Abstract
Epilepsy is characterized by the hyperexcitability of various neuronal circuits that results due to the imbalance between glutamate-mediated excitation of voltage-gated cation channels and γ-amino butyric acid (GABA)-mediated inhibition of anion channels leading to aberrant, sporadic oscillations or fluctuations in neuronal electrical activity. Epilepsy with a risk of mortality and around 65 million sufferers of all ages all over the world is limited therapeutically with high rates of adverse reactions, lack of complete seizure control, and over 30% patients with refractory epilepsy. The only alternative to medicines is to identify and surgically remove the seizure foci in the brain or to abort the seizures just as they begin using an implanted cerebral electrode. However, these alternatives are unable to precisely aim aberrant neuronal circuits while leaving others unaltered. Epilepsy animal models also constitute the identical constraint. Thus, a better target-specific approach is needed to study and treat epilepsy. Unicellular green algae Chlamydomonas reinhardtii expresses a channelrhodopsin-2 (ChR2) sodium ion channel protein that controls the phototaxis movement of algae in response to blue light. Similarly, archaeon Natronomonas pharaonis (NpHR) expresses a monovalent Cl− channel protein halorhodopsin that responds to yellow light. These features of ChR2 and NpHR proteins can be used in optogenetic techniques to manipulate the bi-directional firing pattern of neuronal circuits in an attempt to better understand the pathophysiology of epileptic seizures as well as to discover novel potential drugs to treat epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The word epilepsy came from ancient Greek meaning to seize, possess, or afflict. Epilepsy is a mental ailment of the central nervous system (CNS) with characteristic recurring ictogenesis or the generation of seizures. A seizue, a newer term for fit, came from the Latin word sacire, meaning “to take possession of,” is also called convulsion which is an older term for a tonic–clonic seizure. A seizue is an outward appearance of abnormal, synchrounous, recurrent, and spontaneous release of electrical activity that arises from the aberrant excessive excitement of neurons. Seizures may result either due to the upregulation of excitatory circuits or downregulation of inhibitory circuits [1] of a population of neurons or due to the imbalance between glutamate-mediated excitation or the γ-amino butyric acid (GABA)-mediated inhibition of neurons. Clinically, seizures are manifested as an uncontrolable shaking of the body or alterations in sensory perception, autonomic function, motor control, or consciousness, and more. Physiologically, two concurrent events help initiate a seizure: first are high-frequency, low-amplitude bursts of neuronal firing that result due to the relatively extended hyperexcitation of the neuronal membrane and second is the hypersynchronized activity of a population of neurons.
The hyperexcitation of cortical neurons caused by the excitatory contribution of the reticular formation neurons of brainstem is a critical condition of epileptic activity. Nucleus basalis of Meynert as well as cortically triggered oscillatory activity of the reticular thalamic nucleus (RTN) also contributes to the epileptic activity. The RTN is a tightly organized entity located in the thalamus and has a significant inhibitory or modulatory effect on the thalamic relay of information to the cortex [2]. Cytoarchitecturally, RTN is divided into three anatomical sectors: rostral, posterior, and intermediate; each sector is connected with more than one thalamic nucleus [3]. The rostral part of the RTN is implicated in motor and limbic functions. Studies of the cellular composition of the RTN have shown to contain a homogeneous population of GABAergic neurons, besides other neurons, as shown by anti-GABA antibodies [4, 5]. Homogeneous population of inhibitory GABAergic neurons of rostral RTN is central to the genesis of oscillations and synchronous activity during sleep and epileptic seizures [6].
Epilepsy confers a significant burden to the people affected. Despite the best pharmacological treatment and medical therapies, there are around 30% of patients with refractory epilepsy. Neurostimulation via subdural or depth electrodes has emerged recently, but its effectiveness in clinical trials has not been really tested. One alternative treatment is to surgically remove the seizure focus, but there are a large number of epileptics who are not suitable candidates for such type of surgeries. A common limitation to the existing therapies is not being able to target the aberrant neuronal circuits precisely while leaving the healthy cells untouched. Hence, to discover and develop novel anti-epileptic treatments that could specifically cure the sick neurons, whenever required, is a continuous necessity in epileptic research.
