Abstract
Blood–brain barrier preservation plays an important role in attenuating vasogenic brain edema after subarachnoid hemorrhage (SAH). This study was designed to investigate the protective effect and mechanism of artesunate, a traditional anti-malaria drug, on blood–brain barrier after SAH. Three hundred and seventy-seven (377) male Sprague–Dawley rats were subjected to endovascular perforation model for SAH. The rats received artesunate alone or in combination with Sphingosine-1-phosphate receptor-1 (S1P1) small interfering RNA (siRNA), antagonist VPC23019, or phosphatidylinositol 3-kinase inhibitor wortmannin after SAH. Modified Garcia score, SAH grades, brain water content, Evans blue leakage, transmission electron microscope, immunohistochemistry staining, Western blot, and cultured endothelial cells were used to investigate the optimum concentration and the therapeutic mechanism of artesunate. We found that artesunate (200 mg/kg) could do better in raising modified Garcia score, reducing brain water content and Evans blue leakage than other groups after SAH. Moreover, artesunate elevated S1P1 expression, enhanced phosphatidylinositol 3-kinase activation, lowered GSK-3β activation, stabilized β-catenin, and improved the expression of Claudin-3 and Claudin-5 after SAH in rats. These effects were eliminated by S1P1 siRNA, VPC23019, and wortmannin. This study revealed that artesunate could preserve blood–brain barrier integrity and improve neurological outcome after SAH, possibly through activating S1P1, enhancing phosphatidylinositol 3-kinase activation, stabilizing β-catenin via GSK-3β inhibition, and then effectively raising the expression of Claudin-3 and Claudin-5. Therefore, artesunate may be favorable for the blood–brain barrier (BBB) protection after SAH and become a potential candidate for the treatment of SAH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is associated with high morbidity and fatality rate at a comparatively young age [1], resulting in a huge loss to the society. Currently, the pathophysiology of SAH is perceived as referring to the cerebral vascular neural network, which includes all small blood vessels downstream of the major cerebral arteries and cell types associated with these structures [2]. Blood–brain barrier (BBB) is one of the typical components of this vascular neural network. BBB breakdown has close connection with vasogenic brain edema after SAH [3]. Therefore, by targeting BBB breakdown, it may be favorable for the patients with SAH.
Artesunate has become a standard treatment for cerebral malaria and all kinds of severe malaria because of its safety and efficacy. Accumulated research shows that artesunate have multiple effects, including anti-inflammatory [4], antiviral [5], impeding angiogenesis [6], and anticancer [7], etc. Independent of plasmodium killing, recent studies revealed artesunate can ameliorate BBB breakdown, after experimental malaria infection in mice [8]. However, the efficacy of artesunate has not been evaluated after SAH.
Sphingosine-1-phosphate receptor-1 (S1P1), the receptor of Sphingosine-1-phosphate (S1P), mainly expresses on vascular endothelial cells. Previous researches revealed that S1P1 activation could protect injured brain tissue in many stroke models, such as neonatal hypoxic-ischemic brain injury [9], transient middle cerebral artery occlusion [10], SAH [11], and intracerebral hemorrhage [12]. Moreover, S1P1 was closely associated with PI3K/Akt pathway [13], which is reported to possess BBB protective action after intracerebral hemorrhage in mice [14]. Thus, S1P1 may serve as a valuable therapeutic target for SAH research. Recent studies indicated FTY720, the agonist of S1P1, treatment resulted in symptoms against BBB breakdown and thereby increased survival rate to cerebral malaria in mice [15].
Based on these evidences, we purpose that artesunate may be functional via S1P1 activation in the protection of BBB integrity after SAH. By using the rat endovascular perforation model of SAH, the present study sought to investigate the potential protective effects and underlying mechanisms of artesunate on the BBB after SAH.
Materials and Methods
Animal Preparation
All experimental procedures were approved by the Ethic Committee of Southwest Hospital and performed in accordance with the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Total 377 adult male Sprague–Dawley (SD) rats (8–12 weeks, 280 to 330 g) were used in the present study (Experimental Animal Center of Third Military Medical University, Chongqing, China). Due to severe hemorrhagic volume, 59 rats which died within 24 h after SAH were excluded from the present study. Rats were housed in a humidity (50 to 60 % relative humidity) and temperature-controlled (24 ± 1 °C) room with food and water ad libitum. The light was controlled in a 12-h light/dark cycle. After surgery, buprenorphine was used depending on the degree of observed distress or pain and given for 6 h–1 day depending on signs of pain or distress. The first administration of buprenorphine (0.02 mg/kg) was injected subcutaneously before anesthetic recovery, and rats were observed for any sign of pain or distress.
Experimental Design
Three experiments were conducted in the present study.
Experiment I
For outcome evaluation, a total of 163 rats were randomly divided into 7 groups: the sham group (n = 25), the SAH group (n = 25), the SAH + Vehicle group (n = 25), the SAH + artesunate (25, 50, 100, and 200 mg/kg) groups (n = 88). Immediately after SAH, different doses of artesunate (dissolved in 5 % sodium bicarbonate solution, Holley-Wulingshan pharmaceuticals (Chongqing) Corp. Ltd., Chongqing, China) was administered intraperitoneally in the rats of SAH + artesunate groups once a day, and those in the SAH + Vehicle group were given the same volume of 5 % sodium bicarbonate solution. Modified Garcia neurological scores, SAH grading scores and brain water content were used to assess the degree of brain injury in all groups (n = 7 per group) at 24 or 72 h after surgery. Evans blue extravasation was evaluated at 24 h after SAH in all groups (n = 7 per group). Evans blue fluorescence and colloidal gold leakage were also observed for the degree of BBB breakdown in the sham group, the SAH group, the SAH + Vehicle group, and the SAH + 200 mg/kg artesunate group (n = 2 per group) at 24 h after SAH.
