Abstract
Parkinson’s disease (PD) is complex neurological disorder and is prevalent in the elderly population. This is primarily due to loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) region of the brain. The modulators of the selective loss of dopaminergic neurons in PD are still not well understood. The small non-coding RNAs specifically miRNAs fine-tune the protein levels by post-transcriptional gene regulation. The role of miRNAs in PD pathogenesis is still not well characterized. In the current study, we identified the miRNA expression pattern in 6-OHDA-induced PD stress condition in SH-SY5Y, dopaminergic neuronal cell line. The targets of top 5 miRNAs both up- and down regulated were analyzed by using StarBase. The putative pathways of identified miRNAs included neurotrophin signaling, neuronal processes, mTOR, and cell death. The level of miR-5701 was significantly downregulated in the presence of 6-OHDA. The putative targets of miR-5701 miRNA include genes involved in lysosomal biogenesis and mitochondrial quality control. The transfection of miR-5701 mimic decreased the transcript level of VCP, LAPTM4A, and ATP6V0D1. The expression of miR-5701 mimic induces mitochondrial dysfunction, defect in autophagy flux, and further sensitizes SH-SY5Y cells to 6-OHDA-induced cell death. To our knowledge, the evidence in the current study demonstrated the dysregulation of specific pattern of miRNAs in PD stress conditions. We further characterized the role of miR-5701, a novel miRNA, as a potential regulator of the mitochondrial and lysosomal function determining the fate of neurons which has important implication in the pathogenesis of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a second most common neurodegenerative movement disorder in elderly population. This is clinically characterized by resting tremor, rigidity, bradykinesia, and postural instability due to preferential loss of dopamine-producing neurons in the substantia nigra pars compacta region of the midbrain [1]. The other pathological hallmark of PD is the presence of intracytoplasmic proteinaceous deposits termed as Lewy bodies (LBs) and dystrophic neurites (Lewy neurites) in surviving neurons. These aggregates consist of fibrillar α-synuclein, molecular chaperones, ubiquitin, and neurofilaments [2]. The mechanisms of further progression and selective loss of dopaminergic neurons in PD had been a focus of research for the last several years; however, it is still not well understood. There is no effective therapy, and dopamine (DA) supplementation only provides symptomatic relief. It is important to indentify the modulators of selective neuronal loss in PD to find the next generation of therapeutic strategies.
Now there are established evidences suggesting mitochondrial dysfunction is one of the major causative factors of PD [3,4,5]. The first evidence of association of mitochondrial dysfunction with PD came from the observation of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP), which causes symptoms of PD during drug abuse and produces severe parkinsonian syndrome in various animal models. The drug decreases complex 1 activity, leading to ATP depletion, increased level of reactive oxygen species (ROS) [6] and cell death [6]. The dysfunction of mitochondria is observed in tissue derived from PD patient samples as well as the high level of mtDNA deletion was observed in SN neurons from PD patients [3,4,5]. These evidences strongly suggest that regulation mitochondrial homeostasis is critical in maintenance of neuronal health. The mitochondrial homeostasis is maintained through the process of dynamic fusion and fission [7]. The stressed or damaged mitochondria is labeled through ubiquitination and selectively degraded through process of autophagy, also known as mitophagy [7, 8]. Different genome-wide studies suggest that mutations in genes encoding PINK1 (PTEN-induced kinase 1) and Parkin (E3 ubiquitin ligase) is prevalent in familial PD and is associated with mitochondrial function and quality control through mitophagy [9, 10]. This further provided support to the hypothesis of mitochondria being central mediator of PD. The protein aggregates and defective mitochondria observed in PD conditions are targeted to lysosome for degradation hence regulation of autophagy flux and lysosomal function is critical for neuronal health and survival [11]. The regulators of mitochondrial function, autophagy flux, and its implication in PD still need to be further elucidated.
Small non-coding RNAs, specifically miRNAs, play an important role in the regulation of mRNA copy number and protein level in the narrow physiological range [12]. Our group previously demonstrated the association of miRNAs with mitochondria and their role in the regulation of mitochondrial function [13, 14]. The association and translocation of nuclear genome-encoded mRNA/miRNAs to mitochondria is one of the emerging mechanisms to regulate the mitochondrial functions [15, 16]. The role of miRNAs in regulation of different steps of autophagy and mitochondrial homeostasis and its implication in PD stress condition is not well understood.
In the current study, we systematically identified differentially expressed miRNAs in 6-OHDA-induced PD stress condition in DAergic neuronal cell (SH-SY5Y cell line). Interestingly, miR-5701 is significantly downregulated in 6-OHDA-induced PD stress condition. The evidences in the study suggest the role miR-5710 in the regulation of mitochondrial complex I activity, lysosomal function, autophagy flux, and sensitization to 6-OHDA-induced neuronal cell death.