Recently, a new type of light-gated microbial ion channel proteins called opsins have emerged as novel industrial biotechnology hosts or microbial metabolic engineering product to study and treat epilepsy. Brain activity can be controlled and monitored on a range of spatial and temporal resolution from an isolated neuron to complex neuronal circuits [7] when these opsins are transfected into the aberrant neurons by combining the transgenic methods and optogenetic techniques. Although still in infancy using genetic the toolbox, the introduction of light-sensitive opsins into the cultured neurons or freely moving animals has shown to be possible [8, 9]. Delivery of specific wavelength light to these neurons, using optical fibers, results in the opening and closing of ion channel opsins that may lead to either depolarization or hyperpolarization, depending on the type of ions being transported in or out of the neurons. In recent years, optogenetic approach has revolutionized the field of neuroscience, allowing minimally invasive and spatiotemporal control of neuronal activity that has not been achieved with any other technique before, including electrophysiology. So far, animal models have been the subject of optogenetic approaches; however, efforts are being made to use more sophisticated models, such as nonhuman primates. This review highlights the basics and specifics of how microbial proteins can be combined with optogenetic technology to enhance our understanding and the treatment of not only epilepsy but also other mental ailments. In addition, we tried to elaborate the fundamentals of epileptic pathophysiology including action potential.
Nervous System
Under this heading, we will briefly describe the relevant components of the nervous system, neurotransmitters, concept of action potential and its types, brain waves, and how brain waves are recorded to help understand the pathophysiology of epilepsy.
Nervous system with 1010–1011 neurons, each constituting up to 104 excitable synaptic contacts, participates in coordinating the voluntary and involuntary activities of a living organism by transmitting signals to and from different body parts. The nervous system leads to an extraordinarily intricate and highly developed network composed of approximately 100 trillion (1014) synapses. In general, a neuron is composed of a single cell body, one or multiple cellular projections called dendrites, one axon, and one or more axon terminals. The primary function of dendrites is to receive the information, whereas cell body and axon process transmit that information to other neurons respectively. Electrical excitability and being able to communicate with other cells via electrical signals or synapses is the most fundamental characteristic of neuronal cells that distinguish them from other cells.
Anatomical Division of Nervous System
In vertebrates, the nervous system is composed of the CNS and the peripheral nervous system (PNS). The brain and spinal cord constitute the CNS, whereas the PNS is comprised of nerves (cranial and peripheral) and ganglia. The brain has three central divisions: forebrain, midbrain, and hindbrain. The cerebrum and diencephalon constitute the forebrain, the midbrain contains tegmentum and tectum, while the hind brain is the home of pons, medulla oblongata, and cerebellum. The cerebrum is the most anterior part of the brain, a longitudinal fissure divides the cerebrum into two hemispheres. Each hemisphere contains five distinct lobes: frontal, temporal, parietal, and occipital that cover the surface of brain, whereas the fifth lobe insula is hidden under the Sylvian fissure.
Epileptic Neuronal Circuits
Epilepsy represents a challenging mental condition to study because of the involvement of a large number of overactive neuronal circuits that may be the site of seizure initiation or propagation (reviewed in [10, 11]). Additionally, new recurrent excitatory circuits that are formed after brain injuries also contribute heavily to the epileptic seizures [12].
Thalamus
The thalamus is one of the brain regions that interacts directly or indirectly with the seizure network. Along with the epithalamus and hypothalamus, the thalamus constitutes the largest component of diencephalon, the caudal (posterior) part of the forebrain. The thalamus is the primary site of synapse or information relay from all of the sensory pathways except olfaction on their way to the cerebral cortex, with which it is connected together in a thalamocortical loop.
Thalamocortical Circuits
Thalamocortical and corticothalamic pathways are the fundamental network of neurons that participate in initiation and propagation of seizures. They include pyramidal cells with collateral afferents to the sensory and RT neurons and inhibitory projections from the RTN to the GABAergic interneurons of different cortical layers as well as to thalamocortical neurons [13] (Fig. 1a). In the cerebral cortex, the pyramidal cells, amygdala, and hippocampus (the forebrain regions) are considered the key excitation focus. Chronical injuries in the neocortex have also been shown to result in over excitation or epileptogenic activity of pyramidal neurons [14].
The thalamocortical pathway includes pyramidal cells and interneurons of different cortical layers with collateral afferents projected to the reticular thalamic nuclei (RTN) and sensory thalamus and inhibitory projections from the RTN to the GABAergic interneurons of cortex and to thalamocortical neurons (a). The RTN is a thin shell of neurons that forms a capsule or covering the entire lateral aspect of the thalamus (b)
Depending on the status of a voltage-dependent intrinsic membrane conductance, thalamic relay cells in a thalamocortical loop stimulate the cortical pyramidal cells in a burst or tonic response mode. Besides having distinctly different information processing consequences, both burst and tonic modes participate in an efficient relay of information to the cortex in behaving animals [15]. This allows the thalamus not only to facilitate but also to be a part of the dynamic relay that affects the format and nature of information on its way to the cortex. A particular type of network malfunction or defect in the dynamics of thalamocortical circuit and its interaction with other cerebral regions may cause a neurological illness.
