Abstract
Chronic brain hypoperfusion (CBH) induces the accumulation of abnormal cellular proteins, accompanied by cognitive decline, and the autophagic-lysosomal system is abnormal in dementia. Whether CBH accounts for autophagic-lysosomal neuropathology remains unknown. Here, we show that CBH significantly increased the number of autophagic vacuoles (AVs) with high LC3-II levels, but decreased SQSTM1 and cathepsin D levels in the hippocampi of rats following bilateral common carotid artery occlusion (2VO) for 2 weeks. Further studies showed that microRNA-27a (Mir27a) was upregulated at 2 weeks compared with the sham group. Additionally, LAMP-2 proteins were downregulated by Mir27a overexpression, upregulated by Mir27a inhibition, and unchanged by binding-site mutations or miR-masks, indicating that lamp-2 is the target of Mir27a. Knockdown of endogenous Mir27a prevented the reduction of LAMP-2 protein expression as well as the accumulation of AVs in the hippocampi of 2VO rats. Overexpression of Mir27a induced, while the knockdown of Mir27a reduced, the accumulation of AVs and the LC3-II level in cultured neonatal rat neurons. The results revealed that CBH in rats at 2 weeks could induce inefficient lysosomal clearance, which is regulated by the Mir27a-mediated downregulation of LAMP-2 protein expression. These findings provide an insight into a novel molecular mechanism of autophagy at the miRNA level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malfunction of the autophagic-lysosomal system is associated with neurodegenerative diseases, including Parkinson’s disease (PD), Huntington’s disease (HD), and Alzheimer’s disease (AD) [1–5]. It was found that the accumulation of autophagic vacuoles (AVs), which contain highly enriched amyloid precursor protein (APP) and β-cleaved APP, was observed in autopsy brains of AD patients and PS1/APP mice [6–9]. In addition, inefficient lysosomal clearance due to the impaired fusion between autophagosomes and lysosomes was also identified in AD neuropathology [6, 10]. These results suggest that the autophagic-lysosomal system may play an important role in dementia.
Chronic brain hypoperfusion (CBH) is considered to be a preclinical condition of mild cognitive impairment (MCI), which is thought to precede dementia and is related to multiple neurodegenerative diseases [11–13]. Previous studies have demonstrated that CBH could induce the accumulation of abnormal cellular proteins, such as Aβ aggregation and hyperphosphorylation of Tau [14–17], leading to neuronal cell death [18], and affecting dendritic arborizations and synaptic contacts [19]. However, whether CBH-induced cognitive decline is also associated with an abnormal autophagic-lysosomal system has not yet been reported.
A very recent study reported that the increase of miR-373* and 106a* could downregulate chaperone-mediated autophagy (CMA) by binding to the lamp-2a and hsc70 genes, respectively, inducing α-synuclein aggregation [20], which is a hallmark of PD. We previously found that miR-195 was reduced in the hippocampi and cortices of CBH rats and that it could regulate the biogenesis of Aβ40/42 and promote Tau hyperphosphorylation by posttranscriptionally regulating the expression of APP and BACE1 as well as p35 [14, 17]. However, whether miRNAs participate in CBH-generated changes in the autophagic-lysosomal system is largely unknown.
Several studies have reported that microRNA-27a (Mir27a) is not only an oncogenic miRNA [21, 22] but that it also regulated lipid metabolism and inhibited the replication of the hepatitis C virus by repressing the expression of many lipid metabolism-related genes, including FASN, SREBP1, SREBP2, PPARα, and PPARγ, as well as ApoA1, ApoB100, and ApoE3 [23], and it also promoted apoptosis by activating caspase-3 in H9c2 cardiomyocytes during hypoxia/reperfusion injury [24]. These studies indicate the multiple functions of Mir27a in different pathophysiological states. More importantly, Mir27a contributes to the regulation of hypoxia responses in breast and colon cancer cells, lungs, and neurons [25–27], and Mir27a*, the complementary microRNA of Mir27a, targets AKT1 and mTOR in head and neck squamous cell carcinoma, which are two important suppressors of autophagy [28]. The role of Mir27a in the autophagic-lysosomal system of the brain under hypoxia has not yet been reported.
In this study, we used an integrated approach to examine the hypothesis that CBH produced dysfunction of the autophagic-lysosomal system, which is associated with the abnormal expression of Mir27a.
Materials and Methods
Experimental Animals and Tissue Collection
Male SD rats (weight 260–300 g, obtained from the Animal Centre of the Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China) were housed in a temperature-controlled (23 ± 1 °C) room with 50 ± 5 % relative humidity on a 12-h light-dark cycle (lights on at 7:00 A.M.) and allowed free access to food and water. Rats that underwent permanent, bilateral common carotid artery occlusion (2VO) and stereotaxic injection of the antagomir-27a and negative-control antagomir (NC) were anesthetized with chloral hydrate (500 mg/kg) by intraperitoneal injection maintained with 0.5–1.0 % isoflurane. The depth of anesthesia was monitored by detecting reflexes, heart rate, and respiratory rate. Samples for qRT-PCR, western blot assay, and immunofluorescence staining were obtained from the hippocampi of rats after they had been anesthetized with chloral hydrate (500 mg/kg, intraperitoneal), followed by the confirmation of death by exsanguination. Tissues for primary neuron culturing were obtained from neonatal SD rats after administration of 20 % isoflurane and confirmation of death by cervical dislocation. The number of animals used in the present study was limited to the minimum possible number. All animals and experimental procedures were approved by and carried out in accordance with the Experimental Animal Ethics Committee of Harbin Medical University, China, and the Institute of Laboratory Animal Science of China (A5655-01). All procedures conformed to the Directive 2010/63/EU of the European Parliament.
Synthesis of Mir27a, AMO-27a, and Other Various Oligonucleotides
Mir27a mimics (sense, 5′-UUCACAGUGGCUAAGUUCCGC-3′; antisense, 5′-GGAACUUAGCCACUGUGAAUU-3′), AMO-27a (5′-GCGGAACUUAGCCACUGUGAA-3′), and negative control (NC) (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 5′-ACGUGA CACGUUCGGAGAATT-3′) were synthesized by Shanghai GenePharma Co., Ltd. AMO-27a contains 2′-O-methyl modifications. The lamp-2 and LC3 antisense oligodeoxynucleotides (ODNs) were synthesized from Shanghai Sangon Biological Engineering Technology and Service. Lamp2 masking antisense-ODN was 5′-CCTTATCTCAAATTGTGGGGAC-3′, and LC3 masking antisense-ODN was 5′-CTGAGTGTCACAGTGGGCTCCA-3′. Four nucleotides at both ends of antisense molecular were chemical modification with 2′-OMe. Antagomir-27a and NC containing 21-nucleotide was used in this study (Ribobio Co., China) and modified as specified: antagomir-27a, 5′-gscsggaacuuagccacugusgsasas-Chol-3′; NC, 5′-asascaugauguguuuucausgsascs-Chol-3′. 2′-OMe-modified nucleotides were represented as lower case letters; subscript “s” means the phosphorothioate linkage; “Chol” shows a cholesterol link via the hydroxyprolinol linkage.
