel-wise to achieve a zero mean reading on areas distant from the ventricle. The intensity profile of the corrected F-ghrelin fluorescence was measured perpendicular to the 3V along the length of a 500-μm box, averaging over its 50 μm height. This box was placed either 700 μm or 80 μm dorsal to the ME. Hoechst fluorescence was also recorded and used as a reference for alignment. For peripherally injected mice, immunochemical detection of F-ghrelin was used, as described above. DAB-stained slices were photographed under bright-field illumination. The resulting 16-bit images were flat-field corrected, and the pixel intensity values were converted into optical density data by taking the negative decimal logarithm of the original value divided by the flatfield value. Density profiles were acquired in the dorsal and ventral levels of the hypothalamus similarly as described above. The average optical density of a 25-μm region furthest from the 3V was subtracted to each profile, and the first zero-crossing point of the resulting curve nearest to the 3V was used for alignment. Fiji was used for all image processing and analysis steps. Subsequent analysis of all the aligned profiles was conducted using the average value of 20-μm-long contiguous blocks. D1/2 was estimated as the distance from the 3V where fluorescent or optical density reached 50% of its maximal level.
CSF Collection
Mice were anesthetized and placed in a stereotaxic frame with their head forming a nearly 135° angle with respect to the body. Afterwards, mice were peripherally injected with vehicle alone or containing 300 pmol/g BW of ghrelin. After 15 min, 6–10 μL of CSF was collected from the cisterna magna as described elsewhere [26]. Briefly, the surgical site was swabbed with 70% ethanol and an incision was made inferior to the occiput. Under a binocular lens, the subcutaneous tissue and muscles were separated by blunt dissection with forceps and a pair of microretractors was used to hold the muscles apart. A 100-μm-point capillary tube was inserted into the cisterna magna through the dura mater, allowing the CSF to flow into the capillary tube. Special care was taken to avoid any blood contamination. CSF samples were immediately frozen on dry ice for later storage at − 80 °C. The mice were euthanized by cervical dislocation at the end of the procedure.
Determination of CSF Ghrelin Levels Using Mass Spectrometry
Initially, a pool of 25 μL of CSF (n = 4, per experimental group) was desalted in an activated ZipTip-C18 and then eluted with 1 μL of acetonitrile. Then, samples were loaded with alpha-cyano-4-hydroxycinnamic acid (HCCA) matrix (10 mg/mL in acetonitrile:H2O:TFA (500:499:1) solution) onto GroundSteel 384 plate using dried droplet method. Profile analyses were performed on an Ultraflex MALDI TOF/TOF spectrometer with FlexControl 3.3 (Bruker Daltonics) data acquisition software. Mass spectra were acquired using the following settings: 600–4000 Da range, reflectron positive mode, ion source 1: 25 kV, ion source 2: 21.85 kV, lens: 9 kV, reflector voltage 1: 26.2 kV, reflector voltage 2: 13.6 kV, pulsed ion extraction of 40 ns, suppression up to 600 m/z. Ionization was achieved by irradiation with a solid-state 360-nm pulsed laser operating at 100 Hz (13% attenuator). All spectra were obtained randomly over the spot surface (900 shots fired per spot) manually. Mass calibration was performed externally using the Peptide Calibration Standard (Bruker Daltonics: angiotensin II, angiotensin I, substance P, bombesin, ACTH clip 1-17, ACTH clip 18-39, somatostatin 28). A standard curve was performed using solutions of ghrelin in aCSF. Data analysis was performed using FlexAnalysis software program (Bruker Daltonics).
Determination of CSF Ghrelin Levels Using ELISA
Ghrelin concentration was assayed in mouse CSF samples with a specific acylated ghrelin ELISA kit (A05117; Bertin Pharma). Here, a pool of 30 μL of CSF (n = 4, per experimental group) was measured. The limit of detection of the assay was 2.0 fmol/mL in the sample (6.7 pg/mL).
Food Intake Studies
Mice exposed to a pre-weighed amount of rodent’s food were centrally injected with 2 μL of aCSF alone or containing Scr-F-ghrelin (60 pmol/mouse) or chicken anti-ghrelin (0.1 pmol/mouse, GeneTex, cat# GTX-78202). After 15 min, mice were peripherally injected with vehicle alone or containing ghrelin (60 pmol/g BW). This peripheral dose of ghrelin induces a reliable increment in food intake that is not maximal; thus, we inferred that the behavioral response is not saturated and, consequently, more susceptible to be blocked. This dose of ghrelin induces a transient increase of plasma ghrelin levels, which is ~2-fold higher that basal levels at 30 min post-treatment and resembles concentrations observed in fasted mice [10]. The central dose of Scr-F-ghrelin was chosen based on its selectivity to exclusively affect cells of the blood-CSF barrier (see the “Results” section). The central dose of anti-ghrelin antibodies was chosen to be at least 100-fold higher than our estimations of the amount of ghrelin present in the CSF after the peripheral administration of the hormone (see the “Results” section). This central dose of anti-ghrelin antibodies does not increase c-Fos levels (unpublished observations) and is similar to the dose shown to be able to reduce food intake after a fasting event [27]. Food intake at 30, 60, and 120 min post ghrelin treatment was calculated by subtracting the weight of the remaining food at each time point to the weight of the initial food. Two hours after ghrelin treatment, mice were anesthetized and perfused as described previously. Here, 45 wild-type mice were used and grouped as follows: vehicle + vehicle (n = 8), vehicle + ghrelin (n = 10), Scr-F-ghrelin + vehicle (n = 8), Scr-F-ghrelin + ghrelin (n = 7), anti-ghrelin + vehicle (n = 5), anti-ghrelin + ghrelin (n = 7).
