Abstract
The hippocampus is one of the most susceptible regions in the brain to be distraught with status epilepticus (SE) induced injury. SE can occur from numerous causes and is more frequent in children and the elderly population. Administration of a combination of antiepileptic drugs can abolish acute seizures in most instances of SE but cannot prevent the morbidity typically seen in survivors of SE such as cognitive and mood impairments and spontaneous recurrent seizures. This is primarily due to the inefficiency of antiepileptic drugs to modify the evolution of SE-induced initial precipitating injury into a series of epileptogenic changes followed by a state of chronic epilepsy. Chronic epilepsy is typified by spontaneous recurrent seizures, cognitive dysfunction, and depression, which are associated with persistent inflammation, significantly waned neurogenesis, and abnormal synaptic reorganization. Thus, alternative approaches that are efficient not only for curtailing SE-induced initial brain injury, neuroinflammation, aberrant neurogenesis, and abnormal synaptic reorganization but also for thwarting or restraining the progression of SE into a chronic epileptic state are needed. In this review, we confer the promise of cannabidiol, an active ingredient of Cannabis sativa, for preventing or easing SE-induced neurodegeneration, neuroinflammation, cognitive and mood impairments, and the spontaneous recurrent seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A neurological condition displaying self-enduring, continuous tonic-clonic seizure lasting ≥ 5 min or a cluster of seizures occurring close together with no recovery between seizures for ≥ 30 min is called status epilepticus (SE) [1]. SE is a severe neurologic ailment. Unremitting seizures can initiate extensive brain damage with functional deficits as well as death in some cases, if not interrupted with a combination of antiepileptic drugs (AEDs) [1,2,3,4]. Multiple neurological conditions including brain trauma, brain infections, brain tumor, febrile seizures, stroke, congenital malformations, Alzheimer’s disease, sleep deprivation, abrupt discontinuation of AED in a seizure-prone individual, alcohol, or street drugs can cause SE. The prevalence of SE varies from 10 to 61 cases per 100,000 individuals in a year, with a mortality rate of ~ 20% [1, 5, 6]. However, higher incidences of SE are seen in children and the elderly.
Many clinical and preclinical studies in the last decade have focused on understanding the causes, pathophysiology, prognosis, treatment, and enduring complications of SE [7, 8]. While SE can cause injury to multiple regions of the brain, the hippocampus is one of the most sensitive areas of the brain to SE-induced adverse long-term alterations. The early phase after SE displays multiple changes. These include neurodegeneration, increased oxidative stress, inflammation, and increased and abnormal neurogenesis in the hippocampus [9,10,11,12,13,14]. This phase may continue for days or weeks, following which multiple epileptogenic changes progress for variable periods [15]. For instance, the abnormal migration of newly born neurons continues into the dentate hilus, which can further enhance abnormal circuitry formation [16,17,18,19,20]. On the other hand, the dentate granule cell axons (mossy fibers) sprout into the inner molecular layer and the CA3 stratum oriens and form abnormal synapses, which can increase hyperexcitability of neurons [21,22,23,24,25]. Moreover, a progressive loss of GABA-ergic interneurons and alterations in GABA receptors can decrease inhibitory neurotransmission [26,27,28,29]. Changes in other neurotransmitters and their receptors can also alter the excitation-inhibition balance in neurotransmission [30,31,32]. Furthermore, astrocytes display structural and functional alterations [33], and elevated oxidative stress and inflammatory conditions endure [34,35,36]. Additionally, neurogenesis and multiple neurotrophic factor levels wane considerably [16]. Dispersion of granule cell layer may also occur [37]. Thus, multiple changes likely contribute to hippocampal hyperexcitability and cognitive and mood impairments after SE.
All epileptogenic changes mentioned above may not occur in every case of SE. The extent of alterations varies considerably with the intensity, duration, and type of SE. Nonetheless, an episode of SE can lead to chronic temporal lobe epilepsy (TLE) characterized by spontaneous recurrent seizures (SRS) and cognitive and mood impairments as comorbidities [38,39,40,41]. The occurrence of TLE after SE may take months, years, or even decades, as it depends on the extent and the speed by which the various epileptogenic alterations reach certain thresholds to tilt excitation-inhibition homeostasis into a state of hyperexcitability. While most cases of TLE can be controlled through the intake of a single or combination of AEDs, over 30% of TLE cases are drug-resistant. Lack of seizure control may lead to continued neurodegeneration, inflammation, severe cognitive deficits, and mood dysfunction. Considering these, effective therapies that not only terminate SE but also thwart and substantially restrain epileptogenic changes occurring after SE are needed. Conventional antiepileptic drug therapy is effective for ending or significantly reducing seizures in most cases [7]. However, AEDs have not shown significant efficacy for preventing or reducing SE-induced epileptogenesis and cognitive and mood impairments. From this perspective, alternative drugs, natural compounds, cells, or cell products that can suppress oxidative stress and neuroinflammation provide neuroprotection and maintain neurogenesis to near normal levels after SE has received significant attention. In this review, we focus on discussing the promise of a natural product cannabidiol for easing SE-induced neuroinflammation, epileptogenesis, chronic seizures, and related comorbidities.