Reticular Thalamic Nucleus
The thalamus consists of various groups of neuronal cell bodies called nuclei. The reticular thalamic nucleus (RTN) is a thin shell of neuronal soma that forms a capsule or covers the entire lateral aspect of the thalamus. Besides other neurons, the RTN consists of hyperpolarized pace making GABAergic neurons that release GABA neurotransmitters (Fig. 1b). Due to the GABAergic neurons, the RTN is called the pacemaker for thalamic oscillations and occupies a distinct governing position in the brain. Bursting properties of the RTN inhibitory cells are analogous to thalamocortical neurons. Epilepsy results due to a specific defect in the excitatory synapses connecting the corticothalamic and GABAergic RTN [12].
It is the RTN input that largely controls the thalamocortical activation mode by hyperpolarizing the relay cells via GABA type B (GABAB) receptors and their own inhibition by adjacent reticular cells via GABA type A (GABAA) receptor activation. The RTN is activated by cortical pyramidal cells in a feed-forward loop. This circuit is modulated by ascending serotonergic, dopaminergic, and noradrenergic inputs from brain stem regions.
Neurotransmitters
Neurotransmitters are substances released by the presynaptic neurons into the synaptic clefts, where they bind to the specific receptors on postsynaptic dendrites or soma. Ligand binding to postsynaptic receptors results in the activation of ion transport via ion pumps. Glutamate, GABA, acetylcholine, dopamine, norepinephrine, histamine, and serotonin are the major neurotransmitters of the brain. Hormones and neuropeptides also perform a key role in modulating the physiological effect of neurotransmission in the long run.
Glutamate
The amino acid glutamate is the major excitatory neurotransmitter. Receptors for glutamate neurotransmitter are usually found on postsynaptic excitatory neurons and inhibitory interneurons. Glutamate receptors have several subtypes. These are N-methyl-d-aspartate (NMDA), kainate, and alpha-amino-2, 3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptors (Fig. 2). The ionotropic subclasses of glutamate receptors allow the transport of sodium (Na+) and potassium ions (K+) across the cellular membrane that contributes to the generation and propagation of action potential and membrane depolarization. Cation permeability and differential binding to various pharmacologically active agonists or antagonists are the distinct properties of glutamate receptor subtypes. Kainite, AMPA, and NMDA agonists have been shown to induce while their antagonists suppress seizure activity in animal epilepsy models. Excessive accumulation of Na+ within the cell, or even systemically, is detrimental as it may result in edema, seizures, or even death in certain conditions.
A typical larger than normal magnified neuron consisting of a couple dendrites, a cell body, and one axon. Binding of excitatory neurotransmitter glutamate (Glu) to its receptors results in the influx of sodium ions (Na+) that changes the resting membrane potential to +30 from −60 mV in a process called depolarization. Immediately after, potassium ion (K+) channels throw positively charged ions out of the cell to bring back the membrane potential to −60 mV in a process called repolarization. Similarly, binding of GABA neurotransmitters to chloride ion (Cl−) channels results in the entry of Cl− into the cell that makes the membrane potential more negative or hyperpolarized. As a result, no action potential is propagated. The action potential moves faster in the myelinated axon compared to the axonal areas not insulated by the myelin sheath (Nodes of Ranvier). In certain mental ailments, aberrant GABAergic neurons lead to the prolonged neuronal depolarization and firing of repetitive action potentials
GABA
The major inhibitory neurotransmitter in vertebrate nervous system, GABA, binds with GABAA and GABAB receptors subtypes. GABAA and GABAB receptors modulate synaptic release from their postsynaptic and presynaptic locations, respectively. In the adult brain, once activated, GABAA receptors allow the influx of chloride ions (Cl−), which hyperpolarizes the membrane and inhibits the generation and propagation of action potentials (Fig. 2). Thus, substances like benzodiazepines and barbiturates, which are agonists of GABAA receptor, suppress seizure activity. Rather than being associated with Cl− channels, GABAB receptors belong to second messenger system due to being the member of ligand-gated channels. Owing to their presynaptic location, GABAB receptors attenuate the release of neurotransmitters. Opening of K+ channels and thus hyperpolarization is often led by the second messenger system. Certain agonists of GABAB receptors such as baclofen are known to aggravate neuronal hyperexcitability and thus cause seizures.