c-Fos Immunohistochemistry
As previously described, brains of perfused mice were removed, post-fixed, cryopreserved, and coronally cut at 40 μm into four equal series on a cryostat. Chromogenic immunostaining was performed exactly as described above but using a rabbit anti-c-Fos (Santa Cruz, cat# sc-7202, 1:2000) as primary antibody and a biotinylated goat anti-rabbit antibody (cat# BA-1000; 1:3000, Vector Laboratories) as a secondary antibody. To assess c-Fos induction following ghrelin treatment, blind quantitative analysis was performed. The number of c-Fos-immunoreactive (-IR) cells in the ventromedial ARC was determined by quantifying the cells containing a distinct nuclear black precipitate between bregma − 1.22 and − 2.46 mm, using the neuroanatomical references of the mouse brain atlas. Quantitative analysis was performed in each side of ventricle and expressed as total c-Fos-IR cells per side and per section.
Statistical Analyses
Data were expressed as mean ± SEM. The food intake data were analyzed by two-way repeated measures ANOVA (time × treatment) test, the delta food intake data, by unpaired t test, and the number of c-Fos-IR cells data, by one-way ANOVA test, for comparison of different mean values. For the ANOVA tests, Bonferroni test was used for multiple comparisons. Differences were considered statistically significant when p < 0.05. Analyses were performed using GraphPad Prism, version 6.0 (GraphPad Software).
Results
Centrally Injected F-Ghrelin Is Internalized by Cells of the Blood-CSF Barrier and Diffuses to the Brain Parenchyma
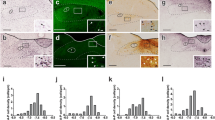
In order to test the ability of the blood-CSF barrier to display ghrelin uptake, we centrally injected F-ghrelin in mice and analyzed the cellular localization of the tracer in their brain at 15 min post-treatment. Since F-ghrelin fluorescence displays a low photostability, brain sections were immunostained using an anti-fluorescein antibody. In addition, brain sections were co-immunostained with an anti-vimentin antibody, which specifically labels hypothalamic tanycytes and some ependymal cells [28, 29]. As we previously showed [10], brains of mice centrally injected with vehicle lacked fluorescent signal while brains of mice centrally injected with F-ghrelin displayed a strong fluorescein-IR signal in neurons as well as other cell types that include cells located at the choroid plexus and at the epithelium of the 3V (Fig. 1). At the choroid plexus, fluorescein-IR signal was observed at the surface of most cuboidal ependymal cells and highly concentrated within some of them, which displayed a scattered patchy distribution within the epithelium (Fig. 1a, b). At the ependymal layer lining the 3V wall, fluorescein-IR signal was highly enriched in hypothalamic tanycytes and in some ependymal cells that were also vimentin-IR (Fig. 1c). Fluorescein-IR ependymal cells displayed a patchy distribution within the epithelium (Fig. 1d). Fluorescein-IR tanycytes were β-type tanycytes since they were located at the floor of the 3V, from where they sent projections to the external zone of the ME (Fig. 1e). Notably, few α-type tanycytes, which are located at the ventral walls of the 3V at the level of the ARC and the VMH, were fluorescein-IR.
Central administered F-ghrelin is internalized by cells of the blood-CSF barrier in mice. Microphotographs show the distribution pattern of the fluorescein-IR (green) and vimentin-IR (red) signals at the choroid plexus (a) or the 3V wall (c) of wild-type mice that were centrally injected with F-ghrelin and perfused 15 min later. b, d, and e show high-magnification images of the areas marked in the low-magnification images. In d and e, upper panels show fluorescein-IR signal, middle panels show vimentin-IR signal, and lower panels show the merge as well as the cell nuclei labeled with Hoechst. Arrowheads point to the cell body of a fluorescein-IR ependymal cell at the choroid plexus, while empty arrowheads point to cells in which the signal was observed only at their surface. Arrows point to the cell body of a fluorescein-IR cell at the 3V wall, and asterisks indicate tanycyte processes. Scale bars, 100 μm (low magnification), 10 μm (high magnification)
Interestingly, brain sections of F-ghrelin-injected mice showed a diffuse fluorescent signal in the periventricular hypothalamic tissue, in close apposition to the ventricular wall, which was not seen in control animals (Fig. 2a–f). Such F-ghrelin signal became more evident using a chromogenic immunostaining against fluorescein, which enhances the IR signal due to the inclusion of enzymatic amplification steps, as seen at either bright-field or pseudo-colored optical density images. In order to estimate the diffusion of F-ghrelin into the brain tissue, we quantified direct fluorescence intensity in the medial-lateral axis of the coronal sections of mice that were injected with F-ghrelin and perfused 7 and 15 min later. Importantly, ependymal cells that line the dorsal part of the 3V lack tight junctions [28] and, as a consequence, molecules present in the CSF diffuse throughout the monolayer to the brain parenchyma; in contrast, the β-type tanycytes that line the ventral part of the 3V possess tight junctions at the apical surface and act as a physical barrier that prevents the diffusion of molecules from the CSF to the ARC. Thus, quantitative analysis of fluorescent signal was performed at two different dorsoventral levels of the hypothalamus for both time points (Fig. 2g−j). Total fluorescence signal was significantly higher at the dorsal, as compared to the ventral, level of the hypothalamus at both time points. In the medial-lateral axis, fluorescence signal profile displayed a rapid decay at the dorsal level (D1/2 52.2 ± 7.3 and 33.3 ± 4.6 μm for 7 and 15 min, respectively) as compared to the ventral level (D1/2 84.6 ± 15.1 and 57.8 ± 6.2 μm for 7 and 15 min, respectively). At the dorsal level, total fluorescence signal showed ~ 50% decrease at 15 min post-treatment, as compared to 7 min post-treatment. At the ventral level, total fluorescence signal did not differ between both time points post-treatment. Notably, chromogenic immunostaining against fluorescein revealed the presence of fluorescein-IR cells in regions distant from the ventricle wall where no significant fluorescence intensity signal was detected (Fig. 2d–f).