Materials and Methods
Cells and Reagents
SH-SY5Y cells were grown at 37 °C, 5% CO2 in Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 (DMEM/F12, Gibco, Invitrogen) supplemented with 15% (v/v) heat-inactivated fetal bovine serum (Gibco, Invitrogen) and 1% penicillin, streptomycin, and neomycin (PSN) antibiotic mixture (Gibco, Invitrogen). mCherry-p62-GFP was provided by Dr. T. Yoshimori (National Institute of Genetics, Shizuoka, Japan) [17]. The primary antibodies used were: rabbit polyclonal against VCP (Cell Signaling, USA), caspase-3 (Cell Signaling, USA), PARP (Cell Signaling, USA), LC3 (Sigma, USA), and β-actin (Abcam, USA). Secondary antibodies: HRP-conjugated anti-rabbit and anti-mouse antibodies (Open Biosystems, USA) were used in the study. 6-OHDA, N-acetyl-cysteine, antimycin A, sodium azide, decylubiquinone, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT), acridine orange, and propidium iodide were purchased from Sigma-Aldrich, USA. SYBR green from Takara (Japan), first-strand cDNA kit, miRCURY miRNA isolation kit from Exiqon (Denmark), and ATP assay kit from ThermoFisher Scientific (USA).
Microarray
SH-SY5Y cells were plated at the density of 5 × 105 cells/per well in 6 well plate. After 24 h of culture, the cells were treated with indicated chemicals for 24 h. The RNA integrity was determined using Agilent 2100 Bioanalyzer (Ambion, USA). The RNAs from both sample and reference were labeled with Hy3™ and Hy5™ fluorescent label, respectively, using the miRCURY LNA™ microRNA Hi-Power Labeling Kit, Hy3™/Hy5™ (Exiqon, Denmark) following the procedure described by the manufacturer. The Hy3™-labeled samples and Hy5™-labeled reference RNA sample were mixed pair-wise and hybridized to the miRCURY LNA™ microRNA Array 7th Gen (Exiqon, Denmark), which contained capture probes targeting all miRNAs for human registered in the miRBASE 18.0. The quantified signals were background corrected (Normexp with offset value 10) and normalized using the global Lowess (LOcally WEighted Scatterplot Smoothing) regression algorithm.
Cell Death Assay
The cell viability was analyzed by MTT assay and acridine/propidium iodide staining. SH-SY5Y cells were plated at the density of 1 × 104 cells/per well in 96 well plate. After 24 h of culture, the cells were treated with indicated chemicals. MTT assay and acridine/propidium iodide staining were performed as described previously [18].
Confocal Microscopy
For confocal microscopy, SH-SY5Y cells were plated at the density of 1.5 × 105 cells per well in 24 well plate having coverslip and co-transfected with mcherry-p62-GFP and miR-5701 mimic using Lipofectamine® RNAiMAX (Thermofisher, USA). After 24 h of transfection, autophagy flux was monitored using Leica TCS-SP5-II confocal microscope (Germany).
Analysis of Reactive Oxygen Species (ROS) and LysoSensor Staining
SH-SY5Y cells were plated at the density of 1.5 × 105 cells per well in 24 well plate and transfected with miR-5701 mimic and control using Lipofectamine® RNAiMAX (ThermoFisher Scientific, USA). After 24 h of transfection, cells were treated with 6-OHDA for 12 h and ROS was quantified as described previously [18].
LysoSensor staining was performed using LysoSensor™ Green DND-189 (ThermoFisher Scientific, USA) as per manufacturer's protocol. Fluorescence intensity was measured using the Fluorescence Spectrophotometer F-7000 (Hitachi, Japan) at Excitation 440 and Emission 510 and images were capture using Leica TCS-SP5-II confocal microscope (Germany).
Analysis of Complex I Activity
Complex I activity was determined by BN-PAGE (Blue Native Page) and spectrophotometrically. SH-SY5Y cells were plated at the density of 2 × 106 cells per well in 100 mm dish and transfected with miR-5701 mimic and control using Lipofectamine® RNAiMAX (ThermoFisher Scientific, USA). BN-PAGE and spectrophotometric assay were performed as described previously [18].
Biotin-Tagged miRNA Pull-Down and Target Validation
The targets of miR-5701 were determined by biotin pull-down as described previously [19] with minor modifications. Briefly, SH-SY5Y cells were transfected with synthetic biotin-labeled miR-5701 duplexes (custom designed from Exiqon, Denmark) and control oligo using HiPerFect Transfection Reagent (QIAGEN, Melbourne, VIC, Australia). After 24 h, cells were harvested and lysed in hypotonic lysis buffer (10 mM KCl, 1.5 mM MgCl2, 10 mM Tris-Cl pH 7.5, 5 mM DTT, 0.5% NP-40, 60 U/ML SUPERase•In (Ambion, Austin, TX, USA), and 1× protease inhibitor (Roche, Brisbane, QLD, Australia). Cell lysate was centrifuged at 10,000g at 4 °C for 2 min, and supernatant was collected and calibrated to 1 M NaCl in fresh vial and further incubated with myOne C1 Dynabeads (ThermoFisher Scientific, USA). The beads were washed and pre-blocked with 1 μg/μl bovine serum albumin and 1 μg/μl yeast tRNA (ThermoFisher Scientific, USA) prior of incubation. The beads were washed again three times with hypotonic lysis buffer and 1 M NaCl and RNA extracted for target identification.
ATP Luciferase Assay
ATP luciferase was performed by using ATP Bioluminescence kit CLS II (ThermoFisher Scientific, USA). SH-SY5Y cells were plated at the density of 1 × 105 cells per well in 12 well plate and transfected with miR-5701 mimic and control using Lipofectamine® RNAiMAX (ThermoFisher Scientific USA). ATP luciferase was performed as described previously [18].
miRNA Isolation and Analysis by qRT-PCR
SH-SY5Y cells were plated at the density of 5 × 105 cells/well in 6 well plate and treated with 6-OHDA for 24 h. miRNA isolation and analysis by qRT-PCR were performed as described previously [18].
