Abstract
Current pharmacological treatments for major depressive disorder (MDD) are severely compromised by both slow action and limited efficacy. RNAi strategies have been used to evoke antidepressant-like effects faster than classical drugs. Using small interfering RNA (siRNA), we herein show that TASK3 potassium channel knockdown in monoamine neurons induces antidepressant-like responses in mice. TASK3-siRNAs were conjugated to cell-specific ligands, sertraline (Ser) or reboxetine (Reb), to promote their selective accumulation in serotonin (5-HT) and norepinephrine (NE) neurons, respectively, after intranasal delivery. Following neuronal internalization of conjugated TASK3-siRNAs, reduced TASK3 mRNA and protein levels were found in the brainstem 5-HT and NE cell groups. Moreover, Ser-TASK3-siRNA induced robust antidepressant-like behaviors, enhanced the hippocampal plasticity, and potentiated the fluoxetine-induced increase on extracellular 5-HT. Similar responses, yet of lower magnitude, were detected for Reb-TASK3-siRNA. These findings provide substantial support for TASK3 as a potential target, and RNAi-based strategies as a novel therapeutic approach to treat MDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high prevalence and increasing socioeconomic impact of major depressive disorder (MDD) is not paralleled by improvements in its treatments [1,2,3]. The antidepressant drugs available for MDD treatment, including the widely prescribed monoamine (serotonin, 5-HT- and norepinephrine, NE-) reuptake inhibitors, are suboptimal and still unsatisfactory. Current antidepressant drugs have a slow onset of action and limited efficacy, which result in a high percentage of MDD patients with poor or no therapeutic responses, thus reducing quality of life and increasing suicide risk [4,5,6]. The reasons for the limited efficacy of monoamine-based treatments are manifold, including neurobiological adaptive mechanisms and genetic factors, resulting in a great individual variability. Understanding the neurobiological basis of MDD and improving the therapeutic strategies currently available remains one of the foremost challenges for modern neuropsychopharmacology.
RNAi strategies may avoid some of the stated limitations once adequate administration routes to the CNS and targeting of selected neuronal populations are established [7, 8]. Previous studies showed that the intracerebroventricular (i.c.v.) or intranasal (i.n.) administration of selective serotonin reuptake inhibitor- (SSRI) conjugated siRNA (small interfering RNA) targeting the serotonin transporter (SERT) or 5-HT1A-autoreceptors induced robust antidepressant-like responses in mice [9, 10]. These effects were similar to those induced by the local application of unmodified siRNA sequence in the raphe nuclei [11, 12]. The presence of sertraline (Ser) in the conjugated siRNA allows its selective accumulation in 5-HT neurons and the targeting of genes expressed by these neurons. Hence, 1-week i.n. administration of small amounts (2.1 nmol/day) of a Ser-conjugated SERT-siRNA evoked antidepressant-like responses comparable to those induced by 1-month treatment with fluoxetine 10 mg/kg/day, i.p. [10]. Likewise, acute 5-HT1A-autoreceptor knockdown also evoked rapid antidepressant-like effects in mice, due to a lesser self-inhibition of serotonergic neurons, thus increasing stress resilience [9, 11].
Here, we extend this strategy to knockdown TASK3, an acid-sensitive two-pore domain potassium channel (K2P) (referred as TWIK-related acid sensitive potassium channel 3, also known as K2P9.1) [13]. TASK3 and other related potassium channel members have been implicated in the pathophysiology of MDD and in the antidepressant drug response as well as in resilience mechanisms. In turn, TASK3 channels may have therapeutic potential in neuropsychiatric disorders due to their ability to control the resting membrane potential and neuronal excitability [14,15,16]. Preclinical studies showed that full deletion of the TASK3 channel in mice markedly reduced REM sleep and evoked antidepressant-like effects [17]. Likewise, non-selective TASK3 antagonist administration increased the active alertness with a concurrent decrease in both REM and delta sleep in wild-type mice, suggesting their therapeutic antidepressant potential [18]. Human and rodent TASK3 channels are abundantly expressed in the cerebral cortex, hippocampus (HPC), thalamic and hypothalamic nuclei, and cerebellum as well as in 5-HT and NE neurons of the dorsal raphe nucleus (DR) and locus coeruleus (LC), respectively [19,20,21,22,23].
Taking advantage of the neuronal selectivity of conjugated siRNAs, we examined the potential antidepressant effects of TASK3 knockdown in 5-HT and NE neurons under the working hypothesis that the reduced TASK3 expression would increase the neuronal excitability and therefore, facilitate the monoaminergic neurotransmission in a similar way to that produced by 5-HT1A-autoreceptor knockdown [9, 11]. Further, the selective reduction of TASK3 expression in 5-HT or NE neurons would avoid potential side effects of TASK3 channel blockers derived from the interaction with cortical, hippocampal, and cerebellar TASK3 channels [24].
Materials and Methods
Animals
Male C57BL/6 J mice (10–14 weeks; Charles River, Lyon, France) were housed under controlled conditions (22 ± 1 °C; 12-h light/dark cycle) with food and water available ad libitum. Animal procedures were conducted in accordance with National (Royal Decree 53/2013) and European legislation (Directive 2010/63/EU on the protection of animals used for scientific purposes, 22 September 2010) and were approved by the Institutional Animal Care and Use Committee of the University of Barcelona.
siRNA Synthesis
Synthesis and purification of naked and conjugated siRNA targeting TASK3 channel (TASK3-siRNA, nt: 1056–1075, GenBank accession NM_001033876) and nonsense siRNA (NS-siRNA) were performed by nLife therapeutics S.L. (Granada, Spain). Both TASK3-siRNA and NS-siRNA sequences were conjugated with the SSRI sertraline (Ser-TASK3-siRNA and Ser-NS-siRNA) as described to target selectively 5-HT neurons [9, 10]. Moreover, TASK3- and NS-siRNA sequences were also conjugated with the selective NE reuptake inhibitor reboxetine (Reb-TASK3-siRNA and Reb-NS-siRNA) to reach NE neurons. Details are shown in the Supplementary Information.
To confirm the in vivo incorporation of conjugated siRNA into 5-HT or NE neurons, Ser-NS-siRNA and Reb-NS-siRNA were additionally labeled with Alexa488 in the antisense strand (A488-Ser-NS-siRNA or A488-Reb-NS-siRNA). Control groups received Alexa488-PBS (A488-PBS) or non-conjugated (naked) NS-siRNA (A488-NS-siRNA) using an identical procedure to Ferrés-Coy et al. and Alarcón-Arís et al. [10, 25]. Stock solutions of all siRNAs were prepared in RNAse-free water and stored at −20 °C until use. Sequences are shown in Suppl. Table 1.