Central administered F-ghrelin diffuses to the brain parenchyma. Microphotographs show the periventricular hypothalamic tissue of mice centrally injected with vehicle (a–c) or F-ghrelin (d–f). a, d Direct fluorescent signal. b, e Chromogenic immunostaining against fluorescein. c, f Pseudo-colored images. Scale bar, 100 μm. Bar graphs representing quantification of direct fluorescent signal in dorsal (g, h) and ventral (i, j) levels, in F-ghrelin treated mice that were perfused 7 min (g, i) or 15 min (h, j) after F-ghrelin administration. Data represent the mean ± SEM. *< 0.05 vs vehicle groups
Afterwards, we tested the selectivity of the blood-CSF barrier to internalize F-ghrelin present in the CSF. For this purpose, we analyzed brain sections of mice centrally injected with either full-length ghrelin, free fluorescein, or Scr-F-ghrelin (Fig. 3). Brain sections of mice injected with vehicle or ghrelin were immunostained using an anti-ghrelin antibody. Here, ghrelin-IR signal was detected in choroid plexus and β-type tanycytes in both groups of mice; however, signal intensity was significantly higher in ghrelin-treated mice, as compared to that observed in vehicle-treated mice, in which detected signal is presumably due to endogenous ghrelin (Fig. 3a). Brain sections of mice injected with fluorescein or Scr-F-ghrelin were immunostained using an anti-fluorescein antibody, as described above. In this case, brains of fluorescein-injected mice lacked fluorescein-IR cells while brains of Scr-F-ghrelin-injected mice displayed fluorescein-IR signal exclusively in cells of the choroid plexus and in β-type tanycytes (Fig. 3b). Notably, Scr-F-ghrelin did not label any cell type in the brain parenchyma suggesting it does not bind to GHSR. Thus, CSF F-ghrelin uptake by the blood-CSF barrier is independent of the fluorescein moiety as well as of the specific order of residues within the sequence of ghrelin.
Selectivity of the blood-CSF barrier to internalize F-ghrelin present in the CSF. Immunofluorescence staining for ghrelin (a) or fluorescein (b), showing the pattern of ghrelin-IR or fluorescein-IR signal at the choroid plexus and the ME of mice centrally injected with vehicle, ghrelin, free fluorescein, or Scr-F-ghrelin. Arrows indicate a positive cell and asterisks a positive process. Scale bar, 10 μm
Peripherally Injected F-Ghrelin Is Detected in Cells of the Blood-CSF Barrier and Reaches the CSF As Well As the Periventricular Regions
In order to test the ability of the blood-CSF barrier to display circulating ghrelin uptake, mice were peripherally injected with F-ghrelin and perfused 15 min later. Fluorescent immunostaining using anti-fluorescein and anti-vimentin antibodies showed that F-ghrelin labeling was evident only at high magnification (Fig. 4). Fluorescein-IR signal was observed with a granular pattern within some cuboidal ependymal cells of the choroid plexus (Fig. 4a, b). In addition, fluorescein-IR signal was detected in a few ependymal cells and in some β-type tanycytes, which were also labeled by anti-vimentin antibodies (Fig. 4d, e). At the intracellular level, fluorescein-IR signal displayed a granular pattern that was enriched at the apical side of both cell types (Fig. 4d, e).
Peripherally administered F-ghrelin is detected in the cells of the blood-CSF barrier in mice. Microphotographs show the fluorescein-IR (green) and vimentin-IR (red) signals at the choroid plexus (a) or the 3V wall (c) of wild-type mice that were peripherally injected with F-ghrelin and perfused 15 min later. b, d, and e show high-magnification images of the areas marked in the low-magnification images. In d and e, upper panels show fluorescein-IR signal, middle panels show vimentin-IR signal, and lower panels show the merge and the cell nuclei labeled with Hoechst. Arrowheads and arrows point to fluorescein-IR signal at the choroid plexus or at the 3V, respectively. Scale bars, 100 μm (low magnification), 10 μm (high magnification)
In order to estimate the passage of circulating F-ghrelin to the brain parenchyma, mice were peripherally injected with F-ghrelin and perfused 7, 15, and 30 min later. Here, brain sections of F-ghrelin-injected mice showed similar fluorescent signal intensity as seen in vehicle-injected mice (Fig. 5a–f). Thus, brain sections were subjected to a chromogenic immunostaining against fluorescein and a quantitative analysis of the fluorescein-IR signal was performed at two different dorsoventral levels of the hypothalamus, as described above. Notably, optical density in brain sections of mice peripherally injected with F-ghrelin was smaller as compared to the values estimated in brain sections of mice centrally injected with the tracer. At the dorsal level, no fluorescein-IR signal was detected at 7 min post F-ghrelin treatment while fluorescein-IR signal was detected in the area located in close apposition to the 3V (from 0 to 100 μm from the ventricle wall) at 15 and 30 min post F-ghrelin treatment (Fig. 5g–i). At the ventral level, fluorescein-IR signal was detected in the analyzed area at all three time points after F-ghrelin treatment (Fig. 5j–l).