Target Analysis
The validated miRNAs were categorized into up- and downregulated columns based on the qPCR results. The target pathways of selected miRNA function were determined by selecting all miRNAs of each category, the combination of all five target prediction tools, and ClipSeq with low stringency and corrected p value <0.05 [20]. The GO terms and pathways were retrieved for each category and tabulated.
Statistical Analysis
Data are shown as mean ± SEM for the number of observations. Comparisons of groups were performed using Student’s t test for repeated measurements to determine the levels of significance for each group. Each experiment has been repeated minimum two times independently and probability values of p < 0.05 were considered as statistically significant.
Result
miRNAs Levels Are Altered in 6-OHDA-Induced PD Stress Model of SH-SY5Y
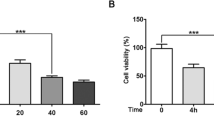
The neurotoxin, 6-hydroxydopamine (6-OHDA), induces apoptosis of DAergic neurons and PD-like conditions in experimental animal models [21]. SH-SY5Y cells express tyrosine hydroxylase and dopamine transporter and can preferentially uptake 6-OHDA [22]. The toxin accumulates in the cytosol and inhibits the mitochondrial respiratory chain complexes I and IV activity, generating ROS leading to cell death [22]. SH-SY5Y cells were treated with different concentration of 6-OHDA, and cell survival was analyzed using MTT (Fig. 1a) and acridine/propidium iodide staining (Fig. S1). It was observed that low-dose (25 and 50 μM) of 6-OHDA induced 25–30% cell death in 24 h. SH-SY5Y cells treated with 100 μM of 6-OHDA showed approximately 65% cell death (necrotic) in 24 h. The concentration of 75 μM of 6-OHDA induced 50% cell death; hence, it was selected for further studies.
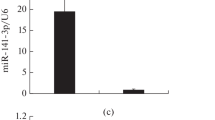
miRNAs levels are altered in 6-OHDA-induced PD stress model of SH-SY5Y. a SH-SY5Y cells were treated with different concentration of 6-OHDA for 24 h and MTT assay was performed. b List of differentially expressed miRNAs in 6-OHDA (75 μM/ml for 24 h) treated conditions analyzed from microarray data. c, d The expression levels of miRNAs which downregulated/upregulated in microarray analysis were determined using 5S rRNA as endogenous control by qPCR. Asterisk (*) indicates that p value <0.05 for SEM
We analyzed the miRNA expression pattern in SH-SY5Y cells treated with 6-OHDA using miRCURY™ array that contained all miRNAs present in the miRBase 18.0. The miRNAs were broadly classified into two categories: upregulated and downregulated upon 6-OHDA treatment (Fig. 1b). The microarray results were further validated by quantitative real-time PCR. It was observed that miRNAs: miR-5701, miR-34a, miR-125a-5p, and miR-92a-3p were significantly downregulated (Fig. 1c), whereas the levels of miR-1287 significantly increased in the presence of 6-OHDA (Fig. 1d). The role of these miRNAs has not yet been characterized in pathogenesis of PD.
miR-5701 Targets the Genes Involved in Lysosomal Degradation Pathways
To identify the putative targets of validated miRNAs and their biological significance, we used starBase v.2.0 and DAVID annotation platform respectively [20, 23]. The putative targets of upregulated miRNAs were involved in regulation of neurotrophin signaling, axonal guidance, and calcium signaling pathway (Fig. S2A). Similarly, the putative targets of downregulated miRNAs included target genes regulating neurotrophin signaling, mTOR signaling, cell cycle, and RNA degradation (Fig. S2B). The cellular processes targeted by identified miRNAs have important implications in the neuronal processes, cues, and progression of PD.
The putative target of miR-5701 included several genes involved in mitochondrial homeostasis, lysosomal degradation pathways (Table 1 and Table S1), autophagosome assembly, mitophagy, protein ubiquitination, mitochondrial fission, endocytosis, synapse assembly, and mitochondrial transport (Tables S1 & S2). Identified targets play crucial role in brain development, neuron projection morphogenesis, axonal guidance, and neuronal differentiation (Table S2). The putative targets of miR-5701 were localized in different sub-cellular sites like neuronal cell body, axon, neuron projection, cell cortex, and synapse (Table S3).
The expression of putative targets of miR-5701 was validated in miR-5701 mimic transfected in SH-SY5Y cells. The quantitative PCR analysis showed that transcript levels of VCP, LAPTM4A, and ATP6V0D1 significantly decreased in the presence of miR-5701 mimic (Fig. 2a–c). All these targets play important role in regulation of lysosomal functions. Interestingly, VCP has been recently shown to be important for mitochondrial function and quality control, lysosomal integrity, and function [24,25,26,27,28]; hence, we further focused on miR-5701-mediated regulation of VCP. SH-SY5Y cells were transfected with miR-5701 mimic, and VCP level was analyzed by Western blotting. The band of 97 kDa corresponding to VCP significantly decreased in the presence of miR-5701 mimic as compared to control (Fig. 2d). To confirm that VCP is the direct target of miR-5701, RNA immunoprecipitation using biotin-labeled-miR-5701 was performed. The transfection of biotin-labeled-miR-5701 in SH-SY5Y cells showed significant decrease in VCP transcript level as compared to control suggesting that biotin-labeled miR-5701 may bind to target mRNAs (Fig. 2e). RNA immunoprecipitation showed significant enrichment of VCP transcripts in streptavidin beads of miR-5701 as compared to negative control (Fig. 2f). This suggests that miR-5701 binds directly to VCP mRNA and negatively regulates its expression.