Permanent 2VO
The method used for the preparation of 2VO rats was similar to that reported in previous studies with minor modifications [14, 29]. Briefly, rats were anaesthetized, and the common carotid arteries were exposed bilaterally via a midline ventral incision. After the arteries were carefully separated from the vagal nerves, permanent ligation was performed with 5-0 silk suture. After the wounds were sutured, the rats were allowed to recover from anesthesia before being returned to their cages. The extent of cerebral ischemia was evaluated by magnetic resonance imaging (MRI). The rats were sacrificed at 1 and 2 weeks after 2VO surgery, respectively.
Stereotactic Injection of Antagomir
The procedure for stereotactic injection was as described in a previous study, with minor modifications [14]. After 2VO surgery for 1 week, rats were anesthetized and placed in a stereotaxic frame (RWB Life Science Co. Ltd). Two injection sites relative to the bregma were selected according to the atlas of Paxinos and Watson (1997), and the detailed injection sites were as follows: site 1—anteroposterior (AP), −4.8 mm; mediolateral (ML), ±3.0 mm; and dorsoventral (DV), −3.5 mm); site 2—AP, −4.8 mm; ML, ±5.2 mm; DV, −5.0 mm. Antagomir-27a (800 ng dilution in 2 μl of PBS) or negative control antagomir (NC) was injected through a microsyringe with a 33-gauge tip needle at a rate of 0.5 μl/min twice every 3 days (Hamilton, Bonaduz). The needle was left in place for an additional 2 min after injection to avoid the backflow of solution.
Primary Hippocampal and Cortical Neuron Culture (NRNs)
The hippocampal and cortical neurons were prepared as our previous study [14]. Briefly, the cerebral hemispheres were dissected from postnatal day 0 rats and dissociated by 0.125 % Trypsin (GIBCO, 15050057) digestion followed by trituration. After pelleted and resuspended, cells were plated onto six-well plate at density of 2 × 106 cells/well or coverslips at density of 0.5 × 105 cells/well precoated with poly-D-lysine (Sigma, P6407). The cells were cultured in Neurobasal A medium (GIBCO, 10888-022) containing 2 % B27 serum-free supplement (GIBCO, 17504-044), 10 % fatal bovine serum (FBS, HyClone) and 1 % penicillin/streptomycin (Invitrogen). After initial plating, half of medium was replaced with fresh medium every 3 days. Neurons were kept at 37 °C humidified incubator with 5 % CO2 and performed for further experiments.
Transfection Procedure
Seventy-five picomoles per milliliter Mir27a mimics or/and lamp-2-ODN or/and LC3-ODN, AMO-27a or negative control (NC) siRNAs were transfected into neonatal rat neurons (NRNs) using X-treme GENE siRNA transfection reagent (Roche, 04476093001) according to the manufacturer’s instructions. The transfected NRNs were collected after 48 h for further detection.
Rapamycin, 3-Methyladenine, and Serum Deprivation Pepstatin A and Bafilomycin A1 Treatments
Rapamycin (Sigma, M9281) was dissolved in DMSO to create a 1-mM stock solution and stored at −20 °C. 3-Methyladenine (3-MA, Sigma, R0395) was dissolved in ddH2O to create a 100-mM solution for fresh use. Pepstatin A (Sigma, P 5318) was diluted in ethanol to create a 1-mM stock solution, in which heat was required for a complete solution. Bafilomycin A1 (Yuanyebio, YY14816) was diluted in DMSO to create the stock solution. NRNs were transfected with Mir27a or NC for 48 h after they were cultured for 4 days in vitro. Then, they were classified into five groups by exposure to fresh culture media with the addition of the following: (1) PBS; (2) 5 nM of rapamycin for 24 h; (3) 5 nM of rapamycin for 24 h first, with the removal of rapamycin for an additional 24 h (Rap/RC); (4) 3 mM of 3-MA for 24 h; and (5) serum-free medium for 24 h. After the NRNs were transfected with NC, Mir27a or AMO for 24 h, 20 μM of pepstatin A were applied for 24 h. The NRNs pretransfected with NC, Mir27a, or AMO for 36 h was incubated with 300 nM of bafilomycin A1 for 12 h.
LysoTracker Treatment
LysoTracker Green DND-26 (Invitrogen, L7526) 1 mM was diluted in growth medium to a final working concentration of 100 nM. When NRNs were transfected with NC, Mir27a, or AMO for 48 h, the old growth medium was removed from the dish, and the prewarmed (37 °C) medium containing LysoTracker Green DND-26 was added. The NRNs were incubated for 30 min under growth conditions and fixed with 4 % PFA. Following blocking and permeabilization, the NRNs were further immunolabeled with SQSTM1/p62 antibody overnight at 4 °C and then visualized with the secondary antibody conjugated to Alexa Fluor 594 donkey anti-rabbit (1:1000, Invitrogen, CA, USA).
Construction of Expression Plasmids
The full length of lamp2 3′-UTR containing Mir27a binding site was cloned into the psi-CHECK 2 dual Luciferase reporter plasmid (Promega), which is located at 3′ end of the coding sequence of R. reniformis luciferase, to produce psi-CHECK2-WT-lamp-2 3′-UTR using PCR (forward primer, 5′-CCGCTCGAGCACCTACAATCTGATTGAATATATTAC-3′; reverse primer, 5′-ATAAGAATGCGGCCGCTTTGGTGGTGAAGCAGTGTTTATTAATTC-3′). To produce the deletion mutant of Mir27a targeting sequence, psi-CHECKTM2-MT-Lamp-3′-UTR was performed with the following conditions: 95 °C for 5 min, followed by 18 cycles of 95 °C at 30 s, 50 °C at 30 s, 68 °C at 8 min, and 68 °C for 5 min. The lamp2 mutagenesis is as follows: forward primer, 5′-GAACTTTTATTTGGAAATCAGTCCGGTGAATTACTGATAAGGCTTTTTTTGAAAAAAAAC-3′, and reverse primer, 5′-GTTTTTTTTCAAAAAAAGCCTTATCAGTAATTCACCGGACTGATTTCCAAATAAAAGTTC -3′. All constructs were completely sequence-verified.
Dual Luciferase Reporter Assay
For the report assay, HEK293T cells were cultured approximately 80 % confluence in a six-well plate and transfected with 0.5 μg either psi-CHECK2-WT-lamp-2 3′-UTR (firefly luciferase vector) or psi-CHECK2-MT-lamp-2 3′-UTR vector as well as 20 μmol/L Mir27a mimics, AMO-27a, or NC siRNAs and 1 μL blank plasmid using Lipofectamine 2000 (Invitrogen) transfection reagent according to the manufacturer’s instructions. After 72 h of transfection, luciferase activities were measured with a dual luciferase reporter assay kit (Promega, E1910) and luminometer (GloMax 20/20; Promega). Nucleotide substitution mutagenesis was carried out using direct oligomer synthesis for the 3′-UTRs of lamp-2. Renilla luciferase activity was normalized to firefly luciferase activity.