Experiment II
In order to clarify the correlation between artesunate and S1P1, 48 rats were randomly assigned into 8 groups (n = 5 per group), consisting of sham (24 and 72 h), SAH (24 and 72 h), SAH + Vehicle (24 and 72 h), and SAH + 200 mg/kg artesunate (24 and 72 h). And then, cerebral vascular endothelial cells were cultured in the medium containing 10 μM oxyhemoglobin for 24 h, which were treated with different concentrations of artesunate (2, 4, 6, 8, 12, and 16 nmol/L) or same volume of 5 % sodium bicarbonate solution. Western blots of ipsilateral/right hemisphere were harvested to detect the expression of S1P1 both in vivo and in vitro after artesunate treatment. Immunohistochemistry staining of S1P1 was also conducted in sham group, SAH group, SAH + Vehicle group, and SAH + 200 mg/kg artesunate group (n = 2 per group) at 24 h after SAH.
Experiment III
To ensure that artesunate exhibits its functions via S1P1, 90 rats were divided into 8 groups: sham, SAH, SAH + Vehicle, SAH + 200 mg/kg artesunate, SAH + 200 mg/kg artesunate + S1P1 scrambled small interfering RNA (siRNA), SAH + 200 mg/kg artesunate + S1P1 siRNA, SAH + 200 mg/kg artesunate + N, N-dimethylsphingosine (DMS, Sigma-Aldrich, St. Louis, MO; dissolved in 1 % DMSO at final concentration of 0.17ug/0.5ul, 0.4 mg/kg was given intraperitoneally at 30 min after SAH), and SAH + 200 mg/kg artesunate + vehicle 3 (the same volume of 1 % DMSO was given intraperitoneally at 30 min after SAH). Modified Garcia neurological scores, SAH grading scores, brain water content, and Evans blue extravasation were evaluated at 24 h after SAH (n = 7 per group). The data of these tests in sham, SAH, SAH + Vehicle, and SAH + 200 mg/kg artesunate groups were shared with experiment I. Evans blue fluorescence was detected at 24 h after SAH (n = 2 per group). And sphingosine kinase (SphK) types 1 and 2, Claudin-3, Claudin-5 expressions were also detected in right hemisphere by Western blot at 24 h after SAH (n = 5 per group).
Experiment IV
For mechanism study, 76 rats in all were randomly separated into 8 groups: sham, SAH, SAH + Vehicle, SAH + 200 mg/kg artesunate, SAH + 200 mg/kg artesunate + S1P1 antagonist VPC23019 (VPC, Avanti Polar Lipids, Inc. Alabaster, AL, dissolved in the mixture of 3 % bovine serum albumin (BSA) + DMSO + 1 N HCL, 0.5 mg/kg was given intraperitoneally at 30 min after SAH,), SAH + 200 mg/kg artesunate + Vehicle 1 (the same volume of the mixture of 3 % BSA + DMSO + 1 N HCL was given intraperitoneally at 30 min after SAH), SAH + 200 mg/kg artesunate + PI3K inhibitor wortmannin (Wort, Sigma-Aldrich, St. Louis, MO, dissolved in 1 % DMSO, 15 μg/kg was given intravenously at 30 min after SAH), and SAH + 200 mg/kg artesunate + Vehicle 2 (the same volume of 1 % DMSO was given intravenously at 30 min after SAH). Modified Garcia neurological scores, SAH grading scores, brain water content, and Evans blue extravasation were used to assess the degree of brain injury in all groups at 24 after SAH (n = 7 per group). The data of these tests in sham, SAH, SAH + Vehicle, and SAH + 200 mg/kg artesunate groups were shared with experiment I. In order to detect the expression of p-Akt/Akt, p-GSK-3β/ GSK-3β, p-β-catenin/β-catenin, Claudin-3, and Claudin-5 in right hemispheres of rats in each group (n = 5 per group), Western blots were conducted at 24 h after SAH.
SAH Model
SAH model was induced by endovascular perforation as described previously [16]. Briefly, all animals were anesthetized with an intraperitoneal injection of chloral hydrate (40 mg/kg). The right external carotid artery was identified through a ventral midline neck incision and transected distally with a 4-mm stump. A 4–0 sharpened monofilament nylon suture (Shadong Biotech Corp. Ltd., Beijing, China) was advanced into the right internal carotid artery through the right external carotid artery until slight resistance was felt (about 18–23 mm) and then was pushed 3 mm further to penetrate the bifurcation of the anterior and middle cerebral artery. The nylon suture was then withdrawn, the stump of the right external carotid artery was ligated, and the internal carotid artery was reperfused. Except for the perforation of the artery, the sham group rats underwent the same procedure with SAH models.
Intracerebroventricular Injection
Intracerebroventricular injection procedure was performed as reported previously [17]. A small burr hole was drilled on the skull according to the following coordinates relative to bregma: 1.5 mm posterior; 1.0 mm lateral. The needle of 10 μL Hamilton syringe (Microliter 701; Hamilton Company, Reno, NV) was stereotactically inserted into the left lateral ventricle through the burr hole 4.0 mm below the horizontal plane of bregma. 500 pmol/5 μL S1P1 or scrambled siRNA (GenePharma Co., Ltd, Shanghai, China) were infused at the same rate at 48 h before SAH surgery. The syringe was left in situ for an additional 10 min before slowly removing. S1P1 siRNA is a pool of three different siRNA duplexes in order to improve the knockdown efficiency. All S1P1 siRNA sequences are provided in 5′ → 3′ orientation:
-
(I) Sense: GCUGCUUGAUCAUCCUAGATT
Antisense: UCUAGGAUGAUCAAGCAGCTT
-
(II) Sense: CCUGUGACAUCCUGUACAATT
Antisense: UUGUACAGGAUGUCACAGGTT
-
(III) Sense: CGCAGCAAAUCAGACAACUTT
Antisense: AGUUGUCUGAUUUGCUGCGTT
Neurological Score
At 24 and 72 h after surgery, all the rats of each group were neurologically evaluated by a blinded observer, using the 18-point scoring system named Modified Garcia neurological scores [18]. This scoring system was composed of six tests, which were spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch. The minimum aggregate neurological score of six tests is 3 and the maximum is 18.
SAH Grade Assessment
The severity of SAH was assessed by using an 18-point SAH grading scale as previously reported [19], which in brief, was based on the amount of subarachnoid blood clot, and the 6 separated parts of the basal cistern could be scored from the scale of 0 to 3 (total 0–18 points). If the scoring result was less than 8, then it would be excluded from the study.