miR-5701 targets the genes involved in lysosomal degradation pathways. a–c SH-SY5Y cells were transfected with miR-5701 mimic, and the expression levels VCP, LAPTM4A, and ATP6V0D1 were determined by real-time PCR using GAPDH as endogenous control. d SH-SY5Y cells were transfected with miR-5701 mimic, and level of VCP was determined by Western blotting. e The levels VCP in presence of biotin-labeled miR-5701 was determined by real-time PCR using 16sRNA as endogenous control. f Biotin tagged miR-5701 was transfected in SH-SY5Y, and RNA IP was performed as described in the method. Fold enrichment analysis of VCP transcript in biotin-labeled miR-5701 was determined by qPCR. Asterisk (*) indicates that p value <0.05 for SEM
miR-5701 Modulates Mitochondrial Function
VCP, target of miR-5701, regulates mitochondrial function and quality control [27, 29]; hence, we further analyzed the role of miR-5701 in the regulation of mitochondrial function. SH-SY5Y cells were transfected with miR-5701 mimic and analyzed different mitochondrial parameters. The level of ATP significantly decreased miR-5701 mimic transfected cells (Fig. 3a) as compared to control. The transfection of miR-5701 increases ROS levels which further increased in presence of 6-OHDA (Fig. 3b). The activity of mitochondrial respiratory chain complexes regulates bioenergetics capacity and ROS levels in the cells [30]. The dysregulation of mitochondrial complex I results in leakage of electrons and is one of the primary sources of ROS; hence, we further analyzed its regulation by miR-5701. The cells were transfected with miR-5701 mimic, and complex I activity was determined by BN-PAGE and spectrophotometrically. The mitochondrial complex I activity decreases in the presence of miR-5701 as observed by in-gel activity staining (Fig. 3c) as well as spectrophotometrically as compared to control (Fig. 3d).
miR-5701 modulates mitochondrial function. a SH-SYSY cells were transfected with miR-5701 and ATP levels were measured by ATP-dependent luciferase activity. b SH-SYSY cells were transfected with miR-5701. After 24 h of transfection treated with 6-OHDA (75 μM/ml) for 12 h and ROS levels were analyzed using CM-H2DCFDA by spectrofluorometry. Complex I activity was measured after 24 h of miR-5701 transfection c by in-gel staining of Blue Native page of complex I and d by monitoring the decrease in absorbance of NADH at 340 nm using spectrophotometer. Asterisk (*) indicates that p value <0.05 for SEM
Mitochondrial complex I activity and assembly is regulated by mitochondrial-DNA encoded transcripts; hence, we further analyzed the role of miR-5701 in the regulation of mitochondrial number and mitochondrial DNA-encoded transcripts. Interestingly, the transfection of miR-5701 significantly increases all mitochondrial DNA (mt DNA) encoded transcripts except COX1 (Fig. 4a, b). The level of mitochondrial DNA-encoded transcripts further increased in the presence of 6-OHDA (Fig. S3). The mitochondrial DNA level was analyzed by quantification of a unique mitochondrial fragment relative to a single copy region of the nuclear gene RNaseP [31]. The unique region of the human mitochondrial sequence between positions 241 and 390 has been used in the study, which was previously shown to be “least similar” to any human nuclear genomic region [31]. Primers were designed to RNaseP and B2 M genes; the primers and amplicons were tested using blast to ensure that they were present only once in the nuclear genome and did not show similarity to any other regions of the genome. The cells were transfected with miR-5701, and mtDNA level was monitored by qPCR. The mtDNA levels increased significantly in presence of miR-5701 as compared to mimic control (Fig. 4c).
Expression of miR-5701 increases mitochondrial DNA and transcripts. a, b SH-SYSY cells were transfected with miR-5701, and relative expression level of mitochondrial DNA encoded transcripts were measured by qRT-PCR. c SH-SYSY cells were transfected with miR-5701, and relative mtDNA was monitored by qRT-PCR. d SH-SYSY cells were transfected with miR-5701, and relative expression level of NRF1 and NRF2 was measured by qRT-PCR. An asterisk (*) indicates that p value <0.05 for SEM
The defect in mitochondrial function leads to its degradation by mitophagy [32], and thereafter, mitochondrial biogenesis is initiated for maintaining a healthy population of mitochondria and cellular homeostasis [32]. Increased mtDNA and transcript levels in presence of miR-5701 suggest that mitochondrial biogenesis may be initiated. To confirm this, we analyzed the levels of transcription factors like NRF1 and NRF2, which regulate mitochondrial biogenesis. There was no change in the transcript of both transcriptional factors NRF1 and NRF2 in the presence of miR-5701 (Fig. 4d). This suggests that increased level miR-5701 in neuronal cells leads to accumulation of defective mitochondria and not mitochondrial biogenesis.