Membrane Potential
One of the functions of neurons is to preserve a voltage difference in the exterior and interior of the cell called membrane potential. As long as there is no perturbation to maintain the resting membrane potential, the interior of the neuron keeps a negative voltage as compared to the exterior. At the axon hillock, the resting membrane potential is typically about −60 to −70 mV (mV) whereas threshold potential is maintained at around −55 mV (reviewed in [16, 17]).
Action Potential
Action potential is the characteristic of various excitable cell types, such as endocrine cells, muscle cells, neuronal cells, and some plant cells as well. In neural cells, action potentials are key to neuronal communications. In physiological terms, there is a rapid rise and fall in action potential of a cell in a short-lasting event, following a steady path while communicating, as follows (Fig. 2):
-
1.
When a stimulus from a postsynaptic neuron of an afferent pathway reaches the end of a presynaptic axon as excitatory postsynaptic potentials from a presynaptic neuron, it causes the release of neurotransmitter molecules into the synaptic cleft.
-
2.
These neurotransmitters are then bound to various types of ion channels or pumps or voltage-gated receptors embedded in the cell membrane of soma or dendrites of postsynaptic neuron.
-
3.
Neurotransmitter binding opens the channels, which are closed at the resting membrane potential, by switching their configuration from closed to open states.
-
4.
Opening of channels allows an influx of extracellular Na+ or calcium ions (Ca2+) within the cells, which increases the electrochemical gradient or voltage difference by increasing the interior concentration of positively charged cations relative to the exterior of the cell in the vicinity of the channels. This causes an additional increase in the local cellular permeability of ions.
-
5.
If the binding of neurotransmitters raise the voltage then the cell’s resting potential, the synapse or the neurotransmitter is called excitatory. However, the neurotransmitter is called inhibitory if its binding decreases the membrane voltage.
-
6.
If the excitatory signal is not strong enough, then it decays by the time it reaches the axon hillock, the trigger point of action potential. If the excitatory signal is strong enough and the interior voltage increases past a precisely defined critical threshold value typically 15 mV higher (from −60 mV up to −55 mV) by the time it reaches the axon hillock, then the Na+ current persists and action potential continues.
-
7.
Continuation of action potential leads to a runaway state where the feedback from the Na+ current opens or activates further voltage-dependent Na+ channels and neuronal firing [18]. This explosive process continues until all of the accessible cation channels are activated, raising an even a greater voltage difference of up to about +30 mV across the plasma membrane in a process called depolarization.
-
8.
The rapid influx of Na+ reverses the plasma membrane polarity by rapidly inactivating the cation channels at the peak of the action potential. With the closing of Na+ channels, there is no more influx of Na+ into the neuron.
-
9.
Potassium leak channels are then opened to expel potassium ions out of the cell to create an exterior K+ current in an attempt to return the electrochemical gradient of neurons to the resting state. The K+ efflux lowers the membrane potential, and the membrane begins to repolarize towards its resting potential.
-
10.
The opening of potassium leak channels leads to the opening of voltage-gated potassium channels, which are much slower to open and close but expel comparatively greater amounts of K+ out of the cell. By the time voltage-gated potassium channels are closed, the efflux of the potassium current exceeds the influx of sodium current. This repolarization, typically called after hyperpolarization or the refractory period, overshoots the resting potential and the voltage returns in a more negative territory then resting membrane potential to around −90 mV [19].
-
11.
This transitory shift to negative membrane potential caused by the surplus K+ would seem to be counterproductive; however, this refractory period precludes the traveling back of an action potential, makes sure of unidirectional proceeding of the signal as well as gives depolarization time for completion. Hyperpolarization also prevents any stimulus that previously initiated an axon, from sending additional signals in the reverse direction, or the receipt of another stimulus throughout this period by increasing the threshold for a new stimulus.
-
12.
10. Simultaneous opening of Na+ and K+ channels would result in the neutrality of the system that may preclude the generation of the action potential.
-
13.
After hyperpolarization has occurred, the original resting state of the membrane to −60 mV is maintained by the Na+/K+ pump in due course.
-
14.
The up-and-down voltage cycles of an action potential created by a given cell are usually stereotyped or identical in shape, amplitude, frequency, and time course.
-
15.
Following depolarization, depending on the cell type, GABA receptors mediate the hyperpolarization by modulating the influx of Cl− or efflux of K+.