Brain Water Content
Edema was assessed by the method as follows: after decapitating rats and separating its brain tissues, the left and right cerebral hemispheres were obtained and quickly weighed to get the wet weight. Then, the samples were placed in an oven set at the temperature of 105 °C for 72 h to obtain the dry weight. By using the formula: ([wet weight − dry weight]/wet weight) *100 %, edema could be assessed accurately [20].
Evans Blue Extravasation and Fluorescence
BBB permeability was shown through Evans blue extravasation and staining. Evans blue extravasation was conducted as previously published [21]. Under deep anesthesia, Evans blue dye (Sigma-Aldrich, St. Louis, MO, 2 %, 4 mL/kg was given intravenously at 24 h after SAH) was injected over 2 min into the right femoral vein of rats and allowed to circulate for 60 min. Then, the rats were sacrificed by intracardial perfusion with 0.9 % saline. The left and right cerebral hemispheres were obtained after decapitating rats and its brain tissues were separated. After quickly weighed and homogenized in 5 mL of 0.9 % saline, the cerebral hemispheres were centrifuged (15,000 g, 30 min) at room temperature. After that, the supernatant was extracted and added the same volume of trichloroacetic acid and incubated overnight at 4 °C. The next day, the samples were centrifuged (15,000 g, 30 min) at room temperature and measured by the spectrophotometer at 620 nm for the amount of Evans blue dye.
Auto-fluorescence of Evans blue was performed as previously reported [21]. Four percent of paraformaldehyde was perfused through the rat heart after 0.9 % saline perfusion. Then, the brain tissue was obtained and cut into 30 μm coronal floating sections using a Leica CM1900 cryostat (Leica biosystems Nussloch GmbH, Wetzlar, Germany). The red auto-fluorescence of Evans blue was displayed on the coronal floating sections under a Zeiss LSM780 confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Transmission Electron Microscope
Transmission electron microscope was also used to display BBB permeability of the rats at 24 h after surgery [22]. Three milliliter per kg Colloidal gold nanoparticles (Shengtaier Biological Medicine Technology Co. Ltd., Chengdu, China, 30 nm in diameter) was injected over 5 min into the right femoral vein of rats and allowed to circulate for 60 min at 24 h after surgery. Rats were deeply anesthetized and then sacrificed by intracardial perfusion with 0.9 % saline and 4 % paraformaldehyde. The brain tissues were removed and post-fixed with 2 % glutaraldehyde and 2 % formaldehyde for 30 min. Then, the brain tissues were minced into 1 cubic millimeter pieces and incubated in 2 % glutaraldehyde and 2 % formaldehyde overnight at 4 °C. Samples were impregnated with epoxy resin after dehydration. Sections were treated with lead citrate and uranyl acetate. Electron micrographs were obtained using an H-7100 transmission electron microscope (Hitachi, Ltd., Tokyo, Japan).
Immunohistochemistry Staining
The rats, under deep anesthesia, were sacrificed by an intracardial perfusion with 0.9 % saline and 4 % paraformaldehyde at 24 h after surgery for immunohistochemical staining. The brain tissues were obtained, post-fixed, embedded with paraffin, and cut into 6 μm sections using a vibratome. Immunohistochemistry was conducted as previously reported [16]. In brief, the sections were first treated with 0.3 % Triton X-100 and 3 % H2O2 (Chengdu Kelong Chemical Co. Ltd., Chengdu, China). Secondly, rabbit anti-S1P1 (1:200, Santa Cruz Biotechnology Inc., Shanghai, China) was used to incubate the sections for 24 h at 4 °C. Thirdly, the sections were treated with Streptavidin-Peroxidase kit (Zhongshan Golden-Bridge Biotechnology Co. Ltd., Beijing, China). Finally, the mixture of diaminobenzidine (DAB; Beyotime biotechnology, Co. Ltd., Jiangsu, China) + 0.05 % H2O2 (Chengdu Kelong Chemical Co. Ltd., Chengdu, China) was used to incubate the sections for 2 min. Representative sections of each group were selected, fixed, and photographed.
Western Blot
Western blot was conducted as previously described [23]. The right cerebral hemisphere of rats was acquired and homogenized. Total proteins were extracted by means of a protein extraction kit (Beyotime Biotechnology, Co. Ltd., Jiangsu, China). The equal amount of extracted proteins was loaded on a 10 % SDS-polyacrylamide gels. The proteins in the gels were electrophoresed and then transferred onto PVDF membranes (EMD Millipore Inc., Billerica, MA). Blocking buffer was used to block the membranes, which were incubated afterwards with following antibodies overnight at 4 °C: rabbit anti-S1P1(1:200, Santa Cruz Biotechnology Inc., Shanghai, China), rabbit anti-Sphk1 (1:1000, Abcam plc., Cambridge, MA), rabbit anti-Sphk2 (1:1000, Abcam plc., Cambridge, MA), rabbit anti-Akt (1:200, Cell signaling technology, Inc., Boston, MA), rabbit anti-phospho-Akt (1:200, Cell signaling technology, Inc., Boston, MA), rabbit anti-phospho-GSK-3β (1:200, Abcam plc., Cambridge, MA), rabbit anti-GSK-3β (1:200, Abcam plc., Cambridge, MA), mouse anti-phospho-β-catenin (1:200, Abcam plc., Cambridge, MA), mouse anti-β-catenin (1:200, from Abcam plc., Cambridge, MA), goat anti-Claudin-3 (1:200, Santa Cruz Biotechnology Inc., Shanghai, China), goat anti-Claudin-5 (1:200, Santa Cruz Biotechnology Inc., Shanghai, China), mouse anti-β-Actin (1:200, Santa Cruz Biotechnology Inc., Shanghai, China), mouse anti-GAPDH (1:200, Zhongshan Golden-bridge Biotechnology Co. Ltd., Beijing, China), mouse anti-Tublin (1:200, from Beyotime Biotechnology, Co. Ltd., Jiangsu, China), respectively. The membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:1000; Beyotime biotechnology, Co. Ltd., Jiangsu, China) for 2 h. The membranes were detected with a chemiluminescence reagent kit (Thermo Fisher Scientific Inc., Waltham, MA). All blot bands were quantified using densitometry with Quantity One software (1-D Analysis Software Version 4.4, Bio-Rad Laboratories, Inc., Hercules, CA).