Treatments
Mice received (1) unmodified siRNAs locally infused into dorsal raphe nucleus (DR) or locus coeruleus (LC) or (2) conjugated siRNAs administered i.n. For the intracerebral infusion of TASK3-siRNA and NS-siRNA, mice were anesthetized (pentobarbital, 40 mg/kg, i.p.), and silica capillary microcannulae (110-μm-OD and 40-μm-ID; Polymicro Technologies, Madrid, Spain) were stereotaxically implanted into DR (coordinates in mm: AP, − 4.5; ML, − 1.0; DV, − 4.1; with a lateral angle of 20°) or LC (AP, − 5.2; ML, − 0.9; DV, − 3.5) [26]. Local siRNA microinfusion was performed 24 h after surgery in awake mice using a precision minipump at a 0.5 μl/min as previously described [11, 12]. siRNAs were prepared in a RNase-free artificial cerebrospinal fluid (aCSF) with 5% glucose and infused intra-DR or intra-LC once daily at the dose of 10 μg/μl (0.7 nmol/dose). Mice received two doses in two consecutive days. Control mice received aCSF. Mice were sacrificed 24 h after last infusion.
For i.n. administration, mice were slightly anesthetized with 2% isoflurane inhalation during ~ 1 min and placed in a supine position [9, 10, 25]. A 5-μl-drop of phosphate buffered saline (PBS) or conjugated siRNAs (Ser- or Reb-NS-siRNA or Ser- or Reb-TASK3-siRNA) was applied alternatively to each nostril once daily. Ten microliters of solution containing 30 or 75 μg (2.1 or 5.3 nmol/day) of conjugated siRNA was delivered for 7 days, and mice were sacrificed between 3 and 4 days after last administration.
In Situ Hybridization
In situ hybridization was performed as previously described [9, 10]. Antisense oligoprobes (Göttingen, Germany) were complementary to the following bases: TASK3/839–888 (GenBank accession NM_001033876), TASK1/101–150 (NM_010608), TREK1/592–641 (NM_001159850), SERT/820–863 (NM_010484.1), serotonin-1A receptor-5-HT1AR/1780–1827 (NM_008308), NET/1210–1259 (NM_009209), α2-adrenoreceptor-Adra2/2137–2186 (NM_NC_000085), brain derived neurotrophic factor-BDNF/1188–1238 (NM_007540), vascular endothelial growth factor-VEGF/2217–2267 (NM_001025250), and activity regulated cytoskeletal protein-ARC/1990–2040 (NM_018790). Details are shown in Supplementary Information.
Immunohistochemistry
Mice were anesthetized with pentobarbital and transcardially perfused with 4% paraformaldehyde in sodium-phosphate buffer (pH 7.4). Brains were collected, post-fixed 24 h at 4 °C in the same solution, and then placed in gradient sucrose solution 10–30% for 3 days at 4 °C. After cryopreservation, serial 30-μm thick sections were cut through hippocampal formation (HPC), midbrain raphe nuclei, and LC. Immunohistochemistry procedure was performed for doublecortin (DCX), glial fibrillary acidic protein (GFAP), Iba-1, Ki-67, tyrosine hydroxylase (TH), and tryptophan hydroxylase 2 (TPH2) using biotin-labeled antibody procedure [9, 10]. Details are shown in the Supplementary Information.
Confocal Fluorescence Microscopy
Intracellular Ser- and Reb-NS-siRNA distribution in 5-HT and NE neurons was examined using a Leica TCS SP5 laser scanning confocal microscope (Leica Microsystems Heidelberg GmbH, Manheim, Germany) equipped with a DMI6000 inverted microscope, blue diode (405 nm), Argon (458/476/488/496/514), diode pumped solid state, (561 nm) and HeNe (594/633 nm) lasers. Details are shown in the Supplementary Information.
Western Blot Analysis
Mice were sacrificed with pentobarbital overdose, brains were rapidly removed; and medial prefrontal cortex (mPFC), HPC, DR, and LC were dissected and snap-frozen on dry ice. Tissues were homogenized in RIPA buffer with protease inhibitors, and total protein amount was quantified using a bicinchoninic acid (BCA) kit (Thermo Scientific Scientific Inc., IL, USA). Details are shown in the Supplementary Information.
Drugs
All reagents used were of analytical grade and were obtained from Merck (Germany). 5-HT oxalate, NE bitartrate, (±)-8-hydroxi-2(dipropylamino)tetralin hydrobromide (8-OH-DPAT), and clonidine were from Sigma-Aldrich-RBI (Madrid, Spain). Fluoxetine, reboxetine, citalopram hydrobromide, and desipramine were from Tocris (Madrid, Spain). To assess the local effects in microdialysis procedures, drugs were dissolved in artificial cerebrospinal fluid (aCSF) and were administered by reverse dialysis at the stated concentrations [9, 10]. All other drugs dissolved in saline or aCSF as required. Concentrated solutions (1 mM; pH adjusted to 6.5–7 with NaHCO3 when necessary) were stored at − 80 °C, and working solutions were prepared daily by dilution in aCSF.
Intracerebral Microdialysis
Extracellular 5-HT and NE concentrations were evaluated by in vivo microdialysis as previously described [9, 10, 27,28,29]. A full description is given in Supplementary Information.
Behavioral Studies
Behavioral analyses were performed 24 h after the last dose with an interval of 1 day between tests. All mice were evaluated in two behavioral paradigms including: (1) novelty suppressed-feeding test (NSFT) and (2) tail suspension test (TST) or forced swim test (FST) or marble burying test (MBT). All tests were performed between 10:00 and 15:00 h by an experimenter blind to mouse treatments. On the test day, mice were placed in a dimly illuminated behavioral room and were left undisturbed for at least 1 h before testing. In an additional group, the extracellular 5-HT concentration in mPFC was simultaneously measured during TST paradigm by in vivo microdialysis [11]. Details are shown in the Supplementary Information.
Statistical Analyses
Results are given as mean ± S.E.M. Data were analyzed using GraphPad Prism 7.01 (San Diego, CA). Statistical analyses were performed by two-tailed Student’s t test and one-way or two-way ANOVA followed by Tukey’s post-hoc test as appropriate. In NSFT, we used the Kaplan-Meier survival analysis following Mantel-Cox log-rank [30]. Animals that did not eat during the 10-min testing time were discarded from the analysis. Differences were considered significant when p < 0.05.
Results
Selective Intranasal Delivery of Sertraline- and Reboxetine-Conjugated TASK3-siRNA Downregulates TASK3 Expression in 5-HT and NE Neurons
First, we examined whether a non-conjugated siRNA sequence targeting TASK3 (TASK3-siRNA) could reduce in vivo TASK3 channel expression in the DR and LC. Mice were injected intra-DR or unilaterally intra-LC with 1 μl of: (1) vehicle, (2) nonsense siRNA (NS-siRNA), or (3) TASK3-siRNA (0.7 nmol/dose) for two consecutive days. In situ hybridization experiments revealed reductions of TASK3 mRNA levels to 69 ± 4% (p = 0.0002, DR) and 82 ± 2% (p < 0.0001, LC) compared to control values (vehicle and NS-siRNA) (Suppl. Fig. 1).