These rise and fall cycles of action potentials usually occur in around a thousandth of a second. Some neurons are very quiet and do not emit any action potential for minutes or longer. In contrast, some neurons can generate as much as 10 to 100 action potentials in a second. The rise and fall cycles of voltage difference or action potentials are also called nerve spikes or impulses. The temporal array of action potentials created by a cell at a given time is called a spike train, and the neuron itself is usually said to fire. Firing rate is the frequency at which a neuron produces action potentials. The continuous sequence of depolarization, repolarization, and hyperpolarization is called the paroxysmal depolarizing shift.
Types of Action Potentials
Animal cells produce two major types of action potentials.
Voltage-Gated Sodium Channels
One type of action potential is created by voltage-gated Na+ channels. Such action potentials are typically under one millisecond long. Na+-dependent spiking is initiated when neurons are depolarized to −55 mV from resting membrane potential levels in both in vitro and in vivo conditions and usually persists throughout the length of the membrane depolarization.
Voltage-Gated Calcium Channels
The other type of action potential is created by voltage-gated Ca2+ channels. Such action potentials are typically 100 or more milliseconds long. In certain neuronal types, slow spikes of Ca2+ drive the long bursts of quickly emitted Na+ spikes. De-inactivation of Ca2+ conductance through T-type Ca2+ channels (Cav3. 1–3.3) produces an inward current of depolarization [20], which, if sufficiently large, regenerates Ca2+-dependent spikes in addition to the ones which are activated by high frequency bursts of Na+ action potentials. Ca2+-mediated bursts of low threshold action potentials are the hallmark of the RTN firing at each spike.
Firing Patterns of Action Potential
Thalamic relay neurons exhibit two very distinct response modes or firing patterns of rhythmic activity; tonic and burst.
Tonic Firing
Tonic firing patterns are often characterized by steady regularly spaced spikes at a constant frequency, as background activity at rest, and typically occur without presynaptic input. However, not all neurons have tonic activity at rest. Tonic firing may serve as keeping a steady background level of a certain neurotransmitter or to serve as a mechanism where either an inhibition or an increase in presynaptic input can be transmitted. When a neuron is silent at rest, only an increase in presynaptic activity can be transmitted postsynaptically.
It is generally believed that tonic firing is only a feature of waking state when both thalamic and cortical cells are flooded by the action potentials train [21]. Behaving awaked states are conventionally called desynchronized as compared to slow-wave, large-amplitude oscillations detected in sound sleep with non-rapid eye movement (non-REM) [22, 23].
Clonic Firing
Tonic oscillation is generally evolved into large-amplitude, low-frequency, slow-wave oscillations in a phase called the clonic phase [24] (Fig. 3a).
Electroencephalography (EEG) of a generalized (a) and childhood absence epilepsy (b) patient from the left and right frontal (F), temporal (T), and occipital (O) brain regions. Generalized epilepsy patient (a) shows normal (i) bilateral, low-amplitude, high-frequency tonic spikes at >13 Hz/s (beta waves) (ii) clonic and (iii) postcompulsive coma (iv) phases. Absence epilepsy patient (b) shows a characteristic bilaterally synchronous, spike-wave discharge pattern at 2.5–4 Hz/s (delta waves) lasting for 5 s
Phasic Firing
In contrast, phasic firing occurs after a neuron is activated due to presynaptic activity in addition to any background activity a neuron may have. It is typically restricted to one, a few, or a short burst of action potentials that quickly return to the resting state.
Burst Firing
As the name implies, the burst mode is a dynamic state of lower frequency rhythms or slow rhythmic oscillations characterized by repeated firing of distinct groups of high-frequency bursts of action potentials followed by periods of quiescence before the occurrence of the next burst. Alternatively, burst firing could also be defined as quiescence interspersed with high-frequency bursts of action potentials. Firing patterns in bursting are regular alternations between short and long interspike intervals.
When inhibited, thalamocortical cells go in the burst firing phase and then are released from inhibition. A rebound burst like this is typically known as a low-threshold spike. In response to tonic inhibition, some neurons initiate burst firing spontaneously. The function of the tonic mode is transparent, whereas the function of the burst mode is obscure. It had been thought that bursting is restricted to drowsiness, episodic, or rhythmic slow-wave sleep or in certain neuropathologies. Although not nearly as frequent, wakefulness can also be accompanied with bursting [15, 21].