miR-5701 Regulates the Fusion of Autophagosome with Lysosome
The evidences here suggest that mitochondrial biogenesis is not initiated in the presence of miR-5701; hence, mitophagy may be defective. The earlier reports suggest that VCP plays role in autophagosome–lysosome fusion [33,34,35], and we observed here that VCP is a direct target of miR-5701; hence, autophagy was monitored in the presence of miR-5701. LC3 is conjugated to maturing autophagosome membrane with the help of ATG proteins and is targeted to lysosome [36] for degradation. Bafilomycin (BFA) is specific V-ATPase inhibitor and acts as an inhibitor of autophagosome and lysosome fusion and increases the pH of lysosome [37], leading to inhibition of lysosomal degradation; hence, it was used as positive control. The level of LC3-II significantly increased in presence of miR-5701 and was similar to the levels observed in presence of BFA (Fig. 5a), suggesting either defect in autophagy flux or lysosomal dysfunction. The autophagy flux was further confirmed by analyzing the turnover of p62 using tandem tag mCherry-GFP-p62 reporter. Interestingly, the number of orange puncta of p62 increased in presence of miR-5701 as compared to mimic control (Fig. 5b, c).This strongly suggests that increased level of miR-5701 inhibits the fusion of autophagosome to lysosome or lysosomal activity itself. In a recent study, autophagosome–lysosome fusion has been shown to play an essential role in the regulation of the lysosomal function [36]; hence, we monitored the lysosomal function in the presence of miR-5701 using LysoSensor staining. The LysoSensor staining decreases in the presence miR-5701 as compared to control (Figs. 5d and S4). These data collectively shows that miR-5701 regulates maturation of autophagolysosome and lysosomal function.
miR-5701 regulates the fusion of auotphoagosme with lysosome. a SH-SYSY cells were transfected with miR-5701. After 24 h of transfection treated with BAF for 4 h and LC3 level was analyzed by Western blotting. b SH-SY5Y were co-transfected miR-5701 and with mCherry-p62-GFP, and cells were monitored under confocal microscopy for red and orange puncta. Scale bar, 7.5 μm. c Graphical representations of the numbers red and orange p62 puncta in miR-5701 with control. d SH-SYSY cells were transfected with miR-5701 mimic, and lysosomal acidification was analyzed by LysoSensor Green staining. Asterisk (*) indicates that p value <0.05 for SEM
miR-5701 Sensitizes 6-OHDA-Induced Neuronal Cell Death
All the above experiments suggest the role of miR-5701 in regulation of mitochondrial and lysosomal cross talk to modulate mitophagy. The accumulation of defective mitochondria may sensitize SH-SY5Y cells to 6-OHDA-induced cell death. To study the effect of the miR-5701 on cell death, SH-SY5Y cells were transfected with miR-5701 mimic and treated with 6-OHDA and cell survival was analyzed by MTT assay. The expression of miR-5701 significantly increased 6-OHDA-induced cell death as compared to mimic control transfected cells (Fig. 6a). This was further confirmed by acridine orange/propidium iodide staining. The acridine orange/propidium iodide-positive cells significantly increased in miR-5701-transfected cells as compared to control mimic in presence of 6-OHDA, suggesting the cell death through apoptosis (Fig. S5). The induction of apoptosis was confirmed by monitoring caspase-3 activation by Western blotting. The increased level of 17/19 kDa band corresponding to cleaved subunit of caspase-3 was observed in miR-5701 transfected in the presence of 6-OHDA as compared to mimic control (Fig. 6b). Caspase 3/7 activity was also monitored by caspase 3/7 luciferase assay in the presence of miR-5701. The transfection of miR-5701 significantly increased caspase 3/7 activity in presence of 6-OHDA (Fig. 6c). As observed above, miR-5701 increases ROS level in normal and 6-OHDA stress condition; we further confirmed if ROS is essential for miR-5701-mediated cell death. SH-SY5Y cells were transfected with miR-5701 with mimic control and co-treated with 6-OHDA, and NAC and cell survival was monitored. The cell death in 6-OHDA stress condition in miR-5701 mimic transfected cell was rescued by NAC (Fig. 6d). Collectively, the evidences here suggest that miR-5701 can potentiate ROS level, caspase activation, and apoptosis in PD stress condition.
miR-5701 sensitizes 6-OHDA-induced neuronal cell death. a SH-SYSY cells were transfected with miR-5701 mimic. After 24 h of transfection, cells were treated with 6-OHDA for 16 h and MTT assay was performed. b SH-SYSY cells were transfected with miR-5701 mimic. After 24 h of transfection treated with 6-OHDA (75 μM/ml) for 16 h and caspase-3 cleavage was analyzed by Western blotting. c Caspase-3/7 activation analyzed by caspase-3/7 luciferase assay. SH-SYSY cells were transfected with miR-5701 mimic after 16 h of 6-OHDA treatment, and caspase activity was analyzed by caspase luciferase assay. d SH-SYSY cells were transfected with miR-5701 mimic. After 24 h of transfection, cells were co-treated with 6-OHDA and NAC for 16 h. MTT assay was performed to monitor cell survival. Asterisk (*) indicates that p value <0.05 for SEM
Discussion
The recent studies clearly suggest that miRNAs play an important role in regulation of copy number of target mRNAs in narrow physiological range to maintain the level of proteins at required physiological level. There had been progress in identification of many novel miRNAs by using next-generation sequencing technologies [38]; however, their cellular functions and implication in PD pathogenesis are not understood. Here, we systematically identified several new miRNAs which may regulate inter-organellar cross talk to regulate cell death role in PD pathogenesis.