Peripherally administered F-ghrelin diffuses to the brain parenchyma. Microphotographs show the periventricular hypothalamic tissue of mice peripherally injected with vehicle (a–c) or F-ghrelin (d–f). a, d Direct fluorescent signal. b, e Chromogenic immunostaining against fluorescein. c, f Pseudo-colored images. Scale bar, 100 μm. Bar graphs representing quantification of optical density signal in dorsal (g, h, i) and ventral (j, k, l) levels, in F-ghrelin-treated mice that were perfused 7 min (g, j), 15 min (h, k), or 30 min (i, l) after F-ghrelin administration. Data represent the mean ± SEM. *< 0.05 vs vehicle groups
In order to test if indeed circulating ghrelin is able to access the CSF, we collected CSF samples in mice peripherally injected with ghrelin and investigated its presence using either mass spectrometry or ELISA. No ghrelin was detected in the CSF of vehicle- or ghrelin-injected mice using mass spectrometry, suggesting that the amount of ghrelin in both conditions is less than 60 fmol/mL (200 pg/mL), which was the lowest sensitivity of the mass spectrometer. Similarly, CSF ghrelin was undetectable in vehicle-injected mice using ELISA, indicating that ghrelin concentration in ad libitum fed mice is less than 2 fmol/mL (6.7 pg/mL), which is the lowest detectable level in a sample given the sensitivity of the assay. In contrast, 19.2 fmol/mL (63 pg/mL) of ghrelin was detected in the CSF of mice peripherally injected with this hormone.
To test the selectivity of the blood-CSF barrier to internalize circulating ghrelin, we studied brain sections of mice peripherally injected with full-length ghrelin, fluorescein, or Scr-F-ghrelin (Fig. 6). Similarly as described above, brain sections of mice injected with vehicle or ghrelin were immunostained using an anti-ghrelin antibody. Here, ghrelin-IR signal was detected in both the choroid plexus and β-type tanycytes (Fig. 6a). As also described above, brain sections of mice injected with fluorescein or Scr-F-ghrelin were immunostained using an anti-fluorescein antibody. In this case, brains of both groups of mice lacked fluorescein-IR cells in the choroid plexus or the ME (Fig. 3b). Thus, the blood-CSF barrier selectively internalizes circulating ghrelin.
Selectivity of the blood-CSF barrier to internalize circulating F-ghrelin. Immunofluorescence staining for ghrelin (a) or fluorescein (b), showing the pattern of ghrelin-IR or fluorescein-IR signal at the choroid plexus and the ME of mice peripherally injected with vehicle, ghrelin, free fluorescein, or Scr-F-ghrelin. Arrows indicate positive signal. Scale bar, 10 μm
The Orexigenic Effect of Peripherally Injected Ghrelin Is Impaired by Central Infusion of Scr-F-Ghrelin or Anti-ghrelin Antibodies
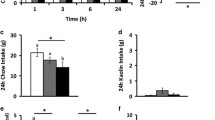
In order to test if the passage of circulating ghrelin through the blood-CSF barrier is required for the orexigenic actions of the hormone, we used two strategies. In one case, we hypothesized that the Scr-F-ghrelin uptake could impair the ability of the cells of the blood-CSF barrier to transport peripherally injected ghrelin into the brain. In the other case, we examined if immuno-neutralization of ghrelin present in the CSF would influence the effect of peripherally injected ghrelin. Thus, we assessed food intake in mice that were first centrally injected with either vehicle, Scr-F-ghrelin, or anti-ghrelin antibodies, and then peripherally injected with vehicle or ghrelin. Importantly, centrally injected Scr-F-ghrelin was found exclusively in cells of the blood-CSF barrier while centrally injected anti-ghrelin antibodies were not found in any type of ependymal cell and did not diffuse into the hypothalamic tissue (not shown). In the absence of ghrelin treatment, food intake was similar in mice centrally injected with vehicle, Scr-F-ghrelin, or anti-ghrelin antibodies (Fig. 7a). In mice centrally injected with vehicle, ghrelin treatment increased food intake mainly within the initial 30 min post-treatment, but also in the 30-to-120-min interval. In mice centrally injected with Scr-F-ghrelin, ghrelin treatment increased food intake 30 min post-treatment; however, food intake was smaller at this time point than ghrelin-induced food intake of mice centrally treated with vehicle, and did not further increase in the 30-to-120-min interval. In mice centrally injected with anti-ghrelin antibodies, ghrelin treatment increased food intake 30 min after treatment, similarly as seen in mice centrally injected with vehicle, and then did not affect it in the 30-to-120-min interval (Fig. 7a, b). Since peripherally injected ghrelin mainly increases food intake by acting in the ARC, c-Fos-positive neurons were quantified in this brain region. In the absence of ghrelin treatment, c-Fos levels in the ARC were similar in mice centrally injected with either vehicle, Scr-F-ghrelin, or anti-ghrelin antibodies (not shown). As compared to mice peripherally injected with vehicle, ghrelin treatment increased the number of c-Fos-IR cells in the ARC of mice centrally injected with vehicle. Notably, ghrelin-induced increase in the number of c-Fos-IR cells in the ARC was smaller in mice centrally injected with Scr-F-ghrelin as compared to the numbers found in ghrelin-treated mice centrally injected with either vehicle or anti-ghrelin antibodies (Fig. 7c, d).