Measurement of Action Potential
The synchronized bursts of brain waves or electricity signals given off at each of thousands of neurons, when cells communicate, could be detected at the scalp or cerebral cortex. Two cortical properties allow the electrical potential of the brain to be recorded. First, relatively identical polarity and orientation of almost all pyramidal neurons. Second, the synchronous activation of most of the pyramidal neurons that produce enough dipole to be detected. The electrical signals from the individual neurons cannot be picked up, since the smaller electrical charge generated by the smaller cells is far too small. The summation of the dipoles or electrical signals is amplified for detection and recording by a device called electroencephalogram (EEG).
Electroencephalography
EEG is one of the most efficient tools in the diagnosis of epilepsy, altered levels of consciousness (coma), sleep, and seizure disorders. However, EEG neither gives out electricity nor interprets the brain messages. The EEG records the currents of the postsynaptic dendrites of cortical pyramidal cells near the brain’s surface, through electrodes placed on the scalp (about 1 cm across) and provides information about spatiotemporal patterns of brain’s electrical activity. A number is given to each electrode; left side of the head is given all odd numbers, and the right side is given even numbers. The electrodes are also given an English letter; according to the brain area, it records the electrical activity from: F for frontal, T for temporal, P for parietal, and O for occipital lobes (Fig. 3). On the EEG, the fluctuations in the voltage often appear as a rapid uphill spike followed by a quick downhill due to the rise or fall in membrane potential that frequently finishes lower than resting membrane potential, where it rests for some time.
High-resolution recordings reveal that besides the spatially limited high-frequency interictal movement, epileptic focus is usually restricted to the cortical area around 1 mm2 in volume or a few tens of thousands of neural population. Such high-frequency brain activity could be best recorded with subdurally inserted micro-wires surrounding the seizure focus and as small as tens of microns in diameter. Each cycle of the oscillation generated by the synchronous activity of neurons comprised of a restricted brain volume, where tiny micro-wires can record high frequency brain signals over 500 Hz. Surface areas of microelectrodes is usually around 10−3 mm2 as compared to the conventional microelectrodes of around 10 mm2 (reviewed in [25].
Patch Clamp
The 1970s and early 1980s emerged as the years with major advancements in cellular electrophysiology with the development of patch clamp technology [26]. Patch clamping allows electrical recordings from the inside or outside patches of the neuronal membrane in addition to whole-cell recording. Patch clamping is carried out with smooth glass pipettes of around a 1-μm tip diameter in order to make them stick to the cell exterior efficiently enough to even measure the leak resistance in giga-ohms as well as to provide a low noise-to-resistance ratio for the signal.
Brain Waves Measured by EEG
The EEG recording shows that the brain produces different types of waves or frequency bands. On the EEG, each wave-type appears differently due to the amplitude, per second number of waves, area of the brain, and physiological time at which wave is originated, such as:
Alpha Waves
Alpha waves usually occur at 8 to 13 or more Hz or waves/s, seen in relaxing adults with their eyes closed. Alpha waves appear more clearly in the brain region responsible for the sight and vision, i.e., occipital lobe.
Beta Waves
Beta waves consist of more than 13 Hz or waves/s, observed more often in awake people. Beta waves are more frequently observed in the brain region involved in conscious thought and movement, i.e., frontal lobe as well as in central brain regions.
Theta Waves
Theta waves vary in frequency from 4 to 7 or more Hz or waves/s, observed in young children or during sleep, however, not in awake adults. Theta waves are also known as slow activity waves.
Delta Waves
Delta waves are the slowest brain waves with only 0 to 3 or more Hz or waves/s; however, delta waves have the strongest signal with highest amplitude. Delta waves are commonly seen in under a year old babies. Delta waves are also observed during some sleeping states.
Gamma Waves
Gamma waves with 26 to 100 Hz or waves/s are considered a biomarker for excitatory activity.
Spikes
Due to their distinct appearance on the EEG and fast occurrence, certain brain waves stand out from other brain activity and are called spikes. Spikes usually last less than one twelfth of a second or less than 80 milliseconds with a slow delta wave like pattern to follow.
Polyspikes
Polyspikes, as the name suggests, is given to a continuous and quick series of spikes.
Spike Waves
When one or more brief spikes are followed by a slow wave, for roughly around three times in a second, they are called spike waves.
Sharp Waves
Sharp waves are observed over a period of 80 to 200 milliseconds.