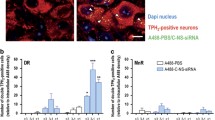
Next, conjugated siRNA molecules were synthesized by the manufacturer using a previously developed strategy, in which the SSRI sertraline was covalently bound to the siRNA (Ser-siRNA) in order to selectively target 5-HT neurons after i.n. or i.c.v. administration [9, 10]. Using the same procedure, siRNA sequences were also conjugated with the selective norepinephrine transporter (NET) inhibitor reboxetine (Reb-siRNA) to promote their accumulation in NE neurons. To assess the distribution of conjugated siRNA in the DR and LC neurons after i.n. administration, mice were treated once daily for 4 days with: (1) A488-PBS, (2) A488-NS-siRNA, (3) A488-Ser-NS-siRNA, or (4) A488-Reb-NS-siRNA (2.1 nmol/dose). Mice were killed 6 h after the last dose. Confocal fluorescence microscopy revealed the presence of double-conjugated molecules in DR and LC. Hence, A488-Ser-NS-siRNA was intracellularly detected in TPH2-positive 5-HT neurons of DR—but not in TH-positive neurons of LC—(Fig. 1a), and A488-Reb-NS-siRNA was found in TH-positive NE neurons of LC—but not in TPH2-positive neurons of DR—(Fig. 1b) indicating the selective incorporation of the oligonucleotides in each monoamine neuronal group expressing the corresponding transporters. Although the traffic mechanisms occurring after the internalization of conjugated siRNA molecules in monoamine neurons are not fully understood, endosomal networks would be involved, since Ser- or Reb-conjugated A488-siRNAs co-localized with the late endosomal marker Rab7 in DR and LC (Fig. 1c). Like antisense oligonucleotide molecules (ASO) conjugated with indatraline (triple monoamine reuptake inhibitor) [25], the conjugated siRNAs used here were scarcely detected in the olfactory bulbs, much closer to the application site than the brainstem monoamine nuclei. Remarkably, A488-Reb-NS-siRNA molecules were not found in TH-positive dopamine (DA) neurons of the olfactory bulb expressing the DA but not the NE transporter (Suppl. Fig. 2).
Selective accumulation of sertraline- or reboxetine-conjugated nonsense siRNA (Ser-NS-siRNA or Reb-NS-siRNA) in serotonin (5-HT) and norepinephrine (NE) neurons after intranasal administration. Mice were intranasally administered with: (1) alexa488-PBS (A488-PBS), (2) alexa488-labeled nonsense siRNA (A488-NS-siRNA), or (3) alexa488-labeled Ser- or Reb-NS-siRNA (A488-Ser-NS-siRNA or A488-Reb-NS-siRNA) at 2.1 nmol/day during 4 days and were sacrificed 6-h post-administration (n = 2 mice/group). Laser confocal images showing the co-localization (yellow) of A488-Ser-NS-siRNA or A488-Reb-NS-siRNA in 5-HT neurons in the dorsal raphe nucleus (DR) identified with a TPH2 marker antibody (red) (a) or in NE neurons of the locus coeruleus (LC) identified with TH marker antibody (red) (b). White arrowheads show the siRNA molecules co-localized with TPH2- and TH-positive cells. However, neither A488-Ser-NS-siRNA was detected in TH-positive cells in LC (a) nor A488-Reb-NS-siRNA in TPH2-positive cells in DR (b) indicating the selective incorporation of the oligonucleotides in each monoamine neuronal group expressing the corresponding transporters. Cell nuclei were stained with Dapi (blue). Scale bars, 10 μm. Confocal images showing the co-localization (yellow) of A488-Ser-NS-siRNA or A488-Reb-NS-siRNA (green) with Rab7 (red) in DR or LC neurons (c). Vesicles are marked with white arrowheads. The boxes include areas of DR or LC shown at higher magnification. Scale bars, low = 10 μm and high = 5 μm
Next step was to examine the effect of Ser-TASK3-siRNA on DR TASK3 mRNA expression. Ser-TASK3-siRNA (2.1 nmol/day) was administered i.n. for 7 days, and two control groups were used treated with PBS or Ser-NS-siRNA. In situ hybridization experiments revealed a significant reduction of DR TASK3 mRNA level to 89 ± 2% of control groups (p < 0.001), without affecting TASK3 expression in LC (p = 0.4656) nor the expression of other genes expressed in 5-HT neurons such as 5-HT1A receptor (p = 0.0922), SERT (p = 0.4021), and other K2P channels (TASK1, p = 0.944, TREK1, p = 0.1777) (Suppl. Fig. 3).
Given the relatively small reduction of DR TASK3 mRNA level, further experiments were performed with a higher daily dose (5.25 nmol/day) also administered during 7 days. TASK3 mRNA density was reduced to 83 ± 2% of PBS group (p < 0.05) levels in the DR (Fig. 2b), without altering TASK3 expression in the hippocampal formation (CA1 and dentate gyrus—DG) or medial prefrontal cortex (mPFC) (Fig. 2c). A more exhaustive histological analysis revealed a greater effect of Ser-TASK3-siRNA on TASK3 mRNA expression. Dipped TASK3 hybridized sections immunostained by using a specific 5-HT neuronal marker (TPH2) showed a reduced number of TPH2-positive cells expressing TASK3 mRNA (60 ± 10% of PBS group) as well as a decreased intracellular TASK3 density (56 ± 6% of PBS group) in TASK3 knockdown mice along the DR anteroposterior axis (Fig. 2a, d, e). Two-way ANOVA showed an effect of group F(1,14) = 14.72, p = 0.0018 and anteroposterior axis F(1,14) = 4.418, p = 0.00541 for double TPH2- and TASK3-positive cells, and an effect of group F(1,14) = 17.86, p = 0.0008 for intracellular TASK3 mRNA density in TPH2-positive neurons. Western blot analysis confirmed the selective silencing at the TASK3 protein level in DR (p = 0.04) (Fig. 2f, g).