The orexigenic effect of peripherally injected ghrelin is partially impaired by central infusion of Scr-F-ghrelin or anti-ghrelin antibodies. Mice were first centrally injected with vehicle, Scr-F-ghrelin, or anti-ghrelin antibody, and 15 min later peripherally injected with ghrelin. a Showing 30, 60, or 120 min food intake. b Showing delta food intake between 30 and 120 min. c Showing representative images of c-Fos immunostaining in the arcuate nucleus (ARC). Scale bar, 100 μm. d Bar graphs show quantitative analysis of the number of c-Fos-IR cells in the ventromedial ARC. Data represent the mean ± SEM. **< 0.01 vs vehicle/vehicle, (a) < 0.05 and (b) < 0.01 vs vehicle/ghrelin group
Discussion
The main target of plasma ghrelin is the brain. Thus, gaining insights about the mechanisms mediating the brain action of ghrelin is essential for the understanding of the physiological role of this hormone. Here, we used F-ghrelin to visualize the brain areas accessible to ghrelin. F-ghrelin allowed us not only to estimate the amount of tracer in the brain by directly quantifying fluorescence but also to perform double-labeling studies in order to characterize the cell types that internalize it. In addition, the immunodetection of F-ghrelin using anti-fluorescein antibodies allowed to specifically amplify the signal, thus increasing the sensitivity of the assays independently of interferences due to the presence of endogenous ghrelin. Performing current experiments using radioactive ghrelin would have been more challenging due not only to cost and safety issues but also because the methods used to visualize the radioactive signal lack adequate spatial resolution. Still, the fact that F-ghrelin possesses structural modifications, as compared to native ghrelin, should be kept in mind when interpreting our observations. F-ghrelin contains the initial 19 residues of the ghrelin sequence with a Dpr-octanoyl replacing the Ser-octanoyl at position 3 and is conjugated to fluorescein at the side-chain amine of the C-terminal Lys residue. F-ghrelin is bioactive presumably because the N-terminal end of the molecule, which interact to GHSR, is conserved; however, the implications of the lack of the C-terminal end are uncertain [14]. Importantly, F-ghrelin is more stable than ghrelin because ghrelin is linked to the octanoyl moiety through an ester bond, while in F-ghrelin, this link takes place through an amide bond, rendering the molecule resistant to esterases [22]. It is also important to stress that F-ghrelin was administered as a single bolus that causes a transient supraphysiological peak of the tracer. We predict that these experimental features, inherent to our methodological approach, did not affect the overall mechanism of ghrelin access into the brain but, in any case, affect some kinetic aspects of the process.
Here, we provide evidence indicating that plasma and CSF ghrelin interacts with the mouse blood-CSF barrier. In particular, we report that centrally injected ghrelin, F-ghrelin, or Scr-F-ghrelin were detected in a subset of ependymal cells of the choroid plexus and in β-type tanycytes. Thus, these molecules were internalized from the CSF independently of its amino acid sequence or the presence of synthetic moieties. In addition, we have already shown that centrally injected des-acyl-ghrelin is also internalized by β-type tanycytes [30]. In contrast, we found that peripherally injected Scr-F-ghrelin or fluorescein were not detected in cells of the blood-CSF barrier while peripherally injected ghrelin or F-ghrelin were similarly internalized by cells of the blood-CSF barrier. Thus, the integrity of the N-terminal sequence of ghrelin seems to be key for the interaction of the hormone with cells of the blood-CSF barrier. These observations suggest that the blood-CSF barrier acts as a low-selectivity scavenger of molecules present in the CSF while it displays a selective uptake of plasma ghrelin. Notably, ghrelin is predominantly synthesized by enteroendocrine cells of the stomach and acts in the brain, where it is not synthesized [31, 32]. Thus, blood-CSF barrier would mainly interact with plasma ghrelin under normal circumstances.