Endothelial Cells Culture and Treatments
Immortalized rat brain microvessel endothelial cells (YS-axbz-229, Yisen Biotechnology, Co. Ltd, Beijing, China) were cultured in RPMI1640 medium (GIBCO, Shanghai, China) plus 10 % FBS, 1 % (v/v) penicillin/streptomycin (Beyotime Biotechnology, Co. Ltd., Jiangsu, China) with/without 10 μM oxyhemoglobin (Sigma-Aldrich, St. Louis, MO) in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C. Viable cells were counted by trypan blue and used for experiments at 24 h after planting. At 1 day after different doses of artesunate (2, 4, 6, 8, 12, and 16 nmol/L) treatment, endothelial cells were harvested and lysed in RIPA buffer (Beyotime Biotechnology, Co. Ltd., Jiangsu, China), which was mixed of protease and phosphatase inhibitors. S1P1 of protein samples were detected by using Western blot as described above.
Statistical Analysis
All data were expressed as means ± standard deviations and analyzed by using SPSS 18.0 software. One-way ANOVA plus Tukey multiple comparisons test were used to compare different groups. Neurological scores were analyzed by using χ 2 test. P < 0.05 was thought as the statistical difference.
Results
Artesunate Alleviated Neurologic Impairment and Brain Edema After SAH in Rats
SAH grading scoring results indicated that no significant differences were found in damage among groups either at 24 or 72 h after SAH (Fig. 1a, b). None of the sham-operated rats died, and 59 rats died within 24 h after SAH caused by severe hemorrhagic volume.
Effects of artesunate treatment on neurological score and brain edema after SAH. a SAH grading score of each group in Experiment I at 24 and 72 h b after SAH (n = 7). c Modified Garcia test results of each group at 24 and 72 h d after SAH (n = 7). e Brain edema assessment of each group at 24 and 72 h f after SAH (n = 7). Arte indicates artesunate; VPC indicates VPC23019; Wort indicates wortmannin. #: vs sham P < 0.05, *: vs SAH P < 0.05, and §: vs SAH + Vehicle P < 0.05
The neurologic score in SAH rats remarkably decreased compared to those in sham group rats at 24 h after SAH (Fig. 1c); but there was not much difference between SAH + artesunate (100 and 200 mg/kg) groups and sham group at 72 h after SAH (Fig. 1d). Compared with SAH + Vehicle group, not only neurologic deficits for SAH + artesunate (100 and 200 mg/kg) groups at 24 h were obviously alleviated, but SAH + artesunate (50, 100, and 200 mg/kg) groups at 72 h after SAH were also alleviated significantly (Fig. 1c, d).
Compared with sham group, the groups of SAH, SAH + Vehicle, and SAH + artesunate (25 and 50 mg/kg) all showed significant brain water content increasing in both hemispheres at 24 and 72 h after SAH (Fig. 1c, d); but, as for the group of SAH + artesunate (100 and 200 mg/kg), brain water content increasing did not rise in the left hemisphere at 24 h and in both hemispheres at 72 h after SAH (Fig. 1c, d). SAH + Artesunate (100 and 200 mg/kg) groups also presented distinct brain edema alleviation in contrast to SAH + Vehicle group at 24 and 72 h after SAH (Fig. 1c, d).
Artesunate Reduced BBB Damage After SAH in Rats
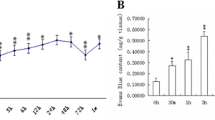
In order to evaluate BBB integrity, we detected the extravasation of Evans blue dye at 24 h after surgery. The results manifested that much more extravasated Evans blue dye was measured in both hemispheres after SAH. However, compared with SAH + Vehicle group, treatment of 100 and 200 mg/kg artesunate significantly reduced the leakage of Evans blue dye in both hemispheres; whereas the same effect was not found in 25 and 50 mg/kg of artesunate treatment groups (Fig. 2a). Representative electron microscope micrographs and confocal imaging of Evans blue also revealed that treatment of 200 mg/kg artesunate obviously reduced the extravasation of colloidal gold nanoparticles and Evans blue dye when compared with SAH + Vehicle group at 24 h after SAH (Fig. 2b, c).
Effects of artesunate treatment on blood–brain barrier permeability at 24 h after SAH. a Evans blue extravasation evaluation at 24 h after SAH (n = 7). b Representative pictures of Evans blue auto-fluorescence of cerebral cortex from sham, SAH, SAH + Vehicle, and SAH + 200 mg/kg artesunate-treated group at 24 h after SAH. c Representative pictures of colloidal gold extravasation in cerebral cortex of each group at 24 h after SAH. Arrow indicates the colloidal gold nanoparticles in the cerebral cortex. Arte indicates artesunate. #: vs sham P < 0.05, *: vs SAH P < 0.05, and §: vs SAH + Vehicle P < 0.05
Artesunate Increased the Expression of S1P1 In Vivo and In Vitro
To find out the relationship of artesunate and S1P1, we detected the expression of S1P1 in vivo and in vitro by using Western blot. Immunohistochemical staining revealed that the expression of S1P1 surrounding microvessels significantly reduced when comparing with the sham group at 24 h after SAH (Fig. 3a). Western blot results showed that the treatment of 200 mg/kg artesunate apparently increased the expression of S1P1 when compared with SAH + Vehicle group at 24 and 72 h after SAH, and there was not much difference between SAH + 200 mg/kg artesunate groups and sham group (Fig. 3b, c). In order to confirm the effects of artesunate in vitro, we administrated cultured endothelial cells with different concentrations of artesunate (2, 4, 6, 8, 12, and 16 nmol/L). Compared with normal and vehicle groups, this dosage of artesunate did not affect the endothelium cell viability (data no shown), while the results of Western blot showed that treatment 6, 8, 12, and 16 nmol/L artesunate significantly increased the expression of S1P1 (Fig. 3d). In addition, the expression of S1P1 in endothelial cells cultured with oxyhemoglobin significantly decreased but reversed by 6 or 8 nmol/L artesunate (Fig. 3e).