Western Blot
Protein samples from the hippocampi of rats or NRNs were lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1 % Tryton X-100; 0.25 % Na-deoxycholate; 0.1 M EDTA; and 1 % SDS) containing protease inhibitor cocktail (Roche Applied Science). The concentration of protein samples were determined spectrophotometrically using a BCA kit (Universal Microplate Spectrophotometer). Proteins were loaded onto 10 or 15 % SDS-PAGE gel, and then transferred onto nitrocellulose membranes. After blocking with 5 % nonfat milk for 2 h at room temperature, the following primary antibodies were used: anti-LC3 (1:2000, L7543, Sigma, Saint Louis, MO, USA), anti-SQSTM1 (1:500, 5114, Cell Signaling Technology, USA), anti-LAMP-2 (1:1000, L0668, Sigma, Saint Louis, MO, USA), anti-cathepsin D (1:100, sc-10725, Santa Cruz Biotechnology, USA), and anti-β-actin (1:1000, sc-47778, Santa Cruz Biotechnology, USA). Secondary antibodies polyclonal goat anti-mouse or goat anti-rabbit IgG conjugated with IRDye 680 (LI-COR, B70920-02) or IRDye 800 (LI-COR, 926-32210) were applied. The films were detected by Odyssey Infrared Imaging System (LI-COR), and Odyssey v3.0 software was used for analysis. β-Actin was used as internal control.
qRT-PCR
Total RNA was purified with the TRIzol Reagent (Invitrogen), according to the manufacturer’s instructions. Mir27a level was quantified by the TaqMan® MicroRNA Reverse Transcription Kit (ABI, 4366596) and TaqMan® Gene Expression Master Mix (Applied Biosystems, 4369016), and U6 was used as an internal control. The TaqMan qRT-PCR probes and primers for Mir27a (ABI, ID: 000408), U6 (ABI, ID: 001973). Lamp2 mRNA level was quantified by the High Capacity cDNA Reverse Transcription Kit (ABI, 4368814) and the SYBR® Gene PCR Master MixApplied Biosystems, 4309115). The specific primer of lamp-2 (forward, 5′-ACAACATCACGCTTTCTTAC-3′; reverse, 5′-CTCACTGTACCATTTTGGAC-3′), LC3 (forward, 5′-ACCAAGTTCCTTGTACCTGA-3′; reverse, 5′-CTCTCTCGCTCTCGTACACT-3′); Sqstm1 (forward, 5′-AGTTCCAGCACAGGCACAGA-3′; reverse, 5′-ACAAATGCGTCCAGTCGTCA-3′), and β-actin (forward, 5′-GGAAATCGTGCGTGACATTA-3′; reverse, 5′-AGGAAGGAAGGCTGGAAGAG-3′). β-Actin was used as an internal control. qPCR was performed on a thermocycler ABI Prism® 7500 fast (Applied Biosystems), and the protocol was as follows: 95 °C for 10 min, followed by 40 cycles with 95 °C for 15 s, 60 °C for 1 min. Results were normalized against the internal control using the δ–δ CT method.
Immunofluorescence Detection
Rats were anesthetized with 10 % chloral hydrate (500 mg/kg, intraperitoneal) and perfused with 4 % paraformaldehyde (pH 7.4) through left ventricle to the whole body. The brains were taken out and fixed in 4 % paraformaldehyde overnight at 4 °C. In the following days, brains were embedded in Tissue-Tek OCT Compound (Sakura) when they sunk to the bottom of dehydration fluid with 30 % sucrose and 40-μm sections cut by freezing microtome (Leica) were mounted on glass slides. The prepared slices were incubated in PBS containing 10 % donkey serum and 0.3 % Triton X-100 for 2 h in room temperature for blocking and permeabilization. The primary antibodies were diluted in PBS with 10 % donkey serum and 0.3 % Triton X-100 overnight at 4 °C.
NRNs in the glass coverslips were fixed with 4 % paraformaldehyde on ice for 30 min. The cells were incubated in PBS containing 10 % donkey serum and 0.1 % Triton X-100 for 1 h at room temperature for blocking and permeabilization. The primary antibodies were diluted in PBS with 10 % donkey serum and 0.1 % Triton X-100 overnight at 4 °C. After rinsed with PBS, the secondary antibody conjugated to Alexa Fluor 594 donkey-anti rabbit (1:1000, Invitrogen, CA, USA) was incubated at room temperature for 1 h.
The following primary antibodies were used in the present study: anti-LC3 antibody (1:1000, L7543, Sigma, Saint Louis, MO,USA), anti-LAMP-2 antibody (1:500, L0668, Sigma, Saint Louis, MO,USA), anti-LAMP-1(1:100, ab25630, Abcam, MA, USA), anti-LAMP-2A(1:100, ab125068, Abcam, MA, USA), anti-CD63(1:100, ab108950, Abcam, MA, USA), and anti-SQSTM1/62 (1:200, 5114, Cell Signaling Technology, USA). After rinsed with PBS, the secondary antibody conjugated to Alexa Fluor 594 donkey-anti rabbit (1:1000, A21207, Invitrogen, CA, USA), Alexa Fluor 488 Donkey Anti-Mouse (1:1000, A-21202, Invitrogen, CA, USA), Alexa Fluor 488 Donkey Anti-Rabbit(1:1000, A-21206, Invitrogen, CA, USA), and Alexa Fluor 594 Donkey Anti-Mouse (1:1000, A-21203, Invitrogen, CA, USA) was incubated at room temperature for 2 h (tissue) or 1 h (primary cultured neurons). Coverslips were mounted with FluorSave™ Reagent (Calbiochem, 345789).
Transmission Electron Microscopy
Hippocampi were removed and fixed in 1 % osmic acid (OsO4) in phosphate-buffered solution with 1.5 % potassium ferricyanide. After dehydration with a concentration gradient of propyl alcohol solutions, tissues were embedded in epon with propylene oxide as an intermediary solvent. Ultrathin sections were processed, mounted onto former-coated slot grids, and stained with uranyl acetate and lead citrate. Images were examined by a Hitachi H-7650 electron microscope (Hitachi, H-7650). Twenty randomly selected EM images per rat were captured, and the number of autophagosomes and lysosomes in each field was counted.
Image Acquisition
Confocal images were captured by FluoView™ FV300 (Olympus) using ×60 objective with the same condition at a resolution of 1024 × 1024 pixels (12 bit). Several random fields were acquired and analyzed with FLV3.0 software in each group.
Statistical Analysis
All data were shown as mean ± SEM. The Student’s t test was used in comparisons between two groups. Multigroup comparisons were performed by one-way ANOVA. SPSS19.0.sorftware was used for all statistical analyses. P < 0.05 was considered as statistically significant.
Results
CBH Induces Abnormal Autophagic Activity
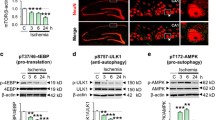
To investigate whether CBH could trigger autophagy, we developed a rat model of CBH by permanent 2VO for 1 and 2 weeks [13, 14]. By western blot analysis, we found that the ratio of phosphorylated p70S6 kinase (p70S6k) to total p70S6k, a sign of mTOR-mediated induction of autophagy [10, 30], declined in the hippocampi of rats during CBH for 1 and 2 weeks (Fig. 1a), which was similar to the results of a previous report in rat brains after MCAO [31]. Further evaluation showed that CBH resulted in the accumulation of microtubule-associated protein 1 light chain 3 II (LC3-II), a marker of autophagosomes [32], in the hippocampi of rats at both 1 and 2 weeks after 2VO surgery (Fig. 1b). This result was further observed with an immunofluorescence signal conjugated with LC3 antibody (Fig. 1c) and quantified by the number of LC3-positive puncta (Fig. 1d).