Intranasal sertraline-conjugated TASK3-siRNA (Ser-TASK3-siRNA) treatment down-regulates TASK3 expression in mouse serotonin (5-HT) neurons. Mice received (1) PBS or (2) Ser-TASK3-siRNA at 5.3 nmol/day for 7 days, and sacrificed 3–4 days after last administration. Photomicrographs showing TPH2-positive neurons expressing TASK3 mRNA (33P-oligonucleotide silver grains) in the dorsal raphe nucleus (DR) at two anteroposterior (AP) coordinates from bregma (AP1, − 4.24 to − 4.48 and AP2, − 4.48 to − 4.72 in mm) of PBS- and Ser-TASK3-siRNA-treated mice (a). Scale bar, 10 μm. Autoradiographic analysis showed that Ser-TASK3-siRNA reduces TASK3 mRNA levels in the DR (b), but not in forebrain areas such as CA1, dentate gyrus (DG), and medial prefrontal cortex (mPFC) (c) (n = 4–6 mice/group). Intranasal Ser-TASK3-siRNA treatment reduced the percentage of TPH2-positive neurons expressing TASK3 mRNA in the DR of mice (n = 4–6 mice/group) (d). However, Ser-TASK3-siRNA did not modify the number of DR TPH2-positive neurons of the same mice (showed in Suppl. Fig. 5a). Dipping analysis also revealed a reduction of intracellular TASK3 mRNA density in TPH2-positive neurons of DR (n = 4–6 mice/group) (e). Western blotting of mPFC, hippocampus (HPC), and DR showing TASK3 and actin protein levels (f). Actin was used as loading control. Note the decreased TASK3 protein density in the DR of Ser-TASK3-siRNA-treated mice. Relative quantification of TASK3 protein level obtained by normalizing TASK3 by actin protein amount (n = 5–7 mice/group) (g). Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 compared to control group treated with PBS
Similarly, i.n. treatment with Reb-TASK3-siRNA (5.25 nmol/day for 7 days) reduced selectively TASK3 mRNA expression in the LC (p = 0.0181) (Fig. 3b), without affecting TASK3 mRNA level in other brain areas such as DR (p = 0.1625), CA1, DG, and mPFC (p = 0.347) nor the expression of NET (p = 0.4063), α2-adrenoreceptors (p = 0.8741), and TASK1 (p = 0.4023) and TREK1 (p = 0.9013) potassium channels in LC (Fig. 3c, Suppl. Fig. 4). Co-localization analysis showed that Reb-TASK3-siRNA significantly reduced the number of TH-positive cells expressing TASK3 mRNA as well as the intracellular density of TASK3 mRNA (76 ± 5% of PBS group) in TASK3 knockdown mice (Fig. 3a, d, e). Two-way ANOVA showed an effect of group for: (1) double TH- and TASK3-positive cells [(F(1,17) = 17.98, p = 0.0006] and (2) density in TPH2-positive neurons [F(1,16) = 30.44, p < 0.0001], but not of anteroposterior axis nor interaction. Likewise, a reduction of TASK3 protein level was found in LC (p = 0.0236), but not in projection brain areas as mPFC and HPC (Fig. 3f, g).
Intranasal reboxetine-conjugated TASK3-siRNA (Reb-TASK3-siRNA) treatment down-regulates TASK3 expression in mouse norepinephrine (NE) neurons. Mice were treated with: (1) PBS or (2) Reb-TASK3-siRNA at 5.3 nmol/day for 7 days, and sacrificed 3–4 days after last administration. Photomicrographs showing TH-positive neurons expressing TASK3 mRNA (33P-oligonucleotide silver grains) in the locus coeruleus (LC) at two anteroposterior (AP) coordinates from bregma (AP1, − 5.52 to − 5.68 and AP2, − 5.68 to − 5.80 in mm) of PBS- and Reb-TASK3-siRNA-treated mice (a). Scale bar, 10 μm. Autoradiographic analysis showed that Reb-TASK3-siRNA reduces TASK3 mRNA levels in the LC (b), but not in forebrain areas such as CA1, dentate gyrus (DG), and medial prefrontal cortex (mPFC) (c) (n = 8 mice/group). Intranasal Reb-TASK3-siRNA treatment reduced the percentage of TH-positive neurons expressing TASK3 mRNA in LC of mice (n = 4–6 mice/group) (d). However, Reb-TASK3-siRNA did not modify the number of LC TH-positive neurons of the same mice (showed in Suppl. Fig. 5a). Dipping analysis also revealed a reduction of intracellular TASK3 mRNA density in TH-positive neurons of LC (n = 4–6 mice/group) (e). Western blotting of mPFC, hippocampus (HPC), and LC showing TASK3 and actin protein levels (f). Actin was used as loading control. Note the decreased TASK3 protein density in the LC of Reb-TASK3-siRNA-treated mice. Relative quantification of TASK3 protein level obtained by normalizing TASK3 by actin protein amount (n = 6 mice/group) (g). Data are presented as the mean ± SEM. ^p < 0.05, ^^p < 0.01, ^^^p < 0.001 compared to control group treated with PBS
Neither treatment with Ser-TASK3-siRNA nor Reb-TASK3-siRNA induced neuronal loss, as evidenced by the presence of the same number of TPH2- or TH-positive neurons in all experimental groups (p = 0.2787 and 0.3266, respectively) and the absence of immune responses such as astrogliosis (GFAP) or microglial activation (Iba-1) (Suppl. Fig. 5).
Seven-Day Treatment with Ser-TASK3-siRNA Evokes Neurochemical, Behavioral, and Cellular Responses, Predictive of Clinical Antidepressant Activity
First, to evaluate the neurochemical impact of i.n. treatment with Ser-TASK3-siRNA (5.25 nmol/day), we examined the effect on extracellular 5-HT concentration in mPFC (one of the projection areas of both DR and LC) using intracerebral microdialysis. There were no significant differences in baseline 5-HT concentration nor on veratridine-stimulated 5-HT values (Suppl. Table 2). However, the reduction of TASK3 expression in 5-HT neurons markedly attenuated the 5-HT1A-autoreceptor-mediated decline in 5-HT release, as shown by the dampened effect of 8-OH-DPAT (1 mg/kg, i.p.) on terminal 5-HT release (Fig. 4a). Two-way ANOVA showed an effect of group F(1,17) = 13.40, p = 0.0019; time F(11,197) = 7.692, p < 0.0001 and interaction group-by-time F(11,187) = 3.44, p = 0.0002. In agreement with lesser 5-HT1A-autoreceptor-mediated self-inhibition of 5-HT neurons, Ser-TASK3-siRNA treatment augmented the effect of fluoxetine (20 mg/kg, i.p.) on extracellular 5-HT in mPFC (Fig. 4b). Two-way ANOVA showed an effect of group F(1,10) = 10.90, p = 0.008; time F(15,150) = 6.965, p < 0.0001 and interaction group-by-time F(15,150) = 2.104, p = 0.0125.