The expression of S1P1 in vivo and in vitro after artesunate treatment. a Representative immunohistochemistry staining slices of S1P1 at 24 h after SAH. Arrow indicates the expression of S1P1 in the cerebral vascular. b Representative bands and quantitative analysis of the expression of S1P1 in right hemisphere of brain specimen at 24 and 72 h c after SAH. e Representative bands and quantitative analysis of the expression of S1P1 in endothelial cells at 24 h after different doses of artesunate treatment. f Representative bands and quantitative analysis of the expression of S1P1 in endothelial cells cultured with oxyhemoglobin at 24 h after different doses of artesunate treatment. Relative density of each protein has been normalized against the sham group. Arte indicates artesunate; Vehi indicates Vehicle. #: vs sham P < 0.05, *: vs SAH P < 0.05, and §: vs SAH + Vehicle P < 0.05
Artesunate Exhibited the Protective Effects via S1P1 Receptors, Not Sphingosine Kinase
To investigate whether artesunate acts through the restoring S1P1 receptor after SAH, we employed the S1P1 siRNA to specifically inhibit the transcription of S1P1 receptor. SAH grading scoring results indicated that no significant differences were found in damages among groups either at 24 h after SAH (Fig. 4a). Compared to the neurologic score of SAH + 200 mg/kg artesunate group, the score of SAH + artesunate 200 mg/kg + S1P1 siRNA group significantly reduced at 24 h after SAH (Fig. 4b). On the other hand, S1P1 siRNA pretreatment also significantly reversed the effects of 200 mg/kg artesunate on reducing brain water content in SAH rats (Fig. 4c). In addition, the SAH + artesunate 200 mg/kg + S1P1 siRNA group showed remarkably increasing on the content of Evans blue extravasation by comparing to SAH + artesunate 200 mg/kg group at 24 h after SAH (Fig. 4d, e).
S1P1 siRNA abolished the protective effects of artesunate. a SAH grading score of each group in Experiment III at 24 h after SAH. Animals pretreated with S1P1 siRNA reversed the effects of artesunate given after SAH, including b modified Garcia test results (n = 7), c brain water content (n = 7), d Evans blue extravasation results (n = 7). e Representative pictures of Evans blue auto-fluorescence of cerebral cortex at 24 h after SAH. Arte indicates artesunate; Vehi indicates Vehicle; Scr siRNA indicates scrambled siRNA. #: vs sham P < 0.05, *: vs SAH P < 0.05, †: vs SAH + Vehicle P < 0.05, &: vs SAH + scrambled siRNA, P < 0.05
To investigate whether artesunate acts through regulating sphingosine kinase, by which the sphingosine 1 phosphate (S1P receptor ligand) was produced, we evaluated the levels of sphingosine kinase (SphK) types 1 and 2, by which the sphingosine 1 phosphate (S1P receptor ligand) was produced. SAH grading scoring results indicated that no significant differences were found in damages among groups either at 24 h after SAH (Fig. 5a). And we found that the sphingosine kinase inhibitor, N, N-dimethylsphingosine (DMS), could not reverse the neuroprotective effects of artesunate on modified Garcia score, brain water content, and Evan’s blue extravasation at 24 h after SAH (Fig. 5b–d). In addition, artesunate had no effects on the Sphk1 and Sphk2 expressions comparing to the SAH + vehicle group at 24 h after SAH (Fig. 5e, f).
Artesunate did not act through sphingosine kinase. a SAH grading score of each group in Experiment III at 24 h after SAH. Animals treated with sphingosine kinase inhibitor, DMS, did not reversed the effects of artesunate given after SAH, including b modified Garcia test results (n = 7), c brain water content (n = 7), d Evans blue extravasation results (n = 7). Representative Western blot bands and quantitative analysis of e Sphk1 and f Sphk2 after artesunate treatment at 24 h after SAH (n = 5). Arte indicates artesunate; DMS indicates N, N-dimethylsphingosine; Vehi indicates Vehicle. #: vs sham P < 0.05, *: vs SAH P < 0.05, †: vs SAH + Vehicle P < 0.05, &: vs SAH + Vehicle 3, P < 0.05
S1P1 or PI3K Inhibition Abolished the Protective Effects of Artesunate
SAH grading scoring results indicated that no significant differences were found in damages among groups either at 24 h after SAH (Fig. 6a). Compared to the neurologic score of SAH + 200 mg/kg artesunate group, the score of SAH + artesunate 200 mg/kg + VPC23019 group significantly reduced at 24 h after SAH. And also, the neuroprotective effect of artesunate 200 mg/kg treatment on neurological deficits was abolished by wortmannin (Fig. 6b).
S1P1 or PI3K inhibition abolished the protective effects of artesunate. a SAH grading score of each group in Experiment IV at 24 h after SAH. Animals treated with VPC23019 and wortmannin reversed the effects of artesunate given after SAH, including b modified Garcia test results (n = 7), c, d brain water content (n = 7), d, e Evans blue extravasation results (n = 7). Arte indicates artesunate; Vehi indicates Vehicle; VPC indicates VPC23019; Wort indicates wortmannin. #: vs sham P < 0.05, *: vs SAH P < 0.05, †: vs SAH + Vehicle P < 0.05, &: vs SAH + Vehicle 1 P < 0.05, and §: vs SAH + Vehicle 2 P < 0.05
On the other hand, VPC23019 or wortmannin treatment also significantly reversed the effects of 200 mg/kg artesunate on reducing brain water content in SAH rats (Fig. 6c, d). In addition, the SAH + artesunate 200 mg/kg + VPC23019 group and SAH + artesunate 200 mg/kg + wortmannin group showed remarkably increasing on the content of Evans blue extravasation by comparing to SAH + artesunate 200 mg/kg group at 24 h or 72 h (Fig. 6e, f).