Alteration of p70S6k, LC3, and SQSTM1 protein expression in sham and 2VO rats. a Total p70S6k and p70S6k phosphorylation levels were quantified by western blot analysis between the sham group and 2VO surgery rats at 1 and 2 weeks; n = 4. b LC3 expression in the hippocampi of rats after 2VO surgery. The left panel is the representative immunoblotting band of LC3-II. The right panel is the digital analysis of LC3-II expression and LC3-II/LC3-I; n = 6. c Immunocytochemical analysis of LC3-positive puncta labeling with LC3 antibody in the hippocampal region of sham and 2VO rats; n = 3. Scale bars, 20μm. d The quantitative analysis of the number of LC3-positive puncta in c; n = 3. Twenty neurons were selected in each hippocampus, and more than 60 neurons in each group were used to count the number of LC3-II. e SQSTM1 protein level in the hippocampi from sham and 2VO rats; n = 6. Values are the mean ± SEM. *P < 0.05 versus sham
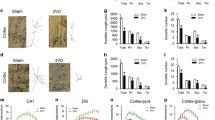
Because the accumulation of LC3-II indicates either increased formation of autophagosomes or the impairment of LC3-II turnover [33], the next step was to investigate whether the foregoing observations were also associated with defects in LC3-II turnover defect. The results showed that the expression of SQSTM1, a ubiquitin-binding protein associated with autophagy clearance by directly binding with Atg8/13 [34, 35], was markedly elevated in the hippocampi of rats during CBH for both 1 and 2 weeks (Fig. 1e), suggesting the impairment of autophagic degradation under CBH. Furthermore, using electron microscopy (EM) analysis, we found that the number of double-membrane-limited AVs in the hippocampi of rats was time dependently increased after 2VO (Fig. 2a). On the contrary, the number of lysosomes was higher in rats at 1 week than those at 2 weeks after 2VO (Fig. 2a). The results were further verified by the changes of lysosome-associated membrane protein-1 (LAMP1) and cathepsin D (CathD) (Fig. 2b, c), which were used to examine the number and activity of lysosomes, respectively [36–38]. These results indicated that abnormal lysosome function may be involved in AV accumulation in the hippocampi of rats at 2 weeks after 2VO.
Alterations of autophagosomes and lysosomes as well as changes in the LAMP-2 protein in sham and 2VO rats. a Electron microscopic analysis of the CA1 subfield of hippocampi from sham and 2VO rats at 1 and 2 weeks. The arrows indicate AVs (red arrows) or lysosomes (blue arrows); n = 3. Scale bars, 1 μm. Lower right panel: Quantitative analysis of AVs and lysosome number in 2VO rats; n = 3. At least ten cells were selected in each hippocampus, and more than 30 cells in each group were used to analyze the number of AVs. b Representative images stained with LAMP1 (red) antibody in the hippocampi from sham and 2VO rats. Nuclei were stained with DAPI. Scale bars, 20 μm. Lower right panel: The corresponding numbers of LAMP1 in each cell between the sham group and 2VO surgery rats. Sixty neurons in each group were counted. c CathD expression in the hippocampi of rats after 2VO; n = 6. d No change in the lamp2 level was detected by qRT-PCR in sham and 2VO rats; n = 6. e LAMP-2 protein expression in the hippocampi of rats in sham and different groups of 2VO rats; n = 6. f Representative images staining with LAMP-2 (red) antibody in the hippocampi from sham and 2VO rats. Nuclei were stained with DAPI. Scale bars, 20 μm. Lower right panel: Quantification of the positive signal area for LAMP-2 in the hippocampi following 2VO surgery; n = 3. At least ten random fields were selected in each hippocampus, and more than 30 fields in each group were used to analyze the ratio of the positive signal/image. g Representative images of neurons immunostained by LAMP-2A antibody and quantification of the positive signal area of LAMP-2A (lower right panel) in the hippocampi of 2VO rats; n = 3. At least ten random fields were selected in each hippocampus, and more than 30 fields in each group were used to analyze the ratio of the positive signal/image. Values are the mean ± SEM. *P < 0.05 versus sham. # P < 0.05 versus 1 week (color figure online)
To further understand whether the accumulation of AVs during CBH was due to the failed fusion of autophagosomes with lysosomes, we investigated whether the expression of LAMP-2, a critical determinant of autophagosomes-lysosome fusion which was reported to induce the accumulation of AVs in LAMP-2-deficient mice [36, 39, 40], was changed in CBH rats. We found that although the mRNA level of LAMP-2 was not changed (Fig. 2d), the expression of LAMP-2 protein was transitional, as indicated by its increase at 1 week, but decrease at 2 weeks in the hippocampi of rats after 2VO (Fig. 2e). This phenomenon was further verified by immunofluorescence analysis for both LAMP-2 (Fig. 2f) and LAMP-2A (Fig. 2g), an isoform of LAMP-2. These results suggested that the change of LAMP-2 was involved in the CBH-induced abnormal autophagosome-lysosome fusion process.
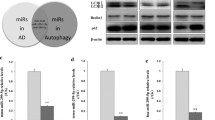
LAMP-2 Is a Target of Mir27a
We next attempted to explore the molecular mechanism in depth. MiRNAs were reported to be changed in either acute ischemic stroke or chronic brain hypoperfusion [14, 41, 42]; in the present study, LAMP-2 expression was changed, whereas its mRNA was not altered (Fig. 2d, e). We thus presumed that miRNAs may be involved in the above observations. By searching the miRNA database RNAhybrid, we found that the lamp2 gene is a potential target of Mir27a (Fig. 3a). Interestingly, Mir27a expression in the hippocampi of rats was distinct at different time points after 2VO. As shown in Fig. 3b, compared with the sham group, the level of Mir27a in the hippocampi of rats was decreased at 1 week and markedly increased at 2 weeks (Fig. 3b). Importantly, the level of Mir27a was matched with LAMP-2 protein expression in a negative pattern (Fig. 2e). Therefore, we established a cell model to overexpress Mir27a via the transfection of Mir27a mimics or to knock down Mir27a by the delivery of 2′-O-methyl antisense oligoribonucleotides to Mir27a (AMO-27a) in cultured NRNs (Fig. 3c). We observed that the overexpression of Mir27a decreased, whereas the downregulation of Mir27a by AMO-27a increased LAMP-2 protein expression in NRNs (Fig. 3d, e). However, the mRNA level of LAMP-2 was unaltered (Fig. 3f). Furthermore, according to the luciferase assay, Mir27a suppressed the luciferase activities of lamp2, whereas the mutation of the binding sites abolished the effect of Mir27a (Fig. 3g). These data suggest that lamp2 is a direct target of Mir27a and that the posttranscriptional inhibitory role of Mir27a on LAMP-2 is specific.