Source of Cannabidiol and Historical Background
The use of Cannabis sativa in religious rituals and for recreation has been documented since millenniums. Medicinal formulations of C. sativa have also been used since ancient times for treating multiple conditions. These include menstrual sickness, gout, fever, glaucoma, nausea, muscle spasms, anxiety, Alzheimer’s disease, Huntington’s disease, neuropathic pain, headache, and epilepsy [42,43,44,45,46]. Nonetheless, the use of C. sativa for treating disease conditions is uncommon in the modern age due to ethical and cultural notions about the psychostimulant effect of Δ9-tetrahydrocannabinol (Δ9-THC), one of the active ingredients of C. sativa. However, a fact that was overlooked for long is the presence of hundreds of C21 terpenophenolic compounds, distinguished as phytocannabinoids in C. sativa. These include cannabidiol (CBD), cannabichromene, and cannabigerol, all of which are nonpsychoactive compounds with several medicinal functions [47,48,49].
Effects of Cannabidiol on Spontaneous Seizures in Epilepsy Patients and Animal Models
Although the two principal components of C. sativa (THC and CBD) showed efficacy for preventing seizures as well as reducing mortality in an animal model of SE with low toxicity and high tolerability [39], CBD received much attention by the scientific community because of its nonpsychostimulant property. Many studies have demonstrated that the hippocampus and the adjacent brain areas contain high levels of CBD receptors (CB1 receptors), which are G-protein-coupled receptors regulating several brain functions [50,51,52]. For example, these receptors can regulate calcium influx into neurons during hyperexcitability, a characteristic feature of epilepsy [53]. Indeed, CBD has antiepileptiform and anticonvulsant effects in in vitro and in vivo models of epilepsy [54,55,56,57,58,59,60,61,62]. CBD can also ameliorate social deficits [60, 61]. Besides, CBD can mediate neuroprotective effects in models of hypoxic-ischemic and self-sustained seizures [62, 63].
There have been many reports on the efficacy of cannabis extracts or CBD for controlling seizures in children with epilepsy. These reports are based mostly on surveys, where parents reported the beneficial effects of oral cannabis extracts enriched with CBD in their children afflicted with some form of refractory epilepsy. These include Dravet syndrome (severe myoclonic epilepsy of infancy), Doose syndrome (myoclonic astatic epilepsy), infantile spasms, Lennox-Gastaut syndrome, West syndrome, Ohtahara syndrome, and idiopathic generalized epilepsy. Porter and Jacobson reported that 84% of 19 children with refractory epilepsy who used an average of 12 AEDs earlier displayed reduced frequency of seizures with cannabidiol-enriched cannabis [64]. Hussain and colleagues report a survey that comprised 117 children with epilepsy receiving CBD-enriched cannabis preparations following failed seizure reduction for 5 years with eight AEDs. The median duration of CBD treatment was 6.8 months, and the median dosage was 4.3 mg/kg/day. Interestingly, 85% of children displayed reduced seizure frequency with 14% showing no seizures [65]. Another survey comprised 75 patients that included both children and adolescents with epilepsy [66]. Seizure control was observed in 57% of patients with 33% displaying over 50% reduction in the frequency of seizures. There were also improvements in behavior and alertness (33%), language (10%), and motor skills (10%). However, adverse events were seen in 44% of patients with 13% exhibiting an enhanced frequency of seizures and 12% showing somnolence or fatigue [66]. A survey by Tzadok and associates reports varying levels of seizure reduction in 89% and aggravated seizures in 7% of children with epilepsy [67]. Several recently published surveys also report similar findings in children and adults with refractory epilepsy. These include decreased frequency of seizures in (i) ~ 81% of children (n = 43) [68], (ii) ~ 90% of adults and ~ 71% of children [69], and (iii) 49% in children and adolescents [70]. Some side effects were also seen in ~ 42% of patients, however [68]. These surveys, while interesting, raise issues such as participation bias, lack of blinded outcome analyses, and the accuracy of seizure numbers. The epilepsy patients, parents of patients, and most medical professionals seem to believe that the available evidence on safety and efficacy is sufficient for employing CBD in treating epilepsy [71]. However, majority of epileptologists like to have additional proof about the safety and efficacy of CBD prior to its widespread clinical use [71].
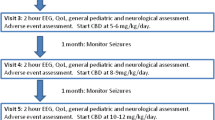
Indeed, the anticonvulsant property of CBD in humans is also evident from some clinical trials (Fig. 1). A first open-label study by Devinsky and colleagues showed the efficacy of CBD in epileptic patients for reducing motor seizures with acceptable toxicity and tolerability [72]. A study by Hess and associates showed the tolerability of CBD as well as its efficacy to reduce the frequency of seizures by ~ 48% in 50% of tuberous sclerosis patients displaying refractory seizures [73]. In another study involving five subjects with Sturge-Weber syndrome, CBD treatment for over a year was well tolerated with milder side effects and induced 50% reduction in seizures [74]. Importantly, a recent randomized, double-blind, placebo-controlled clinical trial performed by Devinsky and colleagues showed the effectiveness of CBD in treating drug-resistant seizures in children afflicted with the Dravet syndrome [75]. In patients receiving CBD treatment at 20 mg/kg/day, the median seizure frequency was reduced from 12.4 to 5.9 seizures per month with 5% of patients reaching seizure-free status, in comparison to placebo-treated patients maintaining 14–15 seizures per month. CBD was well tolerated with reduced side effects than other approved AEDs [76, 77]. Another recent study by Warren and colleagues showed that pharmaceutical grade CBD (Epidiolex; Greenwich Biosciences) is efficacious for reducing seizure severity in three patients with brain tumor-related refractory epilepsy [78]. In addition to the clinical trials described above, there are individual case reports supporting the beneficial effects CBD for reducing seizures [79, 80].