EEG of Generalized Epilepsy
Grand mal is an older term for generalized or tonic–clonic seizure that affects both hemispheres of the brain [27]. On the EEG, it shows a sudden intrinsic rhythmic discharge of electricity that usually occurs throughout or most of the cerebral areas from burst to tonic mode and results in tonic trains of action potentials in the atonic phase. Generalized epileptic seizures usually constitute neural paroxysmal fast activity with low-amplitude, high-frequency spikes of around 13 or more per second (Fig. 3a). Some seizure types in generalized epilepsy demonstrate a rhythmic burst firing pattern in thalamocortical neuronal networks [28, 29].
EEG of Childhood Absence Epilepsy
Non-convulsive absence epilepsy, formerly called petit mal, has an age of onset between 4 and 10 years. The prognosis of childhood absence epilepsy (CAE) is good with ∼75% of children outgrowing the absence seizures during adolescence. On the EEG, absence epilepsy spell shows a characteristic generalized, bilaterally synchronous periodic spike or spike-wave discharge (SWD) or slow-wave discharge [30] at 2.5 to 4 Hz (occipital rhythmic delta) in 15 to 40% of cases (Fig. 3b). After remission of absence epilepsy spells, the interictal EEG is observed in some children. The spike is topographically distributed more profoundly in the frontal cortex, while parietal and occipital cortices are the home to wave oscillations. At onset, the discharge of electrical activity is mostly somewhat faster than 3 Hz that appears to slow down towards the end. When a seizure ends, the patient immediately resumes prior conversation or activity. The seizures start abruptly and generally last from 5 to 20 s. Rodent absence epilepsy models produce spike and slow-wave form of brain activity with rather fast frequency of 5 to 10 Hz a second compared to humans [31].
However, so far, the spatiotemporal topographies of absence and tonic–clonic seizures have not been well understood. Nevertheless, tonic absence seizures and absence tonic–clonic seizure have been named as the two distinctive seizure types [32, 33]. Clinical experiments show that absence seizure may lead to tonic–clonic activity [32], and an absence seizure can accompany a tonic seizure [33]. Thus, in the EEG of epileptics or patients with seizures, both the absence and tonic–clonic seizures show a pathological nonlinear phenomena.
Epilepsy Treatment
Ethosuximide and valproic acid (low-threshold calcium channel blockers) in thalamic neurons [34] are effective for treating absence seizures [35]. In general, epilepsy is limited therapeutically with high rates of adverse reactions and lack of complete seizure control. Alternatively, epilepsy is treated by deep brain stimulation, a strategy similar to implanted cardiac defibrillators, which indiscriminately stimulates all nerve cells in a certain brain region including the ones that are not associated in epileptic seizures, hence reducing efficacy and even results in undesirable side effects. The other alternative to antiepileptic medications is to identify and surgically remove the seizure foci in the brain, such as temporal lobe, amygdala, and hippocampus in the small fraction of patients suffering from temporal lobe epilepsy. In that case, around 86 to 90% of patients experience freedom from seizures at 2 years [36]. Nevertheless, surgery has not been an option in a great majority of epileptics and not all epileptics are candidates for resection.
Animal models of epilepsy are critically vital not only to understand the primary mechanisms of the disease state but also for therapeutic interventions or examining the effectiveness of new drugs [37] that can be used to treat epilepsy [38]. In animal models, epilepsy is usually elicited by chemical and electrical stimulations. Still, with electrical stimulation, it is impossible to target specific neuronal types precisely. Additionally, it creates huge artifacts which interfere with the recording of electrical activity of neurons [39]. Similarly, stereotactical cerebral injections of chemicals lack temporal and spatial precision. Thus, there is a continued need for better target-specific approaches to study and treat epilepsy. Optogenetics is a novel target-specific approach to control electrical activity of genetically modified neurons in bidirectional way with great temporal and spatial resolution.
Optogenetics
Based on the principles of optics and genetics, optogenetics relies on controlling neural activity via microbial-derived, optically active cation or anion channel proteins called opsins. Opsins and cell-type specific promoters are delivered to living cell membranes by gene transfer or introducing viral vectors to modulate distinct populations of neurons by illumination of different wavelengths with an unparalleled level of temporal, spatial, and neurochemical precision. Optogenetics is the only existing technique that can activate specific population of neurons embedded in heterogeneous, dense brain tissue on a millisecond timescale (Fig. 4). Using optogenetics, the firing pattern of specific classes of neurons has been modulated in in vitro [7, 40] and in vivo as well as in vertebrate [39, 41] and invertebrate [42] models [43].