The role of miRNAs is emerging in regulation of many neuronal functions including branching and synaptic remodeling [39,40,41,42]. In the current miRBase 21.0 latest releases, there are approximately 1881 precursors and 2588 mature miRNAs listed in Homo sapiens. The previous report studying the role of miRNAs in PD pathogenesis [43,44,45] have used a limited number of miRNAs probe. In the current study, we used miRNA array that had 1899 capture probes, covering all human annotated in miRBase 18.0. The experiment helped us to identify many novel miRNAs that have not been yet implicated in PD disease. Interestingly, we observed that miR-1287 was upregulated significantly whereas the levels of miR-5701, miR-34a, miR-125a-5p, and miR-92a-3p significantly decreased in 6-OHDA conditions. Furthermore, bioinformatics analysis shows that altered expression of these miRNAs may affect key pathways involved in PD pathogenesis like cell cycle, mRNA transport, proteasomal ubiquitin-dependent protein catabolism, mTOR signaling, neurotrophin signaling, axonal guidance, RNA degradation, calcium signaling, ubiquitination, ubiquitin proteasome system, and mitochondrial import. The further studies are required to elucidate the novel role of differentially expressed miRNAs in neuronal function and its implication in PD.
Interestingly, analysis of the targets of one of the miRNAs, miR-5701, suggest its important role in PD conditions as putative targets included genes involved in lysosomal biogenesis and mitochondrial quality control. We observed decreased expression of VCP, LAPTM4A, and ATP6V0D1 in the presence of miR-5701. In the present study, we identified VCP as the novel target of miR-5701 and its negative regulation by miR-5701 in the neuronal cell. VCP is a type II member of the ATPase protein and its mutation causes multisystem degenerative disease mainly affecting brain, muscle, and bone [28]. Interestingly, mutations also show Parkinsonism-like conditions suggestive of substantia nigra pathology [46, 47]. VCP regulates mitochondrial turnover through the selective form of autophagy called mitophagy which is an important cellular process involved in pathogenesis of PD. VCP/p97 binds to mitofusin-1 and mitofusin-2 following their ubiquitination by the E3 ligase Parkin on outer mitochondrial membrane [48]. VCP not only recognizes defective mitochondria, but it may further help in fusion of autophagosome containing mitochondria with lysosome for degradation [34, 35].
The major focus of all the ongoing studies had been on mitochondrial dysfunction during PD pathogenesis; however, their cross talk with other organelle specifically with lysosome had been still not well characterized. The contact between different organelle is essential for their optimal function. The recent report demonstrated that lysosome contact with mitochondria is the second most prominent after ER [49]. The inhibition of mitochondrial function by deleting the mitochondrial proteins like AIF, PINK1 as well as chemical inhibition of oxidative phosphorylation (OXPHOS) compromises lysosomal function leading to defective autophagy [50]. The impairment of respiration in T cells by knocking down mitochondrial transcription factor A (TFAM) showed reduced lysosomal calcium mobilization and impaired lysosomal degradation capacity. This defect can be restored by inducing lysosome biogenesis through the transcription factor EB (TFEB) [51]. The maintenance of lysosomal function may be important to have healthy pool of mitochondria to prevent the cell death during PD stress conditions. Interestingly, the two other major targets of miR-5701 are LAPTM4A and ATP6V0D1. LAPTM4A is known to encode putative lysosomal protein that has four predicted transmembrane domains. The functional study of the mouse homolog suggests its role in the transport of small molecules across endosomal and lysosomal membranes [52]. Similarly, the other target miR-5701, ATP6V0D1, is a component of vacuolar ATPase (V-ATPase), a multisubunit complex that mediates acidification of eukaryotic intracellular organelles [53]. This may be also one of the causes of lysosomal dysfunction as observed here and needs further validation in cell line and animal model system. During DAergic neurotoxin PD stress conditions, the expression of miR-5701 showed accumulation of defective mitochondria as increased mitochondrial DNA copy number and mitochondrial transcripts was observed. This is further supported by downregulation of VCP which inhibits autophagosome and lysosome fusion hence turnover of mitochondria.
These evidences suggest the important role of miR-5701 in the regulation of mitochondrial function, quality control, and lysosomal function. The observed decrease in miR-5701 during 6-OHDA may be a compensatory mechanism to rescue lysosomal function and mitochondrial biogenesis to increase the chance of neuronal survival. The evidences here further suggest that miR-5701 mediated regulation of mitochondrial and lysosomal cross talk is important for PD pathogenesis. The further evidences in animal model and PD tissue will further strengthen the role of miR-5701, a novel miRNA in PD pathogenesis.
Conclusion
The role of small non-coding RNA is emerging in different patho-physiological conditions. miRNAs play vital role in modulation of target transcript levels in narrow physiological level in different stress conditions. In the current study, we characterized the role of miR-5701, a novel miRNA, which is significantly downregulated in PD stress condition. The target analysis showed that miR-5701 may regulate several proteins of mitochondrial function and quality control and lysosomal degradation pathway. miR-5701 targets VCP level, which modulates autophagy flux and mitochondrial quality control. The increased level of miR-5701 sensitizes 6-OHDA-induced neuronal cell death. This study further showed that miRNAs can regulate inter-organellar cross talk and its dysregulation plays an important role in the pathogenesis of PD.