Summary of clinical trials and animal studies reporting the efficacy of cannabidiol (CBD) as an anticonvulsant and/or antiepileptogenic agent (the upper half) and the potential mechanisms by which CBD provides seizure suppression and neuroprotection (the lower half). Clinical trials have demonstrated the efficacy of CBD to diminish seizure frequency in adult epilepsy patients as well as in children with Dravet syndrome or tuberous sclerosis (see the upper left region). Animal studies have shown the effectiveness of CBD therapy for modulating status epilepticus (SE) and/or SE-induced epilepsy using intrahippocampal pilocarpine, maximal electroshock, corneal kindling, and other acute seizure models (see the upper right region). The properties of CBD that promote seizure suppression include its ability to reduce intracellular calcium in neurons, inhibit the release of excitatory neurotransmitter glutamate, reduce the uptake and degradation of anandamide, and enhance adenosine levels (see the lower left region). The neuroprotective properties of CBD are supported by its ability to activate cannabinoid receptor signaling pathways; reduce oxidative stress, transforming growth factor-alpha (TNFa), interleukin-1 beta (IL-1b), and the associated inflammatory reaction; activate peroxisome-proliferator-activated receptor gamma (PPAR-gamma) signaling pathway; and inhibit nitric oxide (see the lower right region)
Epilepsy is also typified by several comorbidities which interfere with day to day life. These neuropsychiatric symptoms include cognitive and behavioral impairments such as anxiety, depression, and stress [81, 82]. It is of great importance to treat such symptoms in the early stages of life with epilepsy to improve the quality of life and to prevent any unnecessary social discomfort associated with epilepsy. In this context, it is worthy to note that pediatric epileptic patients receiving CBD showed significant improvements in quality of life measures such as energy versus fatigue, cognition, memory, and control versus helplessness [83]. In another study, Press and associates reported improved behavior, alertness, language, and motor skills in ~ 33% of 75 patients (children and adolescents) with refractory epilepsy after oral cannabis extract intake [66]. CBD also seem to promote anxiolytic activity, likely through facilitation of local 5-HT1A receptor-mediated neurotransmission [84].
Because of clinical evidence of anticonvulsant effects as well as several anecdotal cases reported by the media, many countries have approved CBD for the treatment of epilepsy, particularly with regularly employed AEDs not offering satisfactory seizure control. Thus, about controlling seizures in chronic epileptic conditions, CBD has already shown considerable promise. Seizure-suppressing feature of CBD makes this drug a promising candidate for the treatment of drug-resistant epilepsies. However, rigorous clinical studies such as double-blind, placebo-controlled larger trials are required in the future to establish CBD as a drug of choice for treating refractory epilepsy. Furthermore, side effects of CBD with long-term use for controlling seizures in chronic epilepsy is unknown, especially its effects on comorbidities of epilepsy such as cognitive impairments and depression. In this context, studying the various effects of long-term administration of CBD in animal models of chronic epilepsy is critically required.
Prospects of CBD for Easing SE and SE-Induced Epileptogenesis
The efficiency of CBD to modulate epileptogenesis after SE is still unclear. Only a few studies have investigated the efficacy of CBD administration for SE (Fig. 1). In a recent animal study, Do Val-da Silva and colleagues pretreated Wistar rats with intraperitoneal CBD an hour prior to inducing SE through an intrahippocampal microinjection of pilocarpine [63]. In this study, CBD pretreatment increased the latency to SE development, reduced the severity of SE from Racine scale 4 to Racine scale 3, and diminished neurodegeneration in the dentate hilus and CA3 subfield of the hippocampus. Moreover, CBD affected spontaneous local field potentials in the contralateral hippocampus during SE by increasing the latency to epileptiform discharges and reducing powers in delta and theta oscillations. This study provided the baseline data to demonstrate the potential of CBD for reducing neurodegeneration and related changes. However, pretreatment approach has little translational value since CBD is not taken as a daily dietary supplement.
Recently, the National Institute of Neurological Disorders and Stroke funded Epilepsy Therapy Screening Program has examined the effectiveness of intraperitoneal CBD treatment in mouse 6 Hz 44 mA, maximal electroshock (MES), and corneal kindling models, and in a rat MES prototype [85]. In these studies, CBD pretreatment provided dose-dependent protection in acute seizure models as well as in the chronic corneal kindled mice. Furthermore, a recent open-label clinical study in children afflicted with febrile infection-related epilepsy syndrome (an epileptic encephalopathy) showed that CBD significantly reduced seizures that are typically resistant to AED, immune modulatory, and dietary therapies [86]. Importantly, CBD treatment in the subacute or chronic phase led to not only reduction in seizure frequencies but also improvements in motor, cognitive, and verbal function [86]. Collectively, the above studies suggest the potential of CBD for easing SE or SE-induced epileptogenesis. However, detailed long-term studies in several SE models will be required for making definite conclusions on the efficacy of CBD for mitigating SE-induced epileptogenesis. Furthermore, rigorous safety studies in SE models utilizing postnatal, young, adult, and aged rodents examining the different doses of CBD on maximal tolerance, somatic and metabolic changes, motor and sensory function, cognition, and mood function are required. Besides, the efficacy of CBD needs to be tested with commonly used benzodiazepine drugs or other AEDs that terminate SE or prevent the occurrence of SRS in the early phase after SE to understand drug-drug interactions and the possible additive beneficial or adverse effects. These issues are important because CBD can alter the concentration of other drugs [87, 88].