Sertraline-conjugated TASK3-siRNA (Ser-TASK3-siRNA) evokes neurochemical, behavioral, and cellular responses predictive of clinical antidepressant activity. Mice received intranasally (1) PBS or (2) Ser-TASK3-siRNA at 5.3 nmol/day for 7 days and were sacrificed 3–4 days after last administration. Ser-TASK3-siRNA treatment reduced the effect of serotonin-1A (5-HT1A) receptor 8-OH-DPAT agonist (1 mg/kg, i.p.) on serotonin (5-HT) release in medial prefrontal cortex (mPFC) (n = 9–10 mice/group) (a). Fluoxetine (20 mg/kg, i.p.) increased the extracellular 5-HT concentration significantly more in Ser-TASK3-siRNA than in PBS-treated mice (n = 6 mice/group) (b). Ser-TASK3-siRNA treated mice displayed reduced immobility in the tail suspension test (TST, n = 11 mice/group) and forced swim test (FST, n = 7–10 mice/group) (c, d). Effect on novelty suppressed feeding test (NSFT) and survival analysis (e). In two cohorts, DR TASK3 knockdown mice showed a better performance in the NSFT compared to control groups (n = 9–12 mice/group/cohort). PBS and Ser-TASK3-siRNA-treated mice behaved similarly in the marble burying test (MBT, n = 13–14 mice/group) (f). Representative images showing Ki-67- or DCX-positive cells in the dentate gyrus (DG) of Ser-TASK3-siRNA- or PBS-treated mice (g). Scale bar, 100 μm. Ser-TASK3-siRNA significantly increased the number of Ki-67- and DCX-positive cells compared to the PBS group (n = 5–7 mice/group) (h). Representative autoradiograms showing BDNF, ARC, and VEGF mRNA expressions in the hippocampus of control and TASK3 knockdown mice (i). Scale bar, 100 μm. Densitometric analyses of BDNF, ARC, and VEGF mRNA levels were performed in different hippocampal regions (j): CA1, CA2, CA3, and dentate gyrus (DG) shown in the cresyl violet-stained section (left bottom) (n = 5–6 mice/group). Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.0001 versus PBS-treated mice
Next, we evaluated the putative antidepressant-like effects of Ser-TASK3-siRNA by using different behavioral paradigms. Seven-day Ser-TASK3-siRNA i.n. treatment significantly reduced the immobility time in the tail suspension test (TST, p = 0.0295), forced swim test (FST, p = 0.0458), and shortened the latency to eat in the novelty suppressed feeding test (NSFT, Kaplan-Meier analysis showed p = 0.0032) compared to PBS-treated mice (Fig. 4c–e). In contrast, Ser-TASK3-siRNA administration did not affect obsessive/anxiety behavior assessed by the marble burying test (MBT, p = 4834) (Fig. 4f). Moreover, both experimental groups exhibited a similar locomotor activity in the open field test (OFT, data not shown).
Hippocampal neurogenesis and new synapses formation have been associated with clinical antidepressant actions [31, 32]. In agreement with this view, i.n. Ser-TASK3-siRNA treatment (7 days, 5.25 nmol/day) increased the number of Ki-67- (p = 0.0389) (Fig. 4g) and DCX-positive (p = 0.0388) cells in DG, compared to control group (Fig. 4h). The enhanced proliferative and neurogenic activities induced by Ser-TASK3-siRNA were accompanied by a higher expression of neuroplasticity-associated genes, such as BDNF, ARC, and VEGF in different hippocampal subfields (Fig. 4i, j). Two-way ANOVA showed an effect of group F(1,40) = 55.48, p < 0.0001; hippocampal subfields F(3,40) = 4.019, p < 0.01 and interaction group-by-subfields F(3,40) = 4.022, p = 0.01 for BDNF expression, and an effect of group F(1,32) = 44.15, p < 0.0001 and F(1,36) = 30.35, p < 0.0001 for ARC and VEGF expression, respectively.
TASK3 Knockdown in Serotonergic Neurons Enhances 5-HT Release Under Stressful Conditions
Taking into account that (1) DR 5-HT1A-autoreceptor [9, 11] and TASK3 knockdown mice evoked antidepressant-like effects and (2) the existence of a functional interplay between both inhibitory mechanisms as evidenced by the dampened effect of 8-OH-DPAT in TASK3-treated mice, we examined whether the antidepressant-like effect of TASK3 knockdown was associated to an enhanced cortical 5-HT release during TST performance. No significant differences were observed in baseline 5-HT levels between Ser-TASK3-siRNA- and PBS-treated mice (Suppl. Table 2). However, 5-HT release in TASK3 knockdown mice increased to ~ 240% of baseline (effect of group F(1,15) = 2.626, p = 0.0415, and time F(13,195) = 2.928, p = 0.007) (Fig. 5a) in temporal association with reductions in immobility during the exposure to the TST (p = 0.0055) (Fig. 5 b).
Sertraline-conjugated TASK3-siRNA (Ser-TASK3-siRNA) treatment enhanced serotonin (5-HT) release in the medial prefrontal cortex (mPFC) of mice under short-term inescapable stress paradigm. Mice received intranasally (1) PBS or (2) Ser-TASK3-siRNA at 5.3 nmol/day for 7 days and were evaluated 2–3 days after last administration. During a stressful situation induced by the tail suspension test (TST), TASK3 knockdown mice showed a larger increase of extracellular 5-HT in mPFC than PBS-treated mice (n = 8–9 mice/group) (a). Simultaneously, Ser-TASK3-siRNA-treated mice displayed a reduced immobility in the TST (n = 8–9 mice/group) (b). Data are presented as the mean ± SEM. *p < 0.05 and **p < 0.01 versus PBS-treated mice
Knockdown of TASK3 in Norepinephrine Neurons Induces Modest Antidepressant-Like Effects
We next examined the effects of Reb-TASK3-siRNA on variables, which are predictive of antidepressant activity. Intranasal Reb-TASK3-siRNA (5.25 nmol/day, 7 days) treatment attenuated the effect of clonidine on decreasing extracellular NE levels in mPFC, suggesting a lower α2-adrenoceptor functional activity (Fig. 6a). Two-way ANOVA showed an effect of group F(1,17) = 10.12, p = 0.0055; time F(11,187) = 4.466, p < 0.0001 and group-by-time interaction F(11,187) = 2.471, p = 0.0065. However, this effect was not translated into a higher baseline NE concentration (Suppl. Table 2) nor into an enhanced effect of the NET blocker reboxetine on extracellular NE level in mPFC (Fig. 6b).
Mild antidepressant-like effects produced by reboxetine-conjugated TASK3-siRNA (Reb-TASK3-siRNA). Mice received intranasally (1) PBS or (2) Reb-TASK3-siRNA at 5.3 nmol/day for 7 days and were sacrificed 3–4 days after last administration. Reb-TASK3-siRNA treatment reduced the effect of α2-adrenoreceptor clonidine agonist (0.3 mg/kg, i.p.) on extracellular norepinephrine (NE) levels in medial prefrontal cortex (mPFC) (n = 9–10 mice/group) (a). Reboxetine (20 mg/kg, i.p.) increased the extracellular NE levels similarly in both experimental groups (PBS and Reb-TASK3-siRNA) (n = 8 mice/group) (b). Reb-TASK3-siRNA treated mice displayed a reduced immobility in the tail suspension test (TST) versus PBS-treated mice (n = 9–11 mice/group) (c). In two cohorts, both PBS and Reb-TASK3-siRNA mice behaved similarly in the novelty suppressed feeding test (NSFT) (n = 9 mice/group/cohort) (d). No anxiety-related effects were observed on the marble burying test (MBT) (n = 12 mice/group) (e). Representative images showing Ki-67- or DCX-positive cells in the dentate gyrus (DG) of Reb-TASK3-siRNA or PBS-treated mice (f). Scale bar, 100 μm. Reb-TASK3-siRNA did not induce any increase in proliferation (Ki-67-positive cells) or neurogenesis (DCX-positive cells) in DG compared to the PBS group (n = 6 mice/group) (g). Representative autoradiograms showing BDNF, ARC, and VEGF mRNA expressions in the hippocampus of control and TASK3 knockdown mice (h). Scale bar, 100 μm. Densitometric analyses of BDNF, ARC, and VEGF mRNA densities were performed in different hippocampal regions (i): CA1, CA2, CA3, and dentate gyrus (DG) shown in the cresyl violet-stained section (left bottom) (n = 4–5 mice/group). Data are presented as the mean ± SEM. ^p < 0.05 and ^^p < 0.01 versus PBS-treated mice
Regarding to behavioral paradigms, the selective reduction of TASK3 in NE neurons reduced the immobility time in the TST (p = 0.0032) (Fig. 6c), but did not affect the performance in the NSFT (p = 0.6319) (Fig. 6d) and MBT (p = 0.9999) (Fig. 6e) as well as in the FST and OF (data not shown).