S1P1 Stabilized β-catenin and Tight Junction Proteins Through Increased PI3K/Akt Activation and Reduced GSK-3β Activation
To determine the pathway of S1P1 to tight junction proteins, Western blot analyses of the right hemisphere were conducted at 24 h after surgery. Changes in protein expression of the phosphorylated and, therefore, activated Akt (p-Akt), GSK-3β (p-GSK-3β), and β-catenin (p-β-catenin) were quantified as a ratio to total Akt, GSK-3β, and β-catenin. The results indicated that, unlike the rats of sham group, activated Akt (p-Akt, Ser473) was obviously reduced in the right hemisphere of SAH + Vehicle rats, while, 200 mg/kg artesunate treatment could obviously raise the p-Akt/Akt ratio (Fig. 7a, b). This effect was significantly weakened by VPC23019 and reversed by wortmannin (Fig. 7a, b). Activated GSK-3β (p-GSK-3β, Tyr216) evidently went up in the right hemisphere of SAH + Vehicle rats when compared with the rats of sham group (Fig. 7c, d). But 200 mg/kg artesunate treatment obviously reduced the p-GSK-3/GSK-3β ratio when compared with vehicle, which was reversed by VPC23019 and wortmannin (Fig. 7c, d).
The expressions of Akt, GSK-3β, and β-catenin at 24 h after SAH. Representative Western blot bands and quantitative analysis of the expressions of a, b p-Akt/Akt, c, d p-GSK-3β/GSK-3β, and e, f p-β-catenin/β-catenin at 24 h after SAH. Relative density of each protein has been normalized against the sham group. Arte indicates artesunate; VPC indicates VPC23019; Wort indicates wortmannin. N = 5 per group; #: vs sham P < 0.05, *: vs SAH P < 0.05, †: vs SAH + Vehicle P < 0.05, and &: vs SAH + Vehicle 1 or 2 P < 0.05
As we all know, β-catenin phosphorylation promotes its degradation. Compared with the rats of sham group, the level of p-β-catenin was distinctly increased in the rats of the SAH + Vehicle (Fig. 7e, f). The treatment of 200 mg/kg artesunate also obviously reduced the p-β-catenin/β-catenin ratio when compared with vehicle, which was tendentially reversed by VPC23019 and wortmannin (Fig. 7e, f).
Claudin-3 and Claudin-5 were important components of tight junction proteins. The levels of Claudin-3 and Claudin-5 significantly went down at 24 h after SAH; but 200 mg/kg artesunate treatment obviously increased their expression when compared with SAH + Vehicle group (Fig. 8a–d). However, the rats of SAH + 200 mg/kg artesunate + S1P1 siRNA group and SAH + 200 mg/kg artesunate + VPC23019 group presented evidently lowered the levels of Claudin-3 and Claudin-5 when compared with scrambled siRNA or Vehicle 2 rats (Fig. 6a–d). The same situation also incurred in the SAH + 200 mg/kg artesunate + wortmannin groups when compared with Vehicle 3 rats (Fig. 6c, d). Thus, S1P1 siRNA, VPC23019, and wortmannin reversed the initial increasing of Claudin-3 and Claudin-5 by 200 mg/kg artesunate treatment after SAH.
The expressions of tight junction protein Claudin-3 and Claudin-5 at 24 h after SAH. Representative Western blot bands and quantitative analysis of a Claudin-3 and b Claudin-5 after artesunate treatment with/without S1P1 siRNA at 24 h after SAH. c Representative Western blot bands and quantitative analysis of Claudin-3 and Claudin-5 after artesunate treatment with/without VPC23019 at 24 h after SAH. d Representative Western blot bands and quantitative analysis of Claudin-3 and Claudin-5 after artesunate treatment with/without wortmannin at 24 h after SAH. Relative density of each protein has been normalized against the sham group. Arte indicates artesunate; Scr siRNA indicates scrambled siRNA; Vehi indicates Vehicle; VPC indicates VPC23019; Wort indicates wortmannin. N = 5 per group; #: vs sham P < 0.05, *: vs SAH P < 0.05, †: vs SAH + Vehicle P < 0.05, and &: vs SAH + scrambled siRNA or Vehicle 1 or Vehicle 2 P < 0.05
Discussion
This study mainly evaluated the therapeutic effect of artesunate for SAH. We found that artesunate treatment could raise neurological score, reduce brain edema, and decrease the extravasation of Evans blue dye after SAH. Therefore, we believed that artesunate could alleviate SAH-induced brain injury through preserving BBB integrity and thus reduced brain edema. These effects were possible to associate with the improved expression of S1P1 but not sphingosine kinase by which the sphingosine 1 phosphate (S1P receptor ligand) was produced. Our results indicated that S1P1 siRNA and antagonist VPC23019 and PI3K inhibitor wortmannin could reverse the therapeutic effect of artesunate for SAH. Furthermore, artesunate could affect the downstream proteins of S1P1, such as increasing the expression of p-Akt, Claudin-3, and Claudin-5 and reducing the ratio of p-GSK-3/GSK-3β and p-β-catenin/β-catenin at 24 h after SAH, and this effect could be reversed by VPC23019 and wortmannin too.
Artesunate is a semi-synthetic derivative of artemisinin, which is metabolized by CYP P450 2A6 (CYP 2A6) enzymes. It is widely used for the treatment of cerebral malaria and all kinds of severe malaria owing to its safety and efficacy. Yusof W, et al. reported that only patients with the CYP2A6*1B variants were responsible for ultra-rapid metabolism of artesunate and suffered a significantly higher incidence of adverse drug reactions [24]. On the other hand, Zhao F, et al. found that the cytotoxic action of artesunate is specific for retinoblastoma cells in a dose-dependent manner, but with low toxicity in normal retina cells [25]. Also, no direct brain toxicity was detected in Marijon A, et al.’s study [26]. In our present study, we did not observe obvious side effect. And accumulated studies indicated that artesunate has therapeutic effect on many other diseases, such as rheumatoid arthritis [4], cytomegalovirus infection [5], leukemia, and colon cancer [7], etc. Although the BBB protective effect of artesunate in malaria has been reported, the efficacy of artesunate has not been evaluated after SAH. This study confirmed the BBB protective effect of artesunate in SAH. Based on the widely used of artesunate for cerebral malaria and the lipophilicity to cross blood–brain barrier [27], we believed artesunate could be a safety therapeutical strategy for SAH patient. But the S1P1 expression after incubation of higher dosage (16 nM) of artesunate was lower than 8 and 12 nM (Fig. 3d). This result might suggest the toxicity of the high dosage of artesunate and further investigations are still needed for clinical trial.