Analysis of F-ghrelin signal in the periventricular hypothalamic tissue of mice centrally injected with the tracer confirmed that the CSF-brain parenchyma interface at the dorsal and ventral levels of the 3V displays different features. In the dorsal periventricular tissue, F-ghrelin signal was higher at 7 min post-treatment, as compared to 15 min post-treatment, and displayed an exponential-like lateral decay at both time points. Such observations strongly suggest that CSF F-ghrelin freely diffuses across ependymal cells towards the periventricular tissue and agrees with the well-established fact that the ependymal cells that line this part of the 3V lack tight junctions. Quantitative analysis of F-ghrelin signal showed that most centrally injected F-ghrelin penetrated no more than ~ 400 μm deep into the brain from the ventricle wall and that only a small fraction reached deeper areas as suggested by the detection of fluorescein-IR cells in regions distant from the 3V. Thus, we confirm that CSF ghrelin can reach most GHSR-expressing cells, even those targets distant from the ventricles [14], although it seems to mainly diffuse to periventricular areas. Still, we cannot rule out that the high concentration of the transient peak of F-ghrelin in the CSF, as compared to physiological CSF ghrelin levels, may have overwhelmed the normal mechanisms controlling this process. In the ventral periventricular tissue, lower levels of F-ghrelin signal were detected at both time points, as compared to the dorsal periventricular tissue. The mechanism by which CSF ghrelin accesses this part of the hypothalamus, which includes the ARC, remains to be elucidated. However, current evidence suggests that the passive diffusion of CSF ghrelin into the ventral hypothalamic tissue is restricted supporting the notion that the tight junctions connecting the apical surface of β-type tanycytes prevent the free diffusion of molecules from the CSF to the ARC.
Analysis of F-ghrelin signal in the periventricular hypothalamic tissue of mice peripherally injected with the tracer suggests that circulating ghrelin has differential access to the dorsal and ventral regions of the hypothalamus. Here, F-ghrelin signal was detected at 7, 15, and 30 min post-treatment in the ventral hypothalamus, which includes the ARC. Notably, this F-ghrelin signal did not display any obvious pattern of diffusion and, remarkably, was not detected near to the 3V. We and others have already shown that systemically injected fluorescent ghrelin extravasates through fenestrated capillaries of the ME and reaches nearby brain regions including the ARC, which also possess some fenestrated capillaries [10, 15, 16]. Thus, current data confirm that circulating ghrelin can directly diffuse from the bloodstream to the ARC. In the dorsal periventricular tissue, F-ghrelin was absent at 7 min post-treatment but it was detected after 15 and 30 min post-treatment, when it was found in the tissue nearby the 3V. The gradient of F-ghrelin signal, enriched near the ventricular wall, suggests that the tracer is diffusing from the CSF into the parenchyma. In line with this possibility, we found that CSF ghrelin levels were increased 15 min after the peripheral administration of the hormone. Thus, current data suggest that a fraction of circulating ghrelin gains access to the CSF and then, from there, to the periventricular tissue.
Finally, we tested if the passage of ghrelin through the blood-CSF barrier is required for the orexigenic effects of the hormone. Notably, food intake induced by systemically injected ghrelin displays a sharp increase in the initial 30 min post-treatment and then a further moderate increase in the 30-to-120-min post-treatment interval that represents ~ 20% of the total ghrelin-induced food intake [33]. Since we found that centrally injected Scr-F-ghrelin was exclusively accumulated in cells of the blood-CSF barrier, we hypothesized that internalized Scr-F-ghrelin would impair the transport properties of these cells. We found that central injection of Scr-F-ghrelin blocked the orexigenic effect of peripherally injected ghrelin in both time intervals. A previous study successfully used central administration of anti-leptin antibodies in order to show that the blood-to-CSF transport of leptin is required for the hypothalamic actions of this hormone [34]. Thus, we tested if centrally injected anti-ghrelin antibodies can affect ghrelin-induced food intake. We found that immuno-neutralization of CSF ghrelin impaired the orexigenic effect of ghrelin at the 30-to-120-min post-treatment interval. Ghrelin-induced c-Fos in the ARC was only significantly reduced in mice pretreated with Scr-F-ghrelin suggesting that this marker of neuronal activation is a better indicator of the early effects of ghrelin. Thus, it seems reasonable to hypothesize that the initial orexigenic effect of peripherally injected ghrelin mainly depends on its direct access to the ARC through the fenestrated capillaries, while the subsequent orexigenic effect requires its passage through the CSF. The identification of the brain targets mediating this slower orexigenic effect of peripherally injected ghrelin requires further studies. Our previous observation that ARC-ablated mice do not increase food intake in response to peripherally injected ghrelin suggests that sub-regions of the ARC that are more distant from the fenestrated capillaries but near the ventricle, such as the dorsomedial ARC, could mediate these effects [10].
CSF ghrelin levels are lower than plasma ghrelin levels in ad libitum fed conditions. CSF and plasma ghrelin levels were reported to be ~ 4 and ~ 200 pg/mL, respectively, in cows [35] and ~ 24 and ~ 30,000 pg/mL, respectively, in sheep [17]. In healthy individuals, desacyl ghrelin plus ghrelin levels in CSF and plasma were reported to be ~ 400 and ~ 2800 pg/mL or ~ 4 and ~ 260 pg/mL, respectively, in two independent studies [36, 37]. The different CSF and plasma ghrelin levels strongly suggest that the hormone does not freely diffuses in a blood-to-CSF direction. An elegant study in sheep showed that CSF ghrelin levels were increased after endogenous or exogenously induced elevations in plasma ghrelin levels, suggesting that ghrelin can be transported in a blood-to-CSF direction [17]. Investigating the transport of ghrelin through the CSF-blood barrier in small animals, such as mice, is challenging due to the difficulties in obtaining the amount of CSF required for ghrelin assessment. Indeed, we did not find any previous study reporting CSF ghrelin levels in mice. Here, we found that CSF ghrelin levels were less than 6.7 pg/mL in ad libitum fed mice, in which plasma ghrelin levels are ~ 250 pg/mL [10]. CSF ghrelin was detected in all previously investigated species using standard strategies; however, the assessment of CSF ghrelin in mice seems to require the development of ultrasensitive systems assaying a small volume of sample. Importantly, CSF ghrelin becomes detectable after peripheral administration of ghrelin suggesting that plasma ghrelin can gain access to the CSF. The dose of ghrelin used in the current study induces plasma level increases that transitorily resembles plasma ghrelin levels observed in mice upon a severe energy deficit condition [10, 38]. Since caloric restriction induces a sustained increment of plasma ghrelin levels, it can be hypothesized that CSF ghrelin levels would be also increased under energy deficit conditions.