In addition, Reb-TASK3-siRNA i.n. treatment evoked marginally significant effects on the hippocampal cellular proliferation (Ki-67-positive cells) and BDNF expression in the DG (Fig. 6f, g, i), but significantly increased BDNF and ARC levels in the CA3 as well as the last in CA1 (Fig. 6h, i). Two-way ANOVA showed an effect of group F(1,28) = 15.1, p = 0.0006 and F(1,28) = 27.99, p < 0.0001 for BDNF and ARC expression, respectively. Overall, the reduction of TASK3 expression in TH-positive neurons of LC elicited milder antidepressant-like effects than those evoked by TASK3 knockdown in 5-HT neurons.
Discussion
In this study, we report that siRNA-induced knockdown of TASK3 channel in monoaminergic neurons elicits fast antidepressant-like responses in mice more marked when TASK3 expression was reduced in 5-HT neurons. The design of Ser- and Reb-conjugated siRNA molecules allowed us to allocate them selectively in 5-HT and NE neurons, respectively, after i.n. administration. Using this strategy, we were able to reduce the expression of TASK3 channels only in these monoaminergic cell groups, with no signs of neuronal and glial toxicity or compensatory mechanisms involving the expression of other members of K2P channel family as TREK1 and TASK1. These results support TASK3 as a new target for novel antidepressant therapies, which would overcome the limitations of standard antidepressant treatments, including slow clinical action and low efficacy.
Specific accumulation of Ser-conjugated siRNA molecules in 5-HT neurons or of antisense oligonucleotides conjugated with indatraline (triple monoamine reuptake inhibitor) in 5-HT, dopamine (DA), and NE neurons after i.n. administration has been previously reported [9, 10, 25]. Here, we extend this approach to NE neurons of the LC by covalently binding siRNA molecules to Reb, which allows the selective delivery and internalization of oligonucleotides to NE neurons after systemic (intranasal) administration. Delivery mechanism(s) of the conjugated siRNA molecules to monoamine cell bodies remain poorly understood. Given the anatomical proximity of DR and LC to the cerebral aqueduct and the fourth ventricle, respectively, conjugated siRNAs may be rapidly transported via CSF by pulsatile flow, and then taken up by the dense network of axons emerging from monoamine cell bodies in both nuclei, which contain the largest densities of SERT and NET in the brain [33, 34]. This mechanism is supported by the short time (10–20 min) taken by indatraline-conjugated oligonucleotides to reach the monoaminergic nuclei (DR, substantia nigra pars compacta, ventral tegmental area, and LC) after i.n. administration, as assessed by microdialysis [25]. Moreover, the association of conjugated siRNA molecules with endomembrane Rab family in monoaminergic neurons [10, 25, present study] suggests an additional involvement of a complex intracellular trafficking of conjugated oligonucleotides.
Like previous data [9, 10, 25], the presence of sertraline or reboxetine in the conjugated siRNA molecules is required for their selective accumulation in 5-HT and NE neurons, respectively, as observed with the double conjugated A488-Ser-NS-siRNA and A488-Reb-NS-siRNA, respectively. However, conjugated NS-siRNA did not modify the TASK3 mRNA expression in DR and LC, respectively, supporting the specificity of the effect.
Ser-TASK3-siRNA administration evoked significant changes in pre- and postsynaptic markers, which are predictive of clinical antidepressant activity. Likewise, the i.n. treatment was effective in the TST, FST, and NSFT, used to assess antidepressant-like efficacy in mice, but not in the MBT, mainly used to assess anxiolytic and anti-obsessive/compulsivity disorder (OCD) behaviors. Interestingly, the sensitivity of NSFT to chronic—but not acute—standard antidepressant administration and to fast-acting treatments, such as ketamine [30, 35, 36], suggests the superior efficacy of short-term Ser-TASK3-siRNA treatments. In addition, since the Ser-NS-siRNA treatment (7 days, i.n.) did not induced any change in TST, NSFT, or the hedonic state [10]; the antidepressant-like responses found herein should be consequences of downstream changes of reduced TASK3 expression/function in 5-HT (or NE) neurons, but not due to an effect of the minute amounts of sertraline (or reboxetine) contained in the conjugated siRNA. Hence, systemic dose-ranges of 10–20 mg/kg/day (~ 300–600 μg/day) sertraline or reboxetine are necessary to evoke antidepressant responses in rodents [37,38,39,40,41]. However, the dose of sertraline or reboxetine present in the conjugated siRNA was 1.53 μg/day, which represents 200–400 times less than that required to induce antidepressant-like effects.
The antidepressant-like effects of Ser-TASK3-siRNA are likely mediated by an enhancement of forebrain serotonergic neurotransmission associated to a reduced function of 5-HT1A-autoreceptors, thus decreasing the efficacy of negative feedback mechanisms operating at somatodendritic level [42, 43]. Similarly, the efficacy of the α2-adrenoceptor agonist clonidine to reduce NE release was dampened in Reb-TASK3-siRNA-treated mice, indicating a comparable reduction of α2-adrenoceptor sensitivity. However, unlike fluoxetine in Ser-TASK3-siRNA-treated mice, this was not accompanied by a greater effect of reboxetine in increasing extracellular NE in forebrain, as observed with combinations of NET inhibitors and α2-adrenoceptor antagonists [44, 45]. The reduced sensitivity of 5-HT1A-autoreceptors and α2-adrenoceptors in mice treated with conjugated-TASK3-siRNA would be causing changes in membrane potential of monoamine neurons after partial TASK3 inactivation. Indeed, a lower number of constitutively active TASK3 channels would increase the resting membrane potential [46], leaving the 5-HT and NE neurons less sensitive to the hyperpolarizing actions of somatodendritic autoreceptors. Alternatively, an intra-membrane interaction between G protein-coupled inwardly rectifying potassium (GIRK) channels associated to monoamine autoreceptors and TASK3 channels could be involved. Supporting this view, previous studies indicated that activating 5-HT1A-autoreceptors, which primarily open GIRK channels, hyperpolarizing the cell, and reducing firing, also decreases cAMP levels, which may in turn result in a disinhibition of K2P TREK-1 channels—other member of K2P family—also resulting in hyperpolarization [47, 48]. Together, this evidence confirms that K2P channels (TREK-1 and TASK3) play a key role in the regulation of 5-HT and NE neurotransmission.