Furthermore, the mechanism of artesunate against BBB breakdown after SAH had not been elucidated. Previous research had pointed out the BBB protective effect of artesunate in malaria mainly owing to preventing the Plasmodium berghei-induced inflammatory response by inhibiting NF-κB nuclear translocation and the subsequent expression of ICAM-1 [8] in endothelial cells. As we know, NF-κB and ICAM-1 play an important role in leukocyte adhesion and subsequent migration across the brain vascular endothelial cells [8, 28]. However, the early disruption of BBB after SAH is largely due to inter-endothelial tight junction proteins degraded by matrix metalloproteinase [29]. So, the BBB protective effect of artesunate in SAH might have nothing to do with NF-κB and ICAM-1. Previous study revealed that FTY720 (the agonist of S1P1) treatment protected the BBB and increased survival rate in mice subjected to cerebral malaria, and it had better therapeutic effect when treated with artesunate [15]. Hence, we hypothesized that artesunate may protect the BBB via S1P1 in the brain after SAH. The experimental results demonstrated that artesunate could raise the expression of S1P1 in vivo and in vitro after SAH. Further study also confirmed the key role of S1P1 in the BBB protective effect of artesunate after SAH. But we did not find out why the artesunate treatment could improve the expression of S1P1 after SAH. The potential mechanism deserves further study.
S1P1 couples exclusively to the Gi/o family and plays an important role in maintaining the stability of flow-dependent vascular network. Global deletion of S1P1 or deletion of S1P1 in endothelial cells would have serious consequences to the vascular plexus in mice [30]. Previous study showed that increasing the expression of S1P could improve the activation of S1P1 and then delay the development of early brain injury after SAH in mice [11]. The underlying mechanism may be linked with high levels of S1P in blood plasma (∼1 μM) [31]. But, it was not clear about the downstream signal of S1P1 in the BBB protective effect of artesunate after SAH. Previous research showed that S1P1 could activate the PI3K/Akt pathway to inhibit apoptosis [32], prevent activation of FoxO3a and then promote PC12 cell survival [33], enhance adult mouse cardiac myocyte survival during hypoxia [13], and promote the differentiation of adipose-derived stem cells into endothelial nitric oxide synthase expressing endothelial-like cells [34], etc. Therefore, PI3K is a very important downstream effector of S1P1. Taddei, et al. reported that PI3K/Akt activation could inactivate GSK-3β and limit the translocation of β-catenin to the nucleus, which relieved the inhibition and finally increased the expression of Claudin-5 [35]. Relevant research also indicated that PI3K/Akt activation could raise the expression of Claudin-3 and Claudin-5 through reduced GSK-3β activation and stabilized β-catenin after ICH in mice [14]. It is well known that tight junction proteins of Claudin-3 and Claudin-5 are very important to BBB integrity [36]. Effectively preserving the expressions of Claudin-3 and Claudin-5 could alleviate BBB breakdown in various central nervous system diseases. And our data demonstrated that S1P1 stimulation could increase PI3K/Akt activation, then reduce GSK-3β activation and stabilize β-catenin, finally raise the expression of Claudin-3 and Claudin-5 after SAH in rats.
Although, we found that artesunate could raise the expression of S1P1 and then protect the BBB through activating the PI3K/Akt pathway. However, there are some limitations in this study. Firstly, some contradictory evidences showed that artesunate was inhibition rather than activation about the PI3K/Akt pathway [37, 38]. The reason may be that for different cells and diseases, artesunate have different therapeutic mechanisms, but we did not investigate the potential mechanism. Secondly, the experiment was not designed to study other actions of artesunate, such as anti-inflammation. What’s more, the other downstream effectors of S1P1, such as adenylate cyclase, phospholipase C, protein kinase C, intracellular calcium, and the Hippo signaling pathway [30], were not evaluated in this study either. Therefore, the above issues need to be clarified in our future study.
Conclusion
This study found that artesunate, the traditional anti-malaria drug, could protect BBB and finally improve neurological outcomes after SAH in rats via S1P1 signal, which may activate PI3K/Akt pathway, stabilize β-catenin via GSK-3β inhibition, and then effectively raise the expression of Claudin-3 and Claudin-5 (Fig. 9). Therefore, artesunate may be favorable for the BBB protection after SAH and become a potential candidate for the treatment of SAH patients.