Mir27a regulates LAMP-2 expression. a The binding site of the Mir27a seed sequence with the 3′UTR of the lamp2 gene predicted by the RNAhybrid database and the sequence of lamp2-ODN. b Mir27a levels in hippocampi from sham and 2VO rats quantified by qRT-PCR; n = 3. *P < 0.05 versus sham. c Mir27a level in NRNs after transfection with NC, Mir27a mimics, and AMO-27a; n = 3. *P < 0.05 versus NC; # P < 0.05 versus Mir27a mimics. d Western blot analysis of the LAMP-2 protein. NRNs were transfected with NC, Mir27a mimics, or AMO-27a for 24 and 48 h, respectively; n = 3. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. NC scrambled negative control. e Confocal images of the representative neurons treated with Mir27a mimics and AMO-27a and labeled with LAMP-2 antibody (red). Scale bars, 10 μm. Right panel: Quantification of the number of LAMP-2-positive signals per cell in NRNs treated with Mir27a mimics and AMO-27a. At least 15 neurons were selected in each batch, and more than 45 neurons in each group were used to count the number of LAMP-2. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. f Alteration of lamp2 level in NRNs treated with Mir27a mimics or AMO-27a; n = 3. g Luciferase reporter gene assay for interactions between Mir27a and its binding sites (left) or the mutation of the binding site (right) in the 3′-UTR of the lamp2 in HEK293T cells. HEK293T cells were transfected with luciferase-target motif chimeric vector alone, Mir27a mimics, Mir27a + AMO-27a, or NC using Lipofectamine 2000, n = 3. *P < 0.05 versus blank. Values are the mean ± SEM (color figure online)
Overexpression of Mir27a Induces the Accumulation of AVs by Impairing Autophagosome Clearance In Vitro
Next, we tested whether Mir27a has a functional role in the accumulation of AVs by defective LC3-II turnover. As predicted, using immunoblotting and immunofluorescence analyses in cultured NRNs, we observed that Mir27a mimics effectively elevated LC3-II expression and the ratio of LC3-II/LC3-I without changing its mRNA level (Fig. 4a–c), which was further confirmed by increased number of LC3-positive puncta (Fig. 4d). The phenomenon was blocked when NRNs were treated by AMO-27a (Fig. 4a, b, d). Interestingly, in the overexpression of Mir27a inhibited, AMO-27a increased the expression of LAMP-1 (Fig. 4e) as well as CathD (Fig. 4f), suggesting that the role of Mir27a on AV accumulation and LC3-II level may be at least partially associated with abnormal lysosome function.
Overexpression of Mir27a induces the accumulation of AVs and affects the expression of LC3, LAMP1, and cathepsin D proteins in vitro. a The representative bands of LC3-II in NRNs treated with Mir27a mimics or AMO-27a by western blot. The upper panel is the representative band at 24 h after transfection. The lower panel is the representative band at 48 h after transfection. b Alteration of the LC3-II level in NRNs treated with Mir27a mimics or AMO-27a. The upper panel is the analyzed data of the LC3-II level. The lower panel is the result of the LC3II/LC3I ratio; n = 6. *P < 0.05 versus NC. # P < 0.05 versus Mir27a mimics. c No change in the LC3 mRNA level in NRNs treated with Mir27a mimics or AMO-27a was detected by qRT-PCR; n = 3. d Immunofluorescent staining for LC3-positive puncta (red, puncta) in NRNs after treatment with Mir27a mimics or AMO-27a for 48 h. The lower right panel indicates the quantitative analysis of the number of AV-like structures; n = 3. At least 15 neurons were selected in each batch, and a total of more than 45 neurons in each group were used to count the number of LC3-II. Scale bar, 20 μm; mean ± SEM. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. e Immunofluorescent staining for LAMP1 (red, puncta) in NRNs after treatment with Mir27a mimics or AMO-27a for 48 h. The lower right panel indicates the quantitative analysis of the ratio of the positive puncta per image; n = 3. At least 15 neurons were selected in each batch, and a total of more than 45 neurons in each group were used to count the number of LAMP-1. Scale bar, 20 μm. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. f Representative immunoblot and the corresponding quantification of CathD in NRNs treated with Mir27a mimics or AMO-27a; n = 3. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. Values are the mean ± SEM (color figure online)
Because the defective LC3-II turnover occurred under CBH conditions, we speculated that the accumulation of LC3-II induced by the overexpression of Mir27a may also cause the impairment of autophagic turnover. Here, we found that Mir27a mimics, but not AMO-27a, significantly increased the levels of both the protein and mRNA of SQSTM1 (Fig. 5a, b), suggesting that overexpression of Mir27a results in the decrease of the clearance and metabolism of SQSTM1 at both the protein and mRNA levels. To characterize whether the increase in SQSTM1 in Mir27a-overexpressing NRNs was localized to the late endosome/lysosomal compartment due to the impairment of autophagosome-lysosome fusion, we performed two colocalization experiments. First, as illustrated in Fig. 5c, the overexpression of Mir27a mimics, but not the expression of AMO-27a, induced more numerous colocalization of SQSTM1 with CD63, a marker of late endosomes/lysosomes [34]. Second, using the fluorescent dye LysoTracker, which displays intense fluorescence when it faces an acidic environment in the late endosomal/lysosomal compartment [34], we found that LysoTracker-labeled subtractions of the SQSTM1 antibodies were markedly increased in Mir27a-treated NRNs compared with NRNs treated with either NC or AMO-27a (Fig. 5d). These results collectively implied that the accumulation of SQSTM1 by the overexpression of Mir27a was associated with the impaired fusion of autophagosomes with lysosomes.
Effects of Mir27a on the expression and localization of SQSTM1 in vitro. a Alteration of SQSTM1 protein level in NRNs treated with Mir27a mimics or AMO-27a was analyzed by western blot assay; n = 6. *P < 0.05 versus NC. # P < 0.05 versus Mir27a mimics. b The mRNA level of Sqstm1 in NRNs treated with Mir27a mimics or AMO-27a detected by qRT-PCR; n = 3. *P < 0.05 versus NC. # P < 0.05 versus Mir27a mimics. c Representative images showing the colocalization of SQSTM1 (green, left) and CD63 (red, middle) in NRNs treated with Mir27a mimics or AMO-27a. Merged images depicting double positivity (yellow) were shown on the right. Scale bars, 20 μm. NC scrambled negative control. d NRNs treated with NC, Mir27a mimics or AMO-27a was preincubated with LysoTracker and then immunolabeled with SQSTM1 antibodies. The left images were labeled by LysoTracker (green), which displays intense fluorescence when it faces the acidic environment of the late lysosomal compartment. The middle images were labeled by SQSTM1 (red). A merged image depicting double positivity (yellow) is shown on the right. Scale bars, 20 μm. Values are the mean ± SEM (color figure online)
To further validate whether the accumulation of AVs induced by Mir27a overexpression was really due to the impairment of autophagosome clearance, several strategies were implemented to analyze the maturation of autolysosomes. First, in NC-treated cells, rapamycin (5 nM), a specific inhibitor of the mechanistic target of rapamycin (mTOR), which can induce autophagy [7, 43], resulted in markedly elevated LC3-II levels (∼3.2-fold that of NC) (Fig. 6a, c). After withdrawing the rapamycin (Rap/RC) from the culture medium, the LC3-II levels were returned to ∼1.2-fold that of the Ctl group (Fig. 6a, c). Compared with NC-treated cells, the LC3-II level was markedly elevated in NRNs transfected with Mir27a mimics alone (Fig. 6b, c). However, the level of LC3-II in Mir27a-overexpressing NRNs remained at higher levels even when rapamycin was withdrawn (Fig. 6b, c). This phenomenon was also observed after serum starvation (Fig. 6b, c), which can activate autophagy similarly to rapamycin. Next, 3-methyladenine (3MA), an acknowledged selective autophagy inhibitor that suppresses Vps34 activation [7, 44], was used. We found that 3MA was effective induce the increased LC3-II protein when NRNs were treated with NC (Fig. 6a, c); however, its action was inhibited when NRNs were cotreated with Mir27a (Fig. 6b, c). This was further observed after immunofluorescence analysis (Fig. 6d, e). These findings demonstrated that the overexpression of Mir27a resulted in the accumulation of AVs due to the defective clearance of autophagosomes.