Moreover, fluoxetine and its metabolite norfluoxetine have been described as potent inhibitors of TREK-1 channel (IC50 19 and 9 μM, respectively) by a mechanism that involved a decreased dissociation of C-terminal domain from the membrane [49, 50]. Thereby, SSRIs could potentially inhibit TREK-1 in two ways: directly and via increasing 5-HT release onto cAMP-inhibiting 5-HT1A receptors. In contrast, fluoxetine has a less potent inhibitory action on TASK1 and TASK3 channels (IC50 100 μM) [49, 51], indicating that the greater effect of fluoxetine on the extracellular 5-HT concentration in TASK3 knockdown mice would be potentially linked to the inhibition of 5-HT1A autoreceptor-dependent negative feedback loop. To our knowledge, no similar data were reported for reboxetine. Further studies are necessary to understand the nature of the interactions among the monoamine transporters (SERT, NET), autoreceptors (5-HT1A, α2-adrenoceptor), and TASK3 channel antagonism to evoke more rapid antidepressant response than conventional antidepressant drugs.
As observed after the knockdown of 5-HT1A-autoreceptors [11], mice treated with Ser-TASK3-siRNA exhibited a reduced immobility time in the TST, in parallel to an enhanced 5-HT release. The similarity of both effects suggests a common underlying mechanism: both treatments would reduce self-inhibitory inputs onto 5-HT neurons during stressful conditions, thus enhancing serotonergic activity in forebrain and increasing resilience to stress. Conversely, an increased expression/function of 5-HT1A-autoreceptors is associated with poor antidepressant efficacy and increased suicidal behavior [52,53,54,55], and mice with a high expression of 5–HT1A-autoreceptor show a depressive-like phenotype [56]. Overall, these observations support a direct relationship between reduction of self-inhibitory mechanisms in 5-HT neurons and antidepressant activity, to which a reduced TASK3 expression/function can contribute in a significant manner, similarly to what was reported with the 5-HT1A-autoreceptor knockdown [9, 11, 56].
Along with previous presynaptic changes, Ser-TASK3-siRNA and Reb-TASK3-siRNA increased hippocampal cell proliferation (Ki-67- and DCX-positive newborn cells) and the expression of plasticity genes (BDNF, ARC, VEGF). These effects, produced in 3–4 weeks with standard antidepressant treatments [11, 57,58,59], required only a 7-day treatment with Ser-TASK3-siRNA. TASK3 knockdown in 5-HT and NE neurons might act more quickly to alleviate depression, particularly because it does not require a desensitization of presynaptic autoreceptors [43] or an increase in neurogenesis [60], two of the leading hypotheses to explain the delayed onset of action of SSRIs. Moreover, this faster action was also observed with unmodified and conjugated SERT-siRNA [10, 12] and likely reflects the greater effectiveness of RNAi strategies to modulate neuronal function, compared to standard pharmacological treatments.
A key observation of the present study is that behavioral and neurochemical effects evoked by Reb-TASK3-siRNA were less marked than those evoked by Ser-TASK3-siRNA, and in some instances, did not reach statistical significance. This difference may be attributable to different factors. On the one hand, a lesser ability of unmodified and conjugated TASK3-siRNA to reduce TASK3 expression in NE neurons of LC (84 and 76%, respectively) compared to DR 5-HT neurons (69 and 60%, respectively). In the case of the conjugated siRNA, this difference might also be associated with the higher affinity of sertraline for SERT than reboxetine for NET [61, 62]. On the other hand, 5-HT and NE likely play differential roles in the treatment of MDD symptoms, being the 5-HT system more deeply involved in resilience to stress, a key factor in the performance of the behavioral tests used (TST and NSFT).
In summary, the present study shows that the selective reduction of TASK3 expression in monoamine neurons evokes antidepressant-like effects, being more significant when it targets 5-HT neurons than NE neurons. One-week treatments with Ser-TASK3-siRNA evoked behavioral and neurobiological changes comparable to those produced by prolonged SSRI treatments (e.g., 1 month). These effects may be driven by a reduced sensitivity of monoamine neurons to self-inhibitory inputs after TASK3 knockdown, thus enhancing monoamine neurotransmission. Further, the extension of the conjugated-siRNA strategy from 5-HT to NE neurons supports the validity of the present approach as a new therapeutic strategy for MDD treatment.
References
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C et al (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2197–2223
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE et al (2013) Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382:1575–1586
Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet 386:743–800
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH et al (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40
Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA et al (2006) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354:1243–1252
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917
Artigas F, Bortolozzi A (2017) Therapeutic potential of conjugated siRNAs for the treatment of major depressive disorder. Neuropsychopharmacol 42:371
Artigas F, Celada P, Bortolozzi A (2018) Can we increase the speed and efficacy of antidepressant treatments? Part II Glutamatergic and RNA interference strategies. Eur Neuropsychopharmacol 28:457–482. https://doi.org/10.1016/j.euroneuro.2018.01.005
Bortolozzi A, Castañé A, Semakova J, Santana N, Alvarado G, Cortés R, Ferrés-Coy A, Fernández G et al (2012) Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol Psychiatry 17:612–623
Ferrés-Coy A, Galofré M, Pilar-Cuéllar F, Vidal R, Paz V, Ruiz-Bronchal E, Campa L, Pazos Á et al (2016) Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration. Mol Psychiatry 21:328–338
Ferrés-Coy A, Santana N, Castañé A, Cortés R, Carmona MC, Toth M, Montefeltro A, Artigas F et al (2013) Acute 5-HT1A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology 225:61–74
Ferrés-Coy A, Pilar-Cuellar F, Vidal R, Paz V, Masana M, Cortés R et al (2013) RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis. Transl Psychiatry 3:11e211
Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C (2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem 275:16650–16657
Bayliss DA, Barrett PQ (2008) Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29:566–575
Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012) Neurobiology of resilience. Nat Neurosci 15:1475–1484
Borsotto M, Veyssiere J, Moha Ou Maati H, Devader C, Mazella J, Heurteaux C (2015) Targeting two-pore domain K(+) channels TREK-1 and TASK-3 for the treatment of depression: a new therapeutic concept. Br J Pharmacol 172:771–784
Gotter AL, Santarelli VP, Doran SM, Tannenbaum PL, Kraus RL, Rosahl TW, Meziane H, Montial M et al (2011) TASK-3 as a potential antidepressant target. Brain Res 1416:69–79
Coburn CA, Luo Y, Cui M, Wang J, Soll R, Dong J, Hu B, Lyon MA et al (2012) Discovery of a pharmacologically active antagonist of the two-pore-domain potassium channel K2P9.1 (TASK-3). Chem Med Chem 7:123–133
Karschin C, Wischmeyer E, Preisig-Müller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A (2001) Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci 18:632–648
Meadows HJ, Randall AD (2001) Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 40:551–559
Medhurst A, Rennie G, Chapman C, Meadows H, Duckworth M, Kelsell R et al (2001) Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol Brain Res 86:101–114
Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA (2001) CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21:7491–7505
Marinc C, Preisig-Müller R, Prüss H, Derst C, Veh RW (2011) Immunocytochemical localization of TASK-3 (K2P 9.1) channels in monoaminergic and cholinergic neurons. Cell Mol Neurobiol 31:323–335
Linden AM, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, Korpi ER (2007) TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther 323:924–934
Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, Pavia-Collado R, Chica R et al (2018) Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol Ther 26:550–567
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Academic Press, New York
Mateo Y, Meana JJ (1999) Determination of the somatodendritic alpha2-adrenoceptor subtype located in rat locus coeruleus that modulates cortical noradrenaline release in vivo. Eur J Pharmacol 379:53–57
Mateo Y, Fernández-Pastor B, Meana JJ (2001) Acute and chronic effects of desipramine and clorgyline on alpha(2)-adrenoceptors regulating noradrenergic transmission in the rat brain: a dual-probe microdialysis study. Br J Pharmacol 133:1362–1370
Ortega JE, Katner J, Davis R, Wade M, Nisenbaum L, Nomikos GG, Svensson KA, Perry KW (2012) Modulation of neurotransmitter release in orexin/hypocretin-2 receptor knockout mice: a microdialysis study. J Neurosci Res 90:588–596
Samuels BA, Hen R (2011) Novelty-suppressed feeding in the mouse. In: Gould TD (ed) Mood and anxiety related phenotypes in mice: characterization using behavioral test, Volume II. Springer, New York, pp. 107–121
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Duman RS, Voleti B (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56
Javitch JA, Strittmatter SM, Snyder SH (1985) Differential visualization of dopamine and norepinephrine uptake sites in rat brain using [3H]mazindol autoradiography. J Neurosci 5:1513–1521
Cortés R, Soriano E, Pazos A, Probst A, Palacios JM (1988) Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience 27:473–496
Wang JW, David DJ, Monckton JE, Battaglia F, Hen R (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384
Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C et al (2016) Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79:776–786
Redrobe JP, Bourin M (1998) Dose-dependent influence of buspirone on the activities of selective serotonin reuptake inhibitors in the mouse forced swimming test. Psychopharmacology 138:198–206
Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP et al (2000) Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry 47:818–829
Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR et al (2004) Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A 101:8186–8891
O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I (2007) Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology 192:357–371
Roni MA, Rahman S (2015) Effects of lobeline and reboxetine, fluoxetine, or bupropion combination on depression-like behaviors in mice. Pharmacol Biochem Behav 139(Pt A):1–6
Artigas F, Romero L, de Montigny C, Blier P (1996) Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 19:378–383
Hervás I, Artigas F (1998) Effect of fluoxetine on extracellular 5-hydroxytryptamine in rat brain. Role of 5-HT autoreceptors. Eur J Pharmacol 358:9–18
Mateo Y, Pineda J, Meana JJ (1998) Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem 71:790–798
Ortega JE, Fernández-Pastor B, Callado LF, Meana JJ (2010) In vivo potentiation of reboxetine and citalopram effect on extracellular noradrenaline in rat brain by α2-adrenoceptor antagonism. Eur Neuropsychopharmacol 20:813–822
Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA (2002) Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci 22:1256–1265
Gordon JA, Hen R (2006) TREKing toward new antidepressants. Nat Neurosci 9:1081–1083
Mazella J, Pétrault O, Lucas G, Deval E, Béraud-Dufour S, Gandin C, el-Yacoubi M, Widmann C et al (2010) Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol 8:e1000355
Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A (2005) Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol 144:821–829
Sandoz G, Bell SC, Isacoff EY (2011) Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc Natl Acad Sci U S A 108:2605–2610
Hajdu P, Ulens C, Panyi G, Tytgat J (2003) Drug- and mutagenesis-induced changes in the selectivity filter of a cardiac two-pore background K+ channel. Cardiovasc Res 58:46–54
Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G (1998) Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci 18:7394–7401
Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD et al (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23:8788–8799
Lemonde S, Du L, Bakish D, Hrdina P, Albert PR (2004) Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 7:501–506
Neff CD, Abkevich V, Packer JC, Chen Y, Potter J, Riley R et al (2009) Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry 14:621–630
Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A et al (2010) 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547
Pei Q, Zetterström TS, Sprakes M, Tordera R, Sharp T (2003) Antidepressant drug treatment induces Arc gene expression in the rat brain. Neuroscience 121:975–982
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA et al (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493
Dranovsky A, Hen R (2006) Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59:1136–1143
Page ME (2003) The promises and pitfalls of reboxetine. CNS Drug Rev 9:327–342
Sanchez C, Reines EH, Montgomery SA (2014) A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int Clin Psychopharmacol 29:185–196
Acknowledgements
We thank María Calvo, Elisenda Coll, and Anna Bosch for outstanding technical support in the confocal microscopy unit (CCiT-UB); and Mireia Galofré and Letizia Campa for their outstanding technical assistance. We also thank J Pablo Salvador and Núria Pascual for the TASK3 antibody production and purification (Institut de Quimica Avançada de Catalunya, CSIC; Parc Cientific de Barcelona, UB; and CIBER in Bioengineering, Biomaterials, and Nanomedicine), and to Nlife Therapeutics S.L. for advice on the design of conjugated siRNA molecules.
Funding
This work was supported by the following grants: SAF2015-68346-P (F.A.); SAF2013-48586-R (J.M.); SAF2016-75797-R (A.B.); Retos-Colaboración Subprograms RTC-2014-2812-1 and RTC-2015-3309-1 (A.B.); Ministry of Economy and Competitiveness (MINECO)—European Regional Development Fund (ERDF), UE; PI13/01390, Instituto de Salud Carlos III co-financed by ERDF (A.B.); IT616-13 Basque Government—ERDF (J.M.); 20003 NARSAD Independent Investigator (A.B.); and Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM). CERCA Programme/Generalitat de Catalunya is also acknowledged. M.N.F. and A.F-C. are recipients of a fellowship from the Spanish Ministry of Education, Culture and Sport.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
F.A. has received consulting honoraria on antidepressant drugs from Lundbeck and he has been PI of grants from Lundbeck. A.B. has been PI of grants from Nlife Therapeutics. S.L., F.A., and A.B. are coauthors of the patent WO/2011/131693 for the siRNA and ASO (antisense oligonucleotides) molecules and the targeting approach related to this work. The rest of authors declare no competing financial interest.
Electronic Supplementary Material
ESM 1
(DOCX 2522 kb)
Rights and permissions
About this article
Cite this article
Fullana, M.N., Ferrés-Coy, A., Ortega, J.E. et al. Selective Knockdown of TASK3 Potassium Channel in Monoamine Neurons: a New Therapeutic Approach for Depression. Mol Neurobiol 56, 3038–3052 (2019). https://doi.org/10.1007/s12035-018-1288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1288-1