Potential Mechanisms by Which CBD Modulates Seizures and Epileptogenesis
The mechanisms by which CBD exerts seizure-suppressing and neuroprotective effects are unknown. It is plausible that the beneficial effects of CBD are mediated through the activation of the cannabinoid receptor signaling in the brain because activation of endocannabinoid system can control the excitability of neurons [89]. Precisely, brief postsynaptic depolarization during neurotransmission can trigger the release of endocannabinoids such as N-arachidonoyl ethanolamide (anandamide) and 2-arachidonoyl glycerol (2-AG) synthesized from postsynaptic membrane phospholipid precursors in response to increased intracellular calcium [90, 91]. The anticonvulsant activity of CBD possibly involves inhibition of the cellular uptake and catabolism of anandamide, which can increase the concentration of endocannabinoids [92, 93]. These endocannabinoids can bind to G-protein-coupled CB1 receptors in presynaptic terminals to reduce the neurotransmitter release [89]. These phenomena have been elegantly demonstrated in excitatory axons synapsing on Purkinje cells of the cerebellum as “depolarization-induced suppression of excitation.” Similar events in inhibitory axons synapsing on pyramidal neurons of the hippocampus are known as “depolarization-induced suppression of inhibition” [89, 94, 95].
Thus, the endocannabinoids act as synaptic circuit breakers by binding to CB1 receptors [96,97,98]. CB1 receptors are found on axon terminals in multiple brain regions [97, 99, 100]. These include the various areas of the neocortex, hippocampus, amygdala, basal ganglia, thalamus, hypothalamus, nucleus accumbens, substantia nigra, ventral tegmental area, cerebellum, and brain stem [89, 96]. From this perspective, exogenous compounds or drugs that can act on CB1 receptors in excitatory axon terminals may be particularly useful for controlling hyperexcitability of neurons in conditions such as SE or chronic epilepsy. However, it is unlikely that the effect of CBD on seizure suppression is solely due to CB1 receptor signaling because CBD has low affinity for CB1 receptors [101, 102]. CBD is likely suppressing seizures through several mechanisms. These may comprise its ability to reduce the intracellular calcium [53, 89], diminish the release of glutamate, and antagonize G protein-coupled receptor 55, a counterpart of the canonical CB1 receptor signaling pathway [103]. Antiseizure effects of CBD may also occur through modulation of potassium ion channels as well as voltage-gated sodium channels [104, 105]. The blockade of sodium channels by CBD may not directly correlate with anticonvulsant effects [106] but may involve activation and desensitization of transient receptor potential vanilloid 1 (TRPV1) channels to reduce hyperexcitability [107]. Alternative mechanisms of CBD action include its ability to hinder the uptake of adenosine, an endogenous anticonvulsant [108, 109], and inhibit the uptake and degradation of anandamide, an endocannabinoid [92, 110].
CBD may restrain epileptogenesis after SE through several mechanisms. For example, CBD can suppress oxidative stress by acting as a potent antioxidant agent and thereby provide neuroprotective effects through CB1 receptor-independent mechanism [111,112,113]. Furthermore, CBD can modulate inflammation through suppression of tumor necrosis factor-alpha, a potent pro-inflammatory cytokine contributing to neuroinflammation after SE [111, 114,115,116]. These antiinflammatory effects need particular attention because neuroinflammation contributes to epileptogenesis after SE, stroke, Alzheimer’s disease, and traumatic brain injury, and the occurrence of seizures in chronic epilepsy [117,118,119,120,121,122,123]. Moreover, through activation of mTOR pathway, CBD can reduce glutamate release and diminish seizure activity [124]. Additional mechanisms such as activation of peroxisome proliferator-activated receptor-gamma and inhibition of the release of nitric oxide and interleukin-1 beta may also be involved [125]. Thus, it is plausible that CBD eases epileptogenesis and related comorbidities after SE through multi-pronged actions described above.
Conclusions and Future Studies
CBD has promise for inhibiting SE and SE-induced epileptogenic modifications through antiseizure and antiepileptogenic effects, based on studies in animal models and its efficacy for reducing seizures in patients with refractory epilepsy. However, a recent case report using CBD whole plant extract (cannabidiol oil) failed to show beneficial effects in a patient with super refractory status epilepticus [126]. Considering these, detailed studies on CBD treatment in distinct animal models of SE are urgently needed to fully understand its efficacy as an effective neuroprotective and antiepileptogenic drug. These may encompass CBD treatment initiating at different time points after SE for comparing the effectiveness of early intervention versus delayed intervention stratagems. Additionally, studying CBD treatment continuing for variable durations after SE in immediate, latent, and chronic phases after SE will be critical to recognize its effects on diverse facets of epileptogenesis and its proficiency to block chronic epilepsy development after SE-induced brain injury. Likewise, the underlying mechanisms by which CBD curbs epileptogenic changes need to be investigated in more detail.