Impaired clearance of LC3-II in overexpressed Mir27a NRNs. a–c Representative immunoblotting bands of LC3-II in NRNs based on the transfection of NC (a) or Mir27a (b) into NRNs first. NRNs were then treated by PBS, rapamycin (5 nM), the removal of rapamycin following rapamycin treatment (Rap/RC), serum-free (ser), and 3MA (3MA, 3 mM). The quantification of LC3-II protein was normalized to β-actin (c); n = 3. *P < 0.05 versus Ctl, # P < 0.05 versus Rap. d Representative immunofluorescence images staining with LC3-II antibody. Red puncta represent LC3-II. The upper panel is NRNs transfected with NC. The lower panel is NRNs transfected with Mir27a mimics. e Quantification of the number of LC3-positive puncta per neuron; n = 3. At least 15 neurons were selected in each batch, and more than 45 neurons in each group were used to count the number of LC3-II. *P < 0.05 versus Ctl, # P < 0.05 versus Rap. $ P < 0.05 versus NC-Ctl. Values are the mean ± SEM (color figure online)
Mir27a Affects Autophagosome Clearance Through LAMP-2
Our aforementioned data showed that the forced expression of Mir27a could increase, whereas AMO-27a could inhibit the expression of LC3-II (Fig. 4a, b) and LAMP-2 (Fig. 3d). Moreover, Mir27a regulated LAMP-2 expression at a posttranscriptional level (Fig. 3g). Using the RNAhybrid database, we found that the 3′UTR of LC3 mRNA also has a binding site for the seed sequence of Mir27a (Fig. 7a). Therefore, we sought to identify whether Mir27a also exerts its function by regulating LC3 expression on a posttranscriptional level, similar to the action of Mir27a on LAMP-2. For this, we designed two miRNA-masking antisense oligodeoxynucleotides (miR-masks) to base pair the Mir27a binding sites in the 3′-UTRs of LC3 (Fig. 7a) and lamp2 genes (Fig. 3a), which were labeled by LC3-ODN and lamp2-ODN, respectively. In contrast to our prediction, lamp2-ODN, but not LC3-ODN itself prevented the increase in LC3-II expression induced by Mir27a treatment according to western blot and immunofluorescence staining analyses (Fig. 7b–d). These results suggest that LC3-II accumulation by overexpressed Mir27a was due to the posttranscriptionally repressed LAMP-2 but not the influence of LC3. To verify this phenomenon experimentally, we transfected LC3-ODN and lamp2-ODN together with Mir27a mimics into NRNs and assessed the expression of LAMP-2 protein. As expected, we found that the downregulation of LAMP-2 induced by Mir27a overexpression was prevented by lamp2-ODN but not LC3-ODN (Fig. 7e). The results were also verified by immunofluorescence imaging (Fig. 7f, g). Accordingly, we found that lamp2-ODN treatment abolished the increased SQSTM1 level by Mir27a (Fig. 7h). Taken together, these results suggest that the increase in both AV number and LC3-II level induced by the overexpression of Mir27a was caused by the impaired clearance of AVs, which was associated with lysosome dysfunction mediated by the downregulation of LAMP-2.
Mir27a regulates autophagy by targeting lamp2 in vitro. a The binding site of the Mir27a seed sequence with the 3′-UTR of LC3 and the sequence of LC3-ODN. b Depression of LC3-II expression by lamp2-ODN but not LC3-ODN was determined by western blot; n = 3. *P < 0.05 versus Ctl, # P < 0.05 versus Mir27a mimics. c Representative images of LC3-positive puncta in different groups treated with Mir27a mimics, Mir27a mimics, and LC3-ODN or lamp2-ODN. Scale bars, 10 μm. d The corresponding quantification of LC3 in c. At least 15 neurons were selected in each batch, and more than 45 neurons in each group were used to count the number of LC3-positive puncta. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. e Representative immunoblots for LAMP-2 in NRNs treated with Mir27a, Mir27a mimics, and LC3-ODN or lamp2-ODN; n = 6. *P < 0.05 versus Ctl, # P < 0.05 versus Mir27a mimics. f Representative images of cell immunostained for LAMP-2 in NRNs treated with Mir27a, Mir27a mimics, and LC3-ODN or lamp2-ODN. Scale bars, 10 μm. g The corresponding quantification of LAMP-2 in f. At least 15 neurons were selected in each batch, and more than 45 neurons in each group were used to count the number of LAMP-2. *P < 0.05 versus NC, # P < 0.05 versus Mir27a mimics. h Lamp-2-ODN blocks the increase of SQSTM1 protein induced by Mir27a mimics; n = 6. *P < 0.05 versus Ctl, # P < 0.05 versus Mir27a mimics. Values are the mean ± SEM
Downregulation of Mir27a mitigates the accumulation of AVs induced by 2VO
Based on above data, the downregulation of LAMP-2 induced by Mir27a overexpression was expected to promote the accumulation of AVs in CBH rats. To test this notion, a small inhibitory RNA anti-miR (antagomir-27a) was designed and directly stereotaxically injected into the bilateral CA1 subfields of the hippocampi of rats to silence endogenously increased Mir27a induced by 2VO at 2 weeks. As illustrated in Fig. 8a, antagomir-27a injection resulted in significantly lower expression of Mir27a (>50 %) in the hippocampi of rats at 2 weeks after 2VO relative to the rats preinjected with the NC oligonucleotide. As predicted, the accumulation of AVs in the hippocampi of 2VO rats was prevented by antagomir-27a treatment (Fig. 8b). Surprisingly, after treatment with antagomir-27a, the number of lysosomes in the hippocampi of rats at 2 weeks after 2VO was dramatically increased, which was similar to the observation in the hippocampi of rats at 1 weeks after 2VO (Figs. 2a and 8b, c). Although antagomir-27a significantly inhibited the increase of LC3-II protein in the hippocampi of rats at 2 weeks after 2VO (Fig. 8d), it failed to eliminate the increased SQSTM1 level (Fig. 8e). In addition, the expression of the LAMP-2 protein after antagomir-27a treatment obviously increased relative to 2VO rats at 2 weeks (Fig. 8f).