Plasma ghrelin has been proposed to gain access to specific brain areas by crossing the blood-brain barrier or diffusing through the fenestrated capillaries of certain CVOs [9,10,11,12, 15, 39, 40]. The current study describes another potential mechanism by which ghrelin could access the brain. A seminal study by Banks and colleagues found that different radioactive ghrelin-related tracers differed in their degree of access into the brain and transport mechanism [9]. The referred study showed that mouse ghrelin displays a low penetration into the mouse brain and rather it is mainly transported in a brain-to-blood direction via a saturable mechanism [9]. Notably, the methodology used in that study to assess the passage of ghrelin to the brain does not actually provide neuroanatomical insights of the process, nor does it discriminate blood-brain barrier proper from other possible routes, such as the blood-CSF barrier. As referred to above, later studies using fluorescently labeled ghrelin showed that systemically injected ghrelin accesses the ME, the ARC, and the AP [10,11,12, 15]. A restricted accessibility of plasma ghrelin into the brain is suggested by the observation that the local administration of ghrelin into a variety of brain nuclei potently induces feeding; however, ARC ablation is sufficient to abrogate the rapid increase of food intake induced by systemically injected ghrelin [10]. Here, we show that peripherally injected ghrelin (1) is found in cells of the blood-CSF barrier, (2) is detected in the CSF, and (3) quickly reaches the hypothalamic tissue nearby the ME and more slowly reaches the periventricular hypothalamic parenchyma adjacent to the dorsal part of the third ventricle. We also show that maneuvers aimed at restricting the access to or the action of CSF ghrelin blocked the slower effect of ghrelin on food intake. Overall, it seems reasonable to interpret that plasma ghrelin can be selectively internalized by cells of the blood-CSF barrier, transported to the CSF, from where it could diffuse to the periventricular tissue in order to mediate some effects. Interestingly, CSF ghrelin can reach GHSR-expressing neurons that would not be reached by plasma ghrelin in normal conditions [14]. Thus, it seems that plasma ghrelin quickly has access to specific areas of the brain in order to mediate rapid effects, while CSF ghrelin could recruit a different set of targets that are involved in slower or long-term effects of the hormone.
References
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660. https://doi.org/10.1038/45230
Yanagi S, Sato T, Kangawa K, Nakazato M (2018) The homeostatic force of ghrelin. Cell Metab 27:786–804. https://doi.org/10.1016/j.cmet.2018.02.008
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL et al (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977
Perello M, Dickson SL (2015) Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 27:424–434. https://doi.org/10.1111/jne.12236
Cummings DE (2006) Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89:71–84. https://doi.org/10.1016/j.physbeh.2006.05.022
Kuo Y-T, Parkinson JRC, Chaudhri OB, Herlihy AH, So PW, Dhillo WS, Small CJ, Bloom SR et al (2007) The temporal sequence of gut peptide–CNS interactions tracked in vivo by magnetic resonance imaging. J Neurosci 27:12341–12348. https://doi.org/10.1523/JNEUROSCI.2391-07.2007
Mason BL, Wang Q, Zigman JM (2014) The central nervous system sites mediating the orexigenic actions of ghrelin. Annu Rev Physiol 76:519–533. https://doi.org/10.1146/annurev-physiol-021113-170310
Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL et al (2014) Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab 3:64–72. https://doi.org/10.1016/j.molmet.2013.10.001
Banks WA (2002) Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302:822–827. https://doi.org/10.1124/jpet.102.034827
Cabral A, Valdivia S, Fernandez G, Reynaldo M, Perello M (2014) Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility. J Neuroendocrinol 26:542–554. https://doi.org/10.1111/jne.12168
Cabral A, De Francesco PN, Perello M (2015) Brain circuits mediating the orexigenic action of peripheral ghrelin: narrow gates for a vast kingdom. Front Endocrinol (Lausanne) 6:44 . doi: https://doi.org/10.3389/fendo.2015.00044
Cabral A, Cornejo MP, Fernandez G, de Francesco PN, Garcia-Romero G, Uriarte M, Zigman JM, Portiansky E et al (2017) Circulating ghrelin acts on GABA neurons of the area postrema and mediates gastric emptying in male mice. Endocrinology 158:1436–1449. https://doi.org/10.1210/en.2016-1815
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548. https://doi.org/10.1002/cne.20823
Cabral A, Fernandez G, Perello M (2013) Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience 253:406–415. https://doi.org/10.1016/j.neuroscience.2013.09.008
Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdié P et al (2013) Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci 110:1512–1517. https://doi.org/10.1073/pnas.1212137110
Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prévot V, Levine JE (2009) Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology 150:5509–5519. https://doi.org/10.1210/en.2009-0584