References
Trinka E, Kalviainen R (2017) 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure 44:65–73. https://doi.org/10.1016/j.seizure.2016.11.001
Fountain NB (2000) Status epilepticus: risk factors and complications. Epilepsia 41(s2):S23–S30. https://doi.org/10.1111/j.1528-1157.2000.tb01521.x
Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA (1998) Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology 50(3):735–741. https://doi.org/10.1212/WNL.50.3.735
Menon R, Radhakrishnan A, Radhakrishnan K (2013) Status epilepticus. J Assoc Physicians India 61(8 Suppl):58–63
Betjemann JP, Lowenstein DH (2015) Status epilepticus in adults. Lancet Neurol 14(6):615–624. https://doi.org/10.1016/S1474-4422(15)00042-3
Sanchez S, Rincon F (2016) Status epilepticus: epidemiology and public health needs. J Clin Med 5(8):71. https://doi.org/10.3390/jcm5080071
Trinka E, Brigo F, Shorvon S (2016) Recent advances in status epilepticus. Curr Opin Neurol 29:189–198
Loscher W (2017) The search for new screening models of pharmacoresistant epilepsy: is induction of acute seizures in epileptic rodents a suitable approach? Neurochem Res 42(7):1926–1938. https://doi.org/10.1007/s11064-016-2025-7
Hattiangady B, Kuruba R, Shetty AK (2011) Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis 2:1–17
Mishra V, Shuai B, Kodali M, Shetty GA, Hattiangady B, Rao X, Shetty AK (2015) Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci Rep 5(17807)
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17(10):3727–3738
Pauletti A, Terrone G, Shekh-AhmadT SA, Ravizza T, Rizzi M et al (2017) Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 140(7):1885–1899. https://doi.org/10.1093/brain/awx117
Rao MS, Hattiangady B, Reddy DS, Shetty AK (2006) Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res 83(6):1088–1105. https://doi.org/10.1002/jnr.20802
Rao MS, Hattiangady B, Shetty AK (2008) Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus 18(9):931–944. https://doi.org/10.1002/hipo.20449
Pitkänen A, Engel J Jr (2014) Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 11:231–241
Hattiangady B, Rao MS, Shetty AK (2004) Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis 17(3):473–490. https://doi.org/10.1016/j.nbd.2004.08.008
Hattiangady B, Shetty AK (2010) Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus 20(1):97–112. https://doi.org/10.1002/hipo.20594
Rotheneichner P, Marschallinger J, Couillard-Despres S, Aigner L (2013) Neurogenesis and neuronal regeneration in status epilepticus. Epilepsia 54:40–42. https://doi.org/10.1111/epi.12274
Scharfman HE, Gray WP (2007) Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia 48(s2):33–41. https://doi.org/10.1111/j.1528-1167.2007.01065.x
Shetty AK (2014) Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav 38:117–124. https://doi.org/10.1016/j.yebeh.2013.12.001
Buckmaster PS (2014) Does mossy fiber sprouting give rise to the epileptic state? Adv Exp Med Biol 813:161–168. https://doi.org/10.1007/978-94-017-8914-1_13
Koyama R (2016) Dentate circuitry as a model to study epileptogenesis. Biol Pharm Bull 39(6):891–896. https://doi.org/10.1248/bpb.b16-00125
Shetty AK, Zaman V, Hattiangady B (2005) Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci 25:8391–8401
Shetty AK, Turner DA (1997) Fetal hippocampal cells grafted to kainate-lesioned CA3 region of adult hippocampus suppress aberrant supragranular sprouting of host mossy fibers. Exp Neurol 143(2):231–245. https://doi.org/10.1006/exnr.1996.6363
Shetty AK, Turner DA (1999) Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol 414(2):238–254. https://doi.org/10.1002/(SICI)1096-9861(19991115)414:2<238::AID-CNE7>3.0.CO;2-A
Shetty AK, Turner DA (2000) Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci 20(23):8788–8801
Shetty AK, Hattiangady B, Rao MS (2009) Vulnerability of hippocampal GABA-ergic interneurons to kainate-induced excitotoxic injury during old age. J Cell Mol Med 13:2408–2423
Marx M, Haas CA, Haussler U (2013) Differential vulnerability of interneurons in the epileptic hippocampus. Front Cell Neurosci 7(167). https://doi.org/10.3389/fncel.2013.00167
Buckmaster PS, Abrams E, Wen X (2017) Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy. J Comp Neurol 525:2592–2610
Szczurowska E, Mares P (2013) NMDA and AMPA receptors: development and status epilepticus. Physiol Res 62:S21–S38
Scharfman HE, Brooks-Kayal AR (2014) Is plasticity of GABAergic mechanisms relevant to epileptogenesis? Adv Exp Med Biol 813:133–150. https://doi.org/10.1007/978-94-017-8914-1_11
Qian F, Tang FR (2016) Metabotropic glutamate receptors and interacting proteins in epileptogenesis. Curr Neuropharmacol 14(5):551–562. https://doi.org/10.2174/1570159X14666160331142228
Coulter DA, Steinhauser C (2015) Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med 5(3):a022434. https://doi.org/10.1101/cshperspect.a022434
Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62:121–131. https://doi.org/10.1016/j.freeradbiomed.2013.02.002
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7(1):31–40. https://doi.org/10.1038/nrneurol.2010.178
Vezzani A, Lang B, Aronica E (2015) Immunity and inflammation in epilepsy. Cold Spring Harb Perspect Med 6:a022699
Orcinha C, Munzner G, Gerlach J, Kilias A, Follo M, Egert U, Haas CA (2016) Seizure-induced motility of differentiated dentate granule cells is prevented by the central Reelin fragment. Front Cell Neurosci 10:183
Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A et al (2013) International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54(7):1315–1329. https://doi.org/10.1111/epi.12220
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, di Marzo V et al (2014) Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55(6):791–802. https://doi.org/10.1111/epi.12631
Peixoto-Santos JE, Velasco TR, Galvis-Alonso OY, Araujo D, Kandratavicius L, Assirati JA, Carlotti CG, Scandiuzzi RC et al (2015) Temporal lobe epilepsy patients with severe hippocampal neuron loss but normal hippocampal volume: extracellular matrix molecules are important for the maintenance of hippocampal volume. Epilepsia 56(10):1562–1570. https://doi.org/10.1111/epi.13082
Rodrigues GR, Kandratavicius L, Peixoto-Santos JE, Monteiro MR, Gargaro AC, Geraldi Cde V, Velasco TR, Leite JP (2015) Increased frequency of hippocampal sclerosis ILAE type 2 in patients with mesial temporal lobe epilepsy with normal episodic memory. Brain 138(6):e359. https://doi.org/10.1093/brain/awu340
Abel EL (1980) Marihuana: the first twelve thousand years. Plenum Press, New York. https://doi.org/10.1007/978-1-4899-2189-5
Brill H (1981) Marihuana: the first twelve thousand years. J Psychoactive Drugs 13(4):397–398. https://doi.org/10.1080/02791072.1981.10471902
Solimini R, Rotolo MC, Pichini S, Pacifici R (2017) Neurological disorders in medical use of cannabis: an update. CNS Neurol Disord Drug Targets 16(5):527–533. https://doi.org/10.2174/1871527316666170413105421
Steenkamp MM, Blessing EM, Galatzer-Levy IR, Hollahan LC, Anderson WT (2017) Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: a literature review. Depress Anxiety 34(3):207–216. https://doi.org/10.1002/da.22596
Tkaczyk M, Florek E, Piekoszewski W (2012) Marihuana and cannobinoids as medicaments. Przegl Lek 69:1095–1097
ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A (2017) Phytochemistry of Cannabis sativa L. Prog Chem Org Nat Prod 103:1–36. https://doi.org/10.1007/978-3-319-45541-9_1
Elsohly MA, Slade D (2005) Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci 78(5):539–548. https://doi.org/10.1016/j.lfs.2005.09.011
Nickels K (2017) Cannabidiol in patients with intractable epilepsy due to TSC: a possible medication but not a miracle. Epilepsy Curr 17(2):91–92. https://doi.org/10.5698/1535-7511.17.2.91
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34(5):605–613
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87(5):1932–1936. https://doi.org/10.1073/pnas.87.5.1932
Lupica CR, Hu Y, Devinsky O, Hoffman AF (2017) Cannabinoids as hippocampal network administrators. Neuropharmacology 124:25–37. https://doi.org/10.1016/j.neuropharm.2017.04.003
Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29(7):2053–2063. https://doi.org/10.1523/JNEUROSCI.4212-08.2009
Chesher GB, Jackson DM, Malor RM (1975) Interaction of delta 9-tetrahydrocannabinol and cannabidiol with phenobarbitone in protecting mice from electrically induced convulsions. J Pharm Pharmacol 27(8):608–609. https://doi.org/10.1111/j.2042-7158.1975.tb09515.x
Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R (1982) Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol 83(3-4):293–298. https://doi.org/10.1016/0014-2999(82)90264-3
Hosseinzadeh M, Nikseresht S, Khodagholi F, Naderi N, Maghsoudi N (2016) Cannabidiol post-treatment alleviates rat epileptic-related behaviors and activates hippocampal cell autophagy pathway along with antioxidant defense in chronic phase of pilocarpine-induced seizure. J Mol Neurosci 58:432–440
Izquierdo I, Orsingher OA, Berardi AC (1973) Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia 28(1):95–102. https://doi.org/10.1007/BF00413961
Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, Weston SE (2012) Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 21(5):344–352. https://doi.org/10.1016/j.seizure.2012.03.001
Rosenberg EC, Patra PH, Whalley BJ (2017) Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav 70(Pt B):319–327. https://doi.org/10.1016/j.yebeh.2016.11.006
Mao K, You C, Lei D, Zhang H (2015) High dosage of cannabidiol (CBD) alleviates pentylenetetrazole-induced epilepsy in rats by exerting an anticonvulsive effect. Int J Clin Exp Med 15:8820–8827
Kaplan JS, Stella N, Catterall WA, Westenbroek RE (2017) Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A 114(42):11229–11234. https://doi.org/10.1073/pnas.1711351114
Alvarez FJ, Lafuente H, Rey-Santano MC, Mielgo VE, Gastiasoro E, Rueda M (2008) Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res 64(6):653–658. https://doi.org/10.1203/PDR.0b013e318186e5dd
Do Val-da Silva RA, Peixoto-Santos JE, Kandratavicius L, De Ross JB, Esteves I, De Martinis BS, Alves MNR, Scandiuzzi RC et al (2017) Protective effects of cannabidiol against seizures and neuronal death in a rat model of mesial temporal lobe epilepsy. Front Pharmacol 8(131)
Porter BE, Jacobson C (2013) Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav 29(3):574–577. https://doi.org/10.1016/j.yebeh.2013.08.037
Hussain SA, Zhou R, Jacobson C, Weng J, Cheng E, Lay J, Hung P, Lerner JT et al (2015) Perceived efficacy of cannabidiol-enriched cannabis extracts fortreatment of pediatric epilepsy: a potential role for infantile spasms andLennox-Gastaut syndrome. Epilepsy Behav 47:138–141. https://doi.org/10.1016/j.yebeh.2015.04.009
Press CA, Knupp KG, Chapman KE (2015) Parental reporting of response to oralcannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 45:49–52. https://doi.org/10.1016/j.yebeh.2015.02.043