Mir27a attenuates lysosomal function deficits in 2VO rat. a Mir27a level in the hippocampi of 2VO rats at 2 weeks after stereotaxic injection of antagomir27a for 1 week determined by qRT-PCR; n = 3. b Representative images of EM showing that stereotaxic injection of antagomir27a reversed the autophagic pathology induced by 2VO. Scale bars, 2 μm. c The quantitative analysis of AVs and lysosome number; n = 3. At least 5 cells were selected in each hippocampus, and more than 15 cells in each group were used to analyze the number of both AVs and lysosomes. d–f Effects of stereotaxic injection of antagomir27a on the expression of LC3-II (d), SQSTM1 (e), and LAMP-2 (f); n = 6. Values are mean ± SEM. *P < 0.05 versus sham, # P < 0.05 versus 2VO
Discussion
Here, we reported that CBH generated by 2VO could result the impairment of lysosomal clearance in the hippocampi of rats at 2 weeks. Further analysis implied that Mir27a was the key regulator of this process through regulating the expression of the LAMP-2 protein at posttranscriptional level. Therefore, our study revealed a novel molecular mechanism of autophagic-lysosomal system at the miRNA level during CBH and provided a new evidence of miRNA functions in autophagy under hypoxia.
As a cellular self-digestive pathway, numerous studies have reported that autophagy is transient during starvation or acute ischemia. In vitro, Yu et al. reported that after 4 h of starvation in multiple cell types, almost all lysosomes were consumed along with the formation of a few large autolysosomes; however, they were largely recovered after 12 h of starvation [45–49]. The dynamic process of autophagy was recognized as the first response for living cells to adapt to the fluctuating environments in a process called autophagic lysosome reformation (ALR) [46, 47]. In this study, using an animal model of CBH by 2VO, but not an acute severe brain ischemia model by MCAO, we observed dynamic autophagy. At 1 week of 2VO, we classified this process as increased autophagy with normal autophagic flux for the following reasons: (1) a significantly decreased ratio of phospho-p70S6k/p70S6k implied activated autophagy, which was similar to a previous report with regard to hypoxia-activated autophagy [50, 51]. (2) A previous study demonstrated that newly formed autophagosomes are normally eliminated efficiently by fusion with lysosomes, thereby avoiding a build-up of autophagic intermediates [10]. In the present study, we found abundant lysosomes with a slightly increased number of AVs and increased CathD expression, a marker of the protease activity of lysosomes [10]. The results suggest that most of the generated AVs by CBH may be efficiently cleared by abundant lysosomes. (3) As a transient event, SQSTM1 was used as a marker of reduced autophagic clearance [34, 52]. However, SQSTM1 was also localized in the late endosome/lysosomal compartment [34], indicating that increased SQSTM1 may result from both impairment of autophagic clearance and increased lysosomes. In the present study, we found an increase in the LC3-II level accompanied by an elevated SQSTM1 level, which suggests that the increased lysosomes overwhelmed the clearance of SQSTM1 by autophagy activation in the hippocampi of rats at 1 week after 2VO. At 2 weeks after 2VO, basally activated autophagic generation was accompanied by reduced autophagic flux based on the following evidence: (1) we observed profuse accumulation of AVs in the hippocampi of rats containing organellar materials in smaller vesicles, which was considered to be the limited proteolysis occurred within these compartments due to the reduced fusion of autophagosomes with the endosomal and lysosomal compartments [10, 53]. This phenomenon was consistent with the decreased expression of CathD (47 kDa) in the hippocampi of rats at 2 weeks after 2VO. (2) There was sustained increase in LC3-II together an increasingly with ongoing high level of SQSTM1. Although the high level of SQSTM1 at 2 weeks was similar to that at 1 week, we thought that should result from both the constitutive generation of AVs and uncleared AVs due to the reduction of lysosomal function in the hippocampi of rats at this time point, as evidenced by the increase of AVs and the decrease in both LAMP-1 and CathD (47 kDa) expression. Importantly, we found that Mir27a levels were significantly increased in the hippocampi of rats at 2 weeks after 2VO and that overexpression of Mir27a induced the elevation of LC3-II and SQSTM1 with CathD reduction in vitro. Furthermore, Mir27a overexpression also blocked LC3-II turnover after NRNs were treated with Rap/RC and eliminated the inhibition effects of 3MA on autophagy. In addition, Mir27a overexpression could induce greater colocalization of SQSTM1 with CD63 and increase the LysoTracker-labeled subtractions of SQSTM1 antibodies, while what is the mechanism of overexpression of Mir27a on affecting the mRNA level of SQSTM1 needs to be studied in the future. All of these data indicate that the increased LC3-II and SQSTM1 in the hippocampi of rats at 2 weeks after 2VO may be due to impaired autophagic clearance. (3) In addition to the above changes, the LAMP-2 level was significantly decreased, which was consistent with previous reports that LAMP-2 deficiency in transgenic mice caused an accumulation of AVs, as well as a reduction in Cath D in many tissues with impaired autophagic flux [40, 54]. Taken together, these phenomena suggest that the impaired clearance function of autophagy was due to the LAMP-2-mediated dysfunction of lysosomes at 2 weeks after 2VO through the inhibited fusion of autophagosomes with lysosomes [39]. Notably, we found that levels of LC3-II in the hippocampi of rats were very similar between 1 and 2 weeks after 2VO, whereas the increase in AVs at 2 weeks was approximately twofold larger than that at 1 week. One possible explanation may be the persistent synthesized LC3-II protein at 1 week, in which mature AVs have not yet formed. A similar phenomenon was also observed in previous studies [55]. In addition, since LAMP-2 is the key membrane element of lysosome, which is a critical determinant of autophagosome-lysosome fusion in macroautophagy as well as the only binding protein of chaperone-substrate protein complex (substrate proteins-HSC70 complex) at the lysosomal membrane in chaperone-mediated autophagy (CMA) [56], abnormal of LAMP-2 would definitely affect the function of CMA too.
In contrast to previous mounting studies associated with miRNAs, CBH induced transitional changes of Mir27a in the hippocampi of rats at different time points after CBH. Based on our data, we found that the Mir27a level decreased at 1 week but increased at 2 weeks in rats after 2VO, which was negatively correlated with the expression of LAMP-2 protein in the hippocampi of rats during CBH. Our study further demonstrated that Mir27a posttranscriptionally regulated LAMP-2 protein expression. Coincidentally, the inhibition of the Mir27a level by antagomir-27a in the hippocampi of rats at 2 weeks after 2VO could also significantly induce an increased number of lysosomes. These results provided additional evidences that Mir27a is the key regulator of the dynamic autophagy process during CBH by regulating LAMP-2 protein expression at the posttranscriptional level.
Prospectively, previous studies have demonstrated that CBH could induce the overproduction of Aβ by upregulating APP and BACE1 [14–16]. Here, we provide evidence that declined lysosomal clearance in the hippocampi of rats at 2 weeks after 2VO may be another cause that leads to the accumulation of either Aβ or the phosphorylation of Tau in early dementia. It is worth mentioning that these findings provide new insight into a novel molecular mechanism of autophagy and drug targets at the miRNA level during CBH.