Proposed pathway in the present study. This study found that artesunate, the traditional anti-malaria drug, could protect BBB and finally improve neurological outcomes after SAH in rats via S1P1 signal, which may activate PI3K/Akt pathway, stabilize β-catenin via GSK-3β inhibition, and then effectively raise the expression of Claudin-3 and Claudin-5. S1P indicates sphingosine 1 phosphate; PI3K indicates phosphatidylinositol 3-kinase; AKT indicates protein kinase B; GSK3β indicates glycogen synthase kinase-3β
References
Feigin VL, Lawes CM, Bennett DA, Anderson CS (2003) Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2(1):43–53
Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ (2012) The vascular neural network—a new paradigm in stroke pathophysiology. Nat Rev Neurol 8(12):711–716. doi:10.1038/nrneurol.2012.210
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J et al (2013) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. doi:10.1016/j.pneurobio.2013.09.002
Mirshafiey A, Saadat F, Attar M, Di Paola R, Sedaghat R, Cuzzocrea S (2006) Design of a new line in treatment of experimental rheumatoid arthritis by artesunate. Immunopharmacol Immunotoxicol 28(3):397–410. doi:10.1080/08923970600927447
Kaptein SJ, Efferth T, Leis M, Rechter S, Auerochs S, Kalmer M, Bruggeman CA, Vink C et al (2006) The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antivir Res 69(2):60–69. doi:10.1016/j.antiviral.2005.10.003
Chen H, Shi L, Yang X, Li S, Guo X, Pan L (2010) Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol 92(4):587–597. doi:10.1007/s12185-010-0697-3
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18(4):767–773
Souza MC, Paixao FH, Ferraris FK, Ribeiro I, Henriques M (2012) Artesunate exerts a direct effect on endothelial cell activation and NF-kappaB translocation in a mechanism independent of plasmodium killing. Malar Res Treat 2012:679090. doi:10.1155/2012/679090
Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH (2010) Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke 41(7):1521–1527. doi:10.1161/strokeaha.110.583757
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH (2010) Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 41(2):368–374. doi:10.1161/strokeaha.109.568899
Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH (2012) Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med 40(6):1908–1913. doi:10.1097/CCM.0b013e3182474bc1
Rolland WB 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, Zhang JH (2011) FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl 111:213–217. doi:10.1007/978-3-7091-0693-8_36
Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO (2007) Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293(5):H3150–H3158. doi:10.1152/ajpheart.00587.2006
Krafft PR, Caner B, Klebe D, Rolland WB, Tang J, Zhang JH (2013) PHA-543613 preserves blood–brain barrier integrity after intracerebral hemorrhage in mice. Stroke 44(6):1743–1747. doi:10.1161/STROKEAHA.111.000427
Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, Cserti-Gazdewich C, Oravecz T, Liles WC et al (2011) S1P is associated with protection in human and experimental cerebral malaria. Mol Med 17(7–8):717–725. doi:10.2119/molmed.2010.00214
Li B, Luo C, Tang W, Chen Z, Li Q, Hu B, Lin J, Zhu G et al (2012) Role of HCN channels in neuronal hyperexcitability after subarachnoid hemorrhage in rats. J Neurosci 32(9):3164–3175. doi:10.1523/jneurosci.5143-11.2012
Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH (2010) Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol 68(5):650–660. doi:10.1002/ana.22102
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634, discussion 635
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334. doi:10.1016/j.jneumeth.2007.08.004
Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH (2010) Mechanisms of osteopontin-induced stabilization of blood–brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 41(8):1783–1790. doi:10.1161/STROKEAHA.110.586537
Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo C, Tang J, Feng H et al (2015) Norrin protected blood–brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke 46(2):529–536. doi:10.1161/strokeaha.114.007265
Yang F, Wang Z, Zhang JH, Tang J, Liu X, Tan L, Huang QY, Feng H (2015) Receptor for advanced glycation end-product antagonist reduces blood–brain barrier damage after intracerebral hemorrhage. Stroke 46(5):1328–1336. doi:10.1161/STROKEAHA.114.008336
Hu SL, Huang YX, Hu R, Li F, Feng H (2014) Osteopontin mediates hyperbaric oxygen preconditioning-induced neuroprotection against ischemic stroke. Mol Neurobiol. doi:10.1007/s12035-014-8859-6
Yusof W, Hua GS (2012) Gene, ethnic and gender influences predisposition of adverse drug reactions to artesunate among Malaysians. Toxicol Mech Methods 22(3):184–192. doi:10.3109/15376516.2011.623331
Zhao F, Wang H, Kunda P, Chen X, Liu QL, Liu T (2013) Artesunate exerts specific cytotoxicity in retinoblastoma cells via CD71. Oncol Rep 30(3):1473–1482. doi:10.3892/or.2013.2574
Marijon A, Bonnot G, Fourier A, Bringer C, Lavoignat A, Gagnieu MC, Bienvenu AL, Picot S (2014) Efficacy of intranasal administration of artesunate in experimental cerebral malaria. Malar J 13:501. doi:10.1186/1475-2875-13-501
Gautam A, Ahmed T, Batra V, Paliwal J (2009) Pharmacokinetics and pharmacodynamics of endoperoxide antimalarials. Curr Drug Metab 10(3):289–306
Wu D, Cerutti C, Lopez-Ramirez MA, Pryce G, King-Robson J, Simpson JE, van der Pol SM, Hirst MC et al (2014) Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-kappaB activation. J Cereb Blood Flow Metab. doi:10.1038/jcbfm.2014.207
Caner B, Hou J, Altay O, Fuj M 2nd, Zhang JH (2012) Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem 123(Suppl 2):12–21. doi:10.1111/j.1471-4159.2012.07939.x
Mendelson K, Evans T, Hla T (2014) Sphingosine 1-phosphate signalling. Development 141(1):5–9. doi:10.1242/dev.094805
Cyster JG, Schwab SR (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30:69–94. doi:10.1146/annurev-immunol-020711-075011
O’Sullivan C, Dev KK (2013) The structure and function of the S1P1 receptor. Trends Pharmacol Sci 34(7):401–412. doi:10.1016/j.tips.2013.05.002
Safarian F, Khallaghi B, Ahmadiani A, Dargahi L (2014) Activation of S1P receptor regulates PI3K/Akt/FoxO3a pathway in response to oxidative stress in PC12 cells. J Mol Neurosci. doi:10.1007/s12031-014-0478-1
Arya D, Chang S, DiMuzio P, Carpenter J, Tulenko TN (2014) Sphingosine-1-phosphate promotes the differentiation of adipose-derived stem cells into endothelial nitric oxide synthase (eNOS) expressing endothelial-like cells. J Biomed Sci 21:55. doi:10.1186/1423-0127-21-55
Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C et al (2008) Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10(8):923–934. doi:10.1038/ncb1752
Gunzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93(2):525–569. doi:10.1152/physrev.00019.2012
Cheng C, Ho WE, Goh FY, Guan SP, Kong LR, Lai WQ, Leung BP, Wong WS (2011) Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One 6(6), e20932. doi:10.1371/journal.pone.0020932
Thanaketpaisarn O, Waiwut P, Sakurai H, Saiki I (2011) Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-kappaB and PI3K/Akt signaling pathways. Int J Oncol 39(1):279–285. doi:10.3892/ijo.2011.1017
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declared that they have no competing interests.
Source of Funding
This study was funded by National Natural Science Foundation of China (No. 81501002 to Yujie Chen, No. 81220108009 to Hua Feng) and the National Basic Research Program of China (973 Program, No. 2014CB541600 to Hua Feng).
Rights and permissions
About this article
Cite this article
Zuo, S., Ge, H., Li, Q. et al. Artesunate Protected Blood–Brain Barrier via Sphingosine 1 Phosphate Receptor 1/Phosphatidylinositol 3 Kinase Pathway After Subarachnoid Hemorrhage in Rats. Mol Neurobiol 54, 1213–1228 (2017). https://doi.org/10.1007/s12035-016-9732-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9732-6