Tzadok M, Uliel-Siboni S, Linder I, Kramer U, Epstein O, Menascu S, Nissenkorn A, Yosef OB et al (2016) CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure 35:41–44. https://doi.org/10.1016/j.seizure.2016.01.004
Aguirre-Velázquez CG (2017) Report from a survey of parents regarding the use of cannabidiol (Medicinal cannabis) in Mexican children with refractory epilepsy. Neurol Res Int 2017(2985729):1–5. https://doi.org/10.1155/2017/2985729
Suraev AS, Todd L, Bowen MT, Allsop DJ, McGregor IS, Ireland C, Lintzeris N (2017) An Australian nationwide survey on medicinal cannabis use for epilepsy: history of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav 70(Pt B):334–340. https://doi.org/10.1016/j.yebeh.2017.02.005
Treat L, Chapman KE, Colborn KL, Knupp KG (2017) Duration of use of oral cannabis extract in a cohort of pediatric epilepsy patients. Epilepsia 58(1):123–127. https://doi.org/10.1111/epi.13617
Mathern GW, Beninsig L, Nehlig A (2015) Fewer specialists support using medical marijuana and CBD in treating epilepsy patients compared with other medical professionals and patients: result of Epilepsia’s survey. Epilepsia 56:1–6
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R et al (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 15(3):270–278. https://doi.org/10.1016/S1474-4422(15)00379-8
Hess EJ, Moody KA, Geffrey AL, Pollack SF, Skirvin LA, Bruno PL, Paolini JL, Thiele EA (2016) Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 57(10):1617–1624. https://doi.org/10.1111/epi.13499
Kaplan EH, Offermann EA, Sievers JW, Comi AM (2017) Cannabidiol treatment for refractory seizures in Sturge-Weber syndrome. Pediatr Neurol 71:18–23. https://doi.org/10.1016/j.pediatrneurol.2017.02.009
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA et al (2017) Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 376(21):2011–2020. https://doi.org/10.1056/NEJMoa1611618
Ridler C (2017) Epilepsy: cannabidiol reduces seizure frequency in Dravet syndrome. Nat Rev Neurol 13(7):383. https://doi.org/10.1038/nrneurol.2017.86
O’Connell BK, Gloss D, Devinsky O (2017) Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav 70:341–348
Warren PP, Bebin EM, Nabors LB, Szaflarski JP (2017) The use of cannabidiol for seizure management in patients with brain tumor-related epilepsy. Neurocase 23(5-6):287–291. https://doi.org/10.1080/13554794.2017.1391294
Saade D, Joshi C (2015) Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol 52:544–547
Crippa JA, Crippa AC, Hallak JE, Martín-Santos R, Zuardi AW (2016) Δ9-THC intoxication by cannabidiol-enriched cannabis extract in two children with refractory epilepsy: full remission after switching to purified cannabidiol. Front Pharmacol 7(359). https://doi.org/10.3389/fphar.2016.00359
Verrotti A, Carrozzino D, Milioni M, Minna M, Fulcheri M (2014) Epilepsy and its main psychiatric comorbidities in adults and children. J Neurol Sci 343:23–29
Rao G, Mashkouri S, Aum D, Marcet P, Borlongan CV (2017) Contemplating stem cell therapy for epilepsy-induced neuropsychiatric symptoms. Neuropsychiatr Dis Treat 13:585–596
Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D (2017) Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia 58:96–100
Gomes FV, Resstel LB, Guimarães FS (2011) The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology 213(2-3):465–473. https://doi.org/10.1007/s00213-010-2036-z
Klein BD, Jacobson CA, Metcalf CS, Smith MD, Wilcox KS, Hampson AJ, Kehne JH (2017) Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res 42(7):1939–1948. https://doi.org/10.1007/s11064-017-2287-8
Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA, Laux L, Marsh ED (2017) Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol 32(1):35–40. https://doi.org/10.1177/0883073816669450
Geffrey AL, Pollack SF, Bruno PL, Thiele EA (2015) Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 56:246–251
Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP (2017) UAB CBD program interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 58(9):1586–1592. https://doi.org/10.1111/epi.13852
Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O (2015) Cannabinoids and epilepsy. Neurotherapeutics 12(4):747–768. https://doi.org/10.1007/s13311-015-0375-5
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90. https://doi.org/10.1016/0006-2952(95)00109-D
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215(1):89–97. https://doi.org/10.1006/bbrc.1995.2437
Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L et al (2015) Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem 290(14):8711–8721. https://doi.org/10.1074/jbc.M114.618447
Vilela LR, Lima IV, Kunsch ÉB, Pinto HPP, de Miranda AS, Vieira ÉLM, de Oliveira ACP, Moraes MFD et al (2017) Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav 75:29–35. https://doi.org/10.1016/j.yebeh.2017.07.014
Llano I, Marty A, Armstrong CM, Konnerth A (1991) Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol 434(1):183–213. https://doi.org/10.1113/jphysiol.1991.sp018465
Pitler TA, Alger BE (1992) Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci 12(10):4122–4132
Katona I, Freund TF (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14(9):923–930. https://doi.org/10.1038/nm.f.1869
Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558
Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL (2004) Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci 24(1):53–62. https://doi.org/10.1523/JNEUROSCI.4503-03.2004
Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26(11):2991–3001. https://doi.org/10.1523/JNEUROSCI.4872-05.2006
Marsicano G, Lutz B (2006) Neuromodulatory functions of the endocannabinoid system. J Endocrinol Investig 29(3 Suppl):27–46
Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, Stephens GJ (2010) Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther 332(2):569–577. https://doi.org/10.1124/jpet.109.159145
Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH (1998) Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther 285(1):285–292
Sylantyev S, Jensen TP, Ross RA, Rusakov DA (2013) Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A 110(13):5193–5198. https://doi.org/10.1073/pnas.1211204110
Shirazi-zand Z, Ahmad-Molaei L, Motamedi F, Naderi N (2013) The role of potassium BK channels in anticonvulsant effect of cannabidiol in pentylenetetrazole andmaximal electroshock models of seizure in mice. Epilepsy Behav 28(1):1–7. https://doi.org/10.1016/j.yebeh.2013.03.009
Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N, Cummins TR (2016) Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain 139:2164–2181
Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, Stephens GJ, Whalley BJ (2014) Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett 30:269–274
Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E et al (2014) Non psychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci 19:1131–1141
Carrier EJ, Auchampach JA, Hillard CJ (2006) Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 103(20):7895–7900. https://doi.org/10.1073/pnas.0511232103
Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi RN, Cunha RA, Kofalvi A (2011) Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol 655(1-3):38–45. https://doi.org/10.1016/j.ejphar.2011.01.013
De Petrocellis L, Di Marzo V (2010) Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J NeuroImmune Pharmacol 5(1):103–121. https://doi.org/10.1007/s11481-009-9177-z
Booz GW (2011) Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 51(5):1054–1061. https://doi.org/10.1016/j.freeradbiomed.2011.01.007
Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX et al (2013) Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology 52:1079–1087
Hill TD, Cascio MG, Romano B, Duncan M, Pertwee RG, Williams CM, Whalley BJ, Hill AJ (2013) Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol 170(3):679–692. https://doi.org/10.1111/bph.12321
Vilela LR, Gomides LF, David BA, Antunes MM, Diniz AB, Moreira Fde A, Menezes GB (2015) Cannabidiol rescues acute hepatic toxicity and seizure induced by cocaine. Mediat Inflamm. https://doi.org/10.1155/2015/523418
Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, Khan S, Khalifa Y (2008) Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci 49(12):5526–5531. https://doi.org/10.1167/iovs.08-2196
Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C (2012) Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 28:e331
Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24
Shimada T, Takemiya T, Sugiura H, Yamagata K (2014) Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat Inflamm 2014:1–8. https://doi.org/10.1155/2014/901902
Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV (2015) Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 46: 2616–2627
Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat 11:97–106. https://doi.org/10.2147/NDT.S65815
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783. https://doi.org/10.1126/science.aag2590
Long Q, Upadhya D, Kim DK, Hattiangady B, An SY, Prockop DJ, Shetty AK (2017) Intranasal MSC-derived A1-exosomes ease inflammation, and preventabnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A 114:E3536–E3545
Rubio M, Valdeolivas S, Piscitelli F, Verde R, Satta V, Barroso E, Montolio M, Aras LM et al (2016) Analysis of endocannabinoid signaling elements and related proteins in lymphocytes of patients with Dravet syndrome. Pharmacol Res Perspect 4(00220):e00220. https://doi.org/10.1002/prp2.220
Gobira PH, Vilela LR, Gonçalves BD, Santos RP, de Oliveira AC, Vieira LB et al (2015) Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: Possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology 50:116–121. https://doi.org/10.1016/j.neuro.2015.08.007
Esposito G, Scuderi C, Savani C, Steardo L Jr, De Filippis D, Cottone P et al (2007) Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 151(8):1272–1279. https://doi.org/10.1038/sj.bjp.0707337
Rosemergy I, Adler J, Psirides A (2016) Cannabidiol oil in the treatment of super refractory status epilepticus. A case report. Seizure 35:56–58
Acknowledgments
The authors are supported by grants from the Department of Defense (CDMRP W81XWH-14-1-0558 to A.K.S.) and the Department of Veterans Affairs (VA Merit Award grant I01BX000883 and VA-BLR&D Research Career Scientist award, 1IK6BX003612 to A.K.S.). Olagide W Castro was supported by a Visiting Scientist Award from CAPES Foundation, Ministry of Education, Government of Brazil (O.W.C).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Upadhya, D., Castro, O.W., Upadhya, R. et al. Prospects of Cannabidiol for Easing Status Epilepticus-Induced Epileptogenesis and Related Comorbidities. Mol Neurobiol 55, 6956–6964 (2018). https://doi.org/10.1007/s12035-018-0898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0898-y