Abbreviations
- miRNA:
-
MicroRNA
- Mir27a:
-
MicroRNA-27a
- AMO-27a:
-
2′-O-Methyl antisense oligoribonucleotides to miR-27a
- NC:
-
Scramble negative control
- ODN:
-
miRNA-masking antisense oligodeoxynucleotides (miR-masks)
- LC3:
-
Microtubule-associated protein 1 light chain 3
- LAMP-2:
-
Lysosomal-associated membrane protein-2
- SQSTM1/P62:
-
Sequestosome1
- MTOR:
-
Mechanistic target of rapamycin
- p70S6k:
-
Ribosomal protein S6 kinase 70kDa
- AVs:
-
Autophagic vaculoses
- AL:
-
Autolysosome
- 3MA:
-
3-Methyladenine
- Rap:
-
Rapamycin
- 3′UTR:
-
3′-Untranslated region
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- 2VO:
-
Bilateral common carotid artery occlusion
References
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. doi:10.1038/nature06639
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci 20:7268–7278
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA (2005) Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171:87–98
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141:1146–1158. doi:10.1016/j.cell.2010.05.008
Funderburk SF, Marcellino BK, Yue Z (2010) Cell “self-eating” (autophagy) mechanism in Alzheimer’s disease. Mt Sinai J Med 77:59–68. doi:10.1002/msj.20161
Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43:38–45. doi:10.1016/j.nbd.2011.01.021
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28:6926–6937. doi:10.1523/JNEUROSCI.0800-08.2008
Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57:789–794
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. doi:10.1161/STROKEAHA.111.634279
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Ai J, Sun LH, Che H, Zhang R, Zhang TZ, Wu WC, Su XL, Chen X, Yang G, Li K, Wang N, Ban T, Bao YN, Guo F, Niu HF, Zhu YL, Zhu XY, Zhao SG, Yang BF (2013) MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J Neurosci 33:3989–4001. doi:10.1523/JNEUROSCI.1997-12.2013
Kitaguchi H, Tomimoto H, Ihara M, Shibata M, Uemura K, Kalaria RN, Kihara T, Asada-Utsugi M, Kinoshita A, Takahashi R (2009) Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Res 1294:202–210. doi:10.1016/j.brainres.2009.07.078
Zhiyou C, Yong Y, Shanquan S, Jun Z, Liangguo H, Ling Y, Jieying L (2009) Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem Res 34:1226–1235. doi:10.1007/s11064-008-9899-y
Sun LH, Ban T, Liu CD, Chen QX, Wang X, Yan ML, Hu XL, Su XL, Bao YN, Sun LL, Zhao LJ, Pei SC, Jiang XM, Zong DK, Ai J (2015) Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation. J Neurochem 134:1139–1151. doi:10.1111/jnc.13212
Farkas E, Institoris A, Domoki F, Mihaly A, Bari F (2006) The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res 1087:168–174
Liu HX, Zhang JJ, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139:169–177
Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM (2013) Influence of microRNA deregulation on chaperone-mediated autophagy and alpha-synuclein pathology in Parkinson’s disease. Cell Death Dis 4:e545. doi:10.1038/cddis.2013.73
Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S (2007) The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res 67:11001–11011
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F, Yu F (2012) MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One 7:e51702. doi:10.1371/journal.pone.0051702
Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, Sakai A, Okada H, Watanabe R, Murakami S, Yi M, Lemon SM, Kaneko S (2013) MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol 87:5270–5286. doi:10.1128/JVI.03022-12
Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW (2012) MicroRNA-27a regulates cardiomyocytic apoptosis during cardioplegia-induced cardiac arrest by targeting interleukin 10-related pathways. Shock 38:607–614. doi:10.1097/SHK.0b013e318271f944
Kulshreshtha R, Davuluri RV, Calin GA, Ivan M (2008) A microRNA component of the hypoxic response. Cell Death Differ 15:667–671. doi:10.1038/sj.cdd.4402310
Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q (2014) MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis 5:e1132. doi:10.1038/cddis.2014.92
Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM (2013) Hypoxia mediates mutual repression between microRNA-27a and PPARgamma in the pulmonary vasculature. PLoS One 8:e79503. doi:10.1371/journal.pone.0079503
Wu X, Bhayani MK, Dodge CT, Nicoloso MS, Chen Y, Yan X, Adachi M, Thomas L, Galer CE, Jiffar T, Pickering CR, Kupferman ME, Myers JN, Calin GA, Lai SY (2013) Coordinated targeting of the EGFR signaling axis by microRNA-27a*. Oncotarget 4:1388–1398
Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A (2008) Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 155:626–639. doi:10.1016/j.neuroscience.2008.06.023
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6:366–377
Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON (2014) Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 45:2438–2443. doi:10.1161/STROKEAHA.114.005183
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221:117–124. doi:10.1002/path.2694
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125:3170–3181. doi:10.1161/CIRCULATIONAHA.111.041814
Bohley P, Seglen PO (1992) Proteases and proteolysis in the lysosome. Experientia 48:151–157
Amritraj A, Wang Y, Revett TJ, Vergote D, Westaway D, Kar S (2013) Role of cathepsin D in U18666A-induced neuronal cell death: potential implication in Niemann-Pick type C disease pathogenesis. J Biol Chem 288:3136–3152. doi:10.1074/jbc.M112.412460
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502
Saftig P, Beertsen W, Eskelinen EL (2008) LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy 4:510–512
Saugstad JA (2010) MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab 30:1564–1576
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK (2010) MicroRNAs induced during ischemic preconditioning. Stroke 41:1646–1651. doi:10.1038/jcbfm.2010.101
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42. doi:10.1016/j.cell.2007.12.018
Seglen PO, Gordon PB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A 79:1889–1892
Massey AC, Follenzi A, Kiffin R, Zhang C, Cuervo AM (2008) Autophagy 4:442–456
Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465:942–946. doi:10.1038/nature09076
Chen Y, Yu L (2013) Autophagic lysosome reformation. Exp Cell Res 319:142–146. doi:10.1016/j.yexcr.2012.09.004
Adhami F, Schloemer A, Kuan CY (2007) The roles of autophagy in cerebral ischemia. Autophagy 3:42–44
Finn PF, Mesires NT, Vine M, Dice JF (2005) Effects of small molecules on chaperone-mediated autophagy. Autophagy 1:141–145
Althausen S, Mengesdorf T, Mies G, Olah L, Nairn AC, Proud CG, Paschen W (2001) Changes in the phosphorylation of initiation factor eIF-2alpha, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischaemia in mice. J Neurochem 78:779–787
Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, Moro MA, Pende M, Calvo S (2009) mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem 284:22067–22078. doi:10.1074/jbc.M109.033100
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163
Kochl R, Hu XW, Chan EY, Tooze SA (2006) Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7:129–145
Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406:902–906
Klionsky DJ, Abdalla FC, Abeliovich H et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Cuervo AM (2010) Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21(3):142–150. doi:10.1016/j.tem.2009.10.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Natural Science Foundation of China (81070882, 81471115, 81271207 to J. A.) and the Creative Research Groups of the National Natural Science Foundation of China (81421063 to Y.B.F.).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Hui Che and Yan Yan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Che, H., Yan, Y., Kang, XH. et al. MicroRNA-27a Promotes Inefficient Lysosomal Clearance in the Hippocampi of Rats Following Chronic Brain Hypoperfusion. Mol Neurobiol 54, 2595–2610 (2017). https://doi.org/10.1007/s12035-016-9856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9856-8