References
Beitz JM (2014) Parkinson's disease: a review. Front Biosci 6:65–74
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. doi:10.1038/42166
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS et al (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5):515–517. doi:10.1038/ng1769
Lang AE, Lozano AM (1998) Parkinson's disease. Second of two parts. N Engl J Med 339(16):1130–1143. doi:10.1056/NEJM199810153391607
Schapira AH (1993) Mitochondrial complex I deficiency in Parkinson's disease. Adv Neurol 60:288–291
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80(14):4546–4550
Chen H, Chan DC (2009) Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18(R2):R169–R176. doi:10.1093/hmg/ddp326
Gomes LC, Scorrano L (2013) Mitochondrial morphology in mitophagy and macroautophagy. Biochim Biophys Acta 1833(1):205–212. doi:10.1016/j.bbamcr.2012.02.012
Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85(2):257–273. doi:10.1016/j.neuron.2014.12.007
Deas E, Wood NW, Plun-Favreau H (2011) Mitophagy and Parkinson's disease: the PINK1-parkin link. Biochim Biophys Acta 1813(4):623–633. doi:10.1016/j.bbamcr.2010.08.007
Kinghorn KJ, Asghari AM, Castillo-Quan JI (2017) The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson's disease. Neural Regen Res 12(3):380–384. doi:10.4103/1673-5374.202934
Tran N, Hutvagner G (2013) Biogenesis and the regulation of the maturation of miRNAs. Essays Biochem 54:17–28. doi:10.1042/bse0540017
Sripada L, Singh K, Lipatova AV, Singh A, Prajapati P, Tomar D, Bhatelia K, Roy M et al (2017) hsa-miR-4485 regulates mitochondrial functions and inhibits the tumorigenicity of breast cancer cells. J Mol Med 95(6):641–651. doi:10.1007/s00109-017-1517-5
Sripada L, Tomar D, Prajapati P, Singh R, Singh AK, Singh R (2012) Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA. PLoS One 7(9):e44873. doi:10.1371/journal.pone.0044873
Duarte FV, Palmeira CM, Rolo AP (2014) The role of microRNAs in mitochondria: small players acting wide. Genes 5(4):865–886. doi:10.3390/genes5040865
Li P, Jiao J, Gao G, Prabhakar BS (2012) Control of mitochondrial activity by miRNAs. J Cell Biochem 113(4):1104–1110. doi:10.1002/jcb.24004
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728. doi:10.1093/emboj/19.21.5720
Prajapati P, Sripada L, Singh K, Bhatelia K, Singh R, Singh R (2015) TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta 1852(3):451–461. doi:10.1016/j.bbadis.2014.11.019
Orom UA, Lund AH (2007) Isolation of microRNA targets using biotinylated synthetic microRNAs. Methods 43(2):162–165. doi:10.1016/j.ymeth.2007.04.007
Li JH, Liu S, Zhou H, Qu LH, Yang JH (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42(Database issue):D92–D97. doi:10.1093/nar/gkt1248
Luthman J, Fredriksson A, Sundstrom E, Jonsson G, Archer T (1989) Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: motor behavior and monoamine alterations at adult stage. Behav Brain Res 33(3):267–277
Berndt N, Holzhutter HG, Bulik S (2013) Implications of enzyme deficiencies on mitochondrial energy metabolism and reactive oxygen species formation of neurons involved in rotenone-induced Parkinson's disease: a model-based analysis. FEBS J 280(20):5080–5093. doi:10.1111/febs.12480
Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH (2011) starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res 39(Database issue):D202–D209. doi:10.1093/nar/gkq1056
Ludtmann MH, Arber C, Bartolome F, de Vicente M, Preza E, Carro E, Houlden H, Gandhi S et al (2017) Mutations in valosin-containing protein (VCP) decrease ADP/ATP translocation across the mitochondrial membrane and impair energy metabolism in human neurons. J Biol Chem. doi:10.1074/jbc.M116.762898
Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, Chakrapani S, Joshi AU et al (2016) VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington's disease. Nat Commun 7:12646. doi:10.1038/ncomms12646
Johnson AE, Shu H, Hauswirth AG, Tong A, Davis GW. VCP-dependent muscle degeneration is linked to defects in a dynamic tubular lysosomal network in vivo. eLife 2015:4. doi:10.7554/eLife.07366.