Grouselle D, Chaillou E, Caraty A, Bluet-Pajot MT, Zizzari P, Tillet Y, Epelbaum J (2008) Pulsatile cerebrospinal fluid and plasma ghrelin in relation to growth hormone secretion and food intake in the sheep. J Neuroendocrinol 20:1138–1146. https://doi.org/10.1111/j.1365-2826.2008.01770.x
Bolborea M, Dale N (2013) Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 36:91–100. https://doi.org/10.1016/j.tins.2012.12.008
Rodríguez EM, Blázquez JL, Pastor FE et al (2005) Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164. https://doi.org/10.1016/S0074-7696(05)47003-5
Redzic ZB, Preston JE, Duncan JA et al (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52. https://doi.org/10.1016/S0070-2153(05)71001-2
Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V, Bouret SG (2015) Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab 4:15–24. https://doi.org/10.1016/j.molmet.2014.10.003
McGirr R, McFarland MS, McTavish J et al (2011) Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul Pept 172:69–76. https://doi.org/10.1016/j.regpep.2011.08.011
Cabral A, Suescun O, Zigman JM, Perello M (2012) Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS One 7:e31462. https://doi.org/10.1371/journal.pone.0031462
Chuang J-C, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM (2011) Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121:2684–2692. https://doi.org/10.1172/JCI57660
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Liu L, Duff K (2008) A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. JoVE (Journal of Visualized Experiments) e960–e960 . doi: https://doi.org/10.3791/960
Nakazato M, Murakami N, Date Y, et al (2001) A role for ghrelin in the central regulation of feeding. 409:5
Mullier A, Bouret SG, Prevot V, Dehouck B (2010) Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 518:943–962. https://doi.org/10.1002/cne.22273
Sánchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli JL, Lechan RM (2009) Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology 150:2283–2291. https://doi.org/10.1210/en.2008-1643
Fernandez G, Cabral A, Cornejo MP, de Francesco PN, Garcia-Romero G, Reynaldo M, Perello M (2016) Des-acyl ghrelin directly targets the arcuate nucleus in a ghrelin-receptor independent manner and impairs the orexigenic effect of ghrelin. J Neuroendocrinol 28:12349. https://doi.org/10.1111/jne.12349
Kojima M, Kangawa K (2010) Ghrelin: from gene to physiological function. Results Probl Cell Differ 50:185–205. https://doi.org/10.1007/400_2009_28
Cabral A, López Soto EJ, Epelbaum J, Perelló M (2017) Is ghrelin synthesized in the central nervous system? Int J Mol Sci 18. https://doi.org/10.3390/ijms18030638
McFarlane MR, Brown MS, Goldstein JL, Zhao T-J (2014) Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab 20:54–60. https://doi.org/10.1016/j.cmet.2014.04.007
Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A et al (2014) Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19:293–301. https://doi.org/10.1016/j.cmet.2013.12.015
Laeger T, Sauerwein H, Tuchscherer A, Bellmann O, Metges CC, Kuhla B (2013) Concentrations of hormones and metabolites in cerebrospinal fluid and plasma of dairy cows during the periparturient period. J Dairy Sci 96:2883–2893. https://doi.org/10.3168/jds.2012-5909
Popovic V, Svetel M, Djurovic M, Petrovic S, Doknic M, Pekic S, Miljic D, Milic N et al (2004) Circulating and cerebrospinal fluid ghrelin and leptin: potential role in altered body weight in Huntington’s disease. Eur J Endocrinol 151:451–455
Unger MM, Oertel WH, Tackenberg B (2013) Cerebrospinal fluid concentrations of ghrelin in patients with multiple sclerosis. Neuro Endocrinol Lett 34:14–17
Zhao T-J, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM (2010) Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A 107:15868–15873. https://doi.org/10.1073/pnas.1011116107
Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N et al (2006) Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 9:381–388. https://doi.org/10.1038/nn1656
Pan W, Tu H, Kastin AJ (2006) Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides 27:911–916. https://doi.org/10.1016/j.peptides.2005.12.014
Acknowledgements
The MALDI-TOF MS analyses were carried out in the Centro de Estudios Químicos y Biológicos por Espectrometría de Masa (CEQUIBIEM) at University of Buenos Aires. We would like to thank María José Tolosa, Mirta Reynaldo, and Guadalupe García Romero for their technical support and to Dr. Jacques Epelbaum for critically reading the manuscript.
Funding
This work was supported by research grants of the National Agency of Scientific and Technological Promotion of Argentina (PICT2016-1084 to MP), the Natural Sciences and Engineering Research Council of Canada (to LGL), and the Canadian Institutes of Health Research (to LGL). MU, GF, and AC were supported by CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and all efforts were made to minimize suffering. All experimentation received approval from the Institutional Animal Care and Use Committee of the Multidisciplinary Institute of Cell Biology (approval ID 10-0112).
Rights and permissions
About this article
Cite this article
Uriarte, M., De Francesco, P.N., Fernandez, G. et al. Evidence Supporting a Role for the Blood-Cerebrospinal Fluid Barrier Transporting Circulating Ghrelin into the Brain. Mol Neurobiol 56, 4120–4134 (2019). https://doi.org/10.1007/s12035-018-1362-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1362-8