Fang L, Hemion C, Pinho Ferreira Bento AC, Bippes CC, Flammer J, Neutzner A (2015) Mitochondrial function in neuronal cells depends on p97/VCP/Cdc48-mediated quality control. Front Cell Neurosci 9:16. doi:10.3389/fncel.2015.00016
Kim NC, Tresse E, Kolaitis RM, Molliex A, Thomas RE, Alami NH, Wang B, Joshi A et al (2013) VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 78(1):65–80. doi:10.1016/j.neuron.2013.02.029
Bartolome F, Wu HC, Burchell VS, Preza E, Wray S, Mahoney CJ, Fox NC, Calvo A et al (2013) Pathogenic VCP mutations induce mitochondrial uncoupling and reduced ATP levels. Neuron 78(1):57–64. doi:10.1016/j.neuron.2013.02.028
Stowe DF, Camara AK (2009) Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal 11(6):1373–1414. doi:10.1089/ARS.2008.2331
Malik AN, Shahni R, Rodriguez-de-Ledesma A, Laftah A, Cunningham P (2011) Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem Biophys Res Commun 412(1):1–7. doi:10.1016/j.bbrc.2011.06.067
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. doi:10.1038/cdd.2012.81
Dargemont C, Ossareh-Nazari B (2012) Cdc48/p97, a key actor in the interplay between autophagy and ubiquitin/proteasome catabolic pathways. Biochim Biophys Acta 1823(1):138–144. doi:10.1016/j.bbamcr.2011.07.011
Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, Dantuma NP, Taylor JP (2010) VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy 6(2):217–227
Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC (2009) Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol 187(6):875–888. doi:10.1083/jcb.200908115
Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P et al (2013) Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 23(4):508–523. doi:10.1038/cr.2013.11
Crider BP, Xie XS, Stone DK (1994) Bafilomycin inhibits proton flow through the H+ channel of vacuolar proton pumps. J Biol Chem 269(26):17379–17381
Hu Y, Lan W, Miller D (2017) Next-generation sequencing for MicroRNA expression profile. Methods Mol Biol 1617:169–177. doi:10.1007/978-1-4939-7046-9_12
Li H, Mao S, Wang H, Zen K, Zhang C, Li L (2014) MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein & cell 5(2):160–169. doi:10.1007/s13238-014-0022-7
Hu Z, Yu D, Gu QH, Yang Y, Tu K, Zhu J, Li Z (2014) miR-191 and miR-135 are required for long-lasting spine remodelling associated with synaptic long-term depression. Nat Commun 5:3263. doi:10.1038/ncomms4263
Kaplan BB, Kar AN, Gioio AE, Aschrafi A (2013) MicroRNAs in the axon and presynaptic nerve terminal. Front Cell Neurosci 7:126. doi:10.3389/fncel.2013.00126
Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N (2012) microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci. doi:10.1038/nn.3082
Recasens A, Perier C, Sue CM (2016) Role of microRNAs in the regulation of alpha-Synuclein expression: a systematic review. Front Mol Neurosci 9:128. doi:10.3389/fnmol.2016.00128
Marques TM, Kuiperij HB, Bruinsma IB, van Rumund A, Aerts MB, Esselink RA, Bloem BR, Verbeek MM (2016) MicroRNAs in cerebrospinal fluid as potential biomarkers for Parkinson's disease and multiple system atrophy. Mol Neurobiol. doi:10.1007/s12035-016-0253-0
Hoss AG, Labadorf A, Beach TG, Latourelle JC, Myers RH (2016) microRNA profiles in Parkinson's disease prefrontal cortex. Front Aging Neurosci 8:36. doi:10.3389/fnagi.2016.00036
Majounie E, Traynor BJ, Chio A, Restagno G, Mandrioli J, Benatar M, Taylor JP, Singleton AB (2012) Mutational analysis of the VCP gene in Parkinson's disease. Neurobiol Aging 33(1):209 e201–209 e202. doi:10.1016/j.neurobiolaging.2011.07.011
Chan N, Le C, Shieh P, Mozaffar T, Khare M, Bronstein J, Kimonis V (2012) Valosin-containing protein mutation and Parkinson's disease. Parkinsonism Relat Disord 18(1):107–109. doi:10.1016/j.parkreldis.2011.07.006
Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191(7):1367–1380. doi:10.1083/jcb.201007013
Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW et al (2017) Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. doi:10.1038/nature22369
Demers-Lamarche J, Guillebaud G, Tlili M, Todkar K, Belanger N, Grondin M, Nguyen AP, Michel J et al (2016) Loss of mitochondrial function impairs lysosomes. J Biol Chem 291(19):10263–10276. doi:10.1074/jbc.M115.695825
Baixauli F, Acin-Perez R, Villarroya-Beltri C, Mazzeo C, Nunez-Andrade N, Gabande-Rodriguez E, Ledesma MD, Blazquez A et al (2015) Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab 22(3):485–498. doi:10.1016/j.cmet.2015.07.020
Hogue DL, Nash C, Ling V, Hobman TC (2002) Lysosome-associated protein transmembrane 4 alpha (LAPTM4 alpha) requires two tandemly arranged tyrosine-based signals for sorting to lysosomes. The Biochemical journal 365(Pt 3):721–730. doi:10.1042/BJ20020205
Agarwal AK, White PC (2000) Structure of the VPATPD gene encoding subunit D of the human vacuolar proton ATPase. Biochem Biophys Res Commun 279(2):543–547. doi:10.1006/bbrc.2000.4003
Acknowledgements
This work was financially supported by the Department of Biotechnology, Government of India (Grant number BT/PR14206/MED/30/396/2010 to Rajesh Singh). This work constitutes the Ph.D. thesis of Paresh Prajapati. Lakshmi Sripada and Kritarth Singh received their Senior Research fellowship from University Grants Commission (UGC), Govt. of India. Khyati Bhatelia received her Senior Research fellowship from Council of Scientific and Industrial Research (CSIR), Govt. of India. Dhruv Gohel help in maintenance of SH-SY5Y cell and transfection during revision of the manuscript is acknowledged. The authors also acknowledge the instrumentation facility by DBT MSUB ILSPARE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no any competing financial interests in relation to the work described.
Rights and permissions
About this article
Cite this article
Prajapati, P., Sripada, L., Singh, K. et al. Systemic Analysis of miRNAs in PD Stress Condition: miR-5701 Modulates Mitochondrial–Lysosomal Cross Talk to Regulate Neuronal Death. Mol Neurobiol 55, 4689–4701 (2018). https://doi.org/10.1007/s12035-017-0664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0664-6