In optogenetics, optically active proteins (opsins) are isolated from microbes, constructed with a promoter, inserted into a viral carrier, and injected into the male pronucleus of a fertilized egg which is then implanted in a pregnant female mouse. An optrode is implanted into the brains of transgene carrying founder mice. Excitation or inhibition of neurons, containing microbial opsin proteins, can be controlled by illuminating the neurons via optrode at different wavelengths. The technique can then be used to record electrophysiological and behavioral data
Opsins
Owing to the great diversity in cellular architecture and biosynthetic capacity, microorganisms can be commercially exploited in industrial biotechnology for the production of compounds that they make naturally. These products range from the polymers; alginate and pigments; astaxanthin, β-carotene, and food products; agar and carrageenan to fuels to platform chemicals; and a variety of therapeutics and pharmaceuticals. Such metabolic microbial engineering finds it roots into the fermentation practices to make beer, wine, and bread, production of value-added omega-3 fatty acids (docosahexanoic and eicosapentaenoic acids) from Phaeodactylum tricornutum and penicillin from Penicillium notatum fungus (reviewed in [44]).
Microbes are also a very good source of opsins used in optogenetic techniques. Opsins are a family of photosensory proteins or light-sensitive receptors found throughout the animal kingdom, where they perform varied functions ranging from phototaxis in algae to circadian rhythms and eyesight in vertebrates and certain types of photosynthesis in plants. The number of available opsins continues to grow and be optimized for experimental and human applications. In epilepsy research, the most frequently used microbial opsins are light-driven cation channel proteins, such as channelrhodopsins and light-gated pumps such as halorhodopsin or archaerhodopsin (Fig. 4).
Chlamydomonas reinhardtii
In optogenetics, C. reinhardtii is considered one of the best known industrial biotechnology hosts. C. reinhardtii is a unicellular green algae about 10 μM in diameter that swims with two flagella (biflagellate). C. reinhardtii is found on damp soil, in stagnant water, seawater, freshwater, and even in snow as snow algae. C. reinhardtii has a light-sensitive red pigment spot that allows algae to swim photoautotrophically towards light. Commercially, C. reinhardtii is of interest for producing biopharmaceuticals and biofuel, as well as a valuable research tool in making bio-hydrogen [45, 46], channelrhodopsin-1, and channelrhodopsin-2 (ChR2) proteins.
Channelrhodopsin-2 (ChR2)
In neuroscience, ChR2 is the first genetically encoded, type I optogenetic tool. ChR2 is an excitatory, light-gated, monovalent cation channel [47] that controls the phototaxis movement of algae in response to blue light of ~470 nm by allowing Na+ ions to enter the cell [40] and changing the conformation of ChR2 from all-trans configuration to 13-cis-retinal. ChR2 can transduce blue light flashes of millisecond length into distinct spike waves as fast as 50 Hz a second (Fig. 5a).
Algal protein ChR2 fused with fluorescent marker Texas red (TR) allows the entry of positively charged sodium ions (Na+) into the cell upon activation with blue light resulting in seizure induction (a). Archaeon NpHR protein fused with green fluorescent protein (GFP) allows negatively charged chloride ion (Cl−) inflow upon activation with yellow light resulting in seizure reduction (b)
Natronomonas pharaonis
N. pharaonis could also serve as an industrial biotechnology platform in optogenetics. N. pharaonis is an aerobic, extremely haloalkaliphilic archaeon that is isolated from salt-saturated (3.5 M NaCl, pH 8.5) lakes of up to pH 11. Optogenetically, N. pharaonis is of interest for producing halorhodopsin protein (Fig. 5b).
Halorhodopsin (NpHR)
NpHR is a light-sensitive inward anion pump and a major inhibitory opsin [48] that allows the influx of Cl− ions by using the energy of 593 nm yellow light. NpHR is 7-transmembrane domain protein that belongs to the retinylidene family of proteins, has a tertiary structure identical to vertebrate light-sensitive retinal pigment rhodopsin, and homologous to bacteriorhodopsin, the light-gated proton (H+) pump. NpHR also shares sequence similarity to ChR2 and contains all-trans-retinal derivative of essential vitamin A that isomerizes in response to light. NpHR is among the very few membrane proteins that have been crystallized. Like ChR2, NpHR does not need exogenous cofactors to function in mammalian cells. NpHR works either by blocking a single action potential or by continuously knocking the spikes after being activated by yellow light [49].
Original NpHR channel tends to get trapped in the endoplasmic reticulum when expressed in mammalian cells [50]. A C-terminal endoplasmic reticulum export motif was added to the original sequence of NpHR (called eNpHR2.0) in order to resolve the sub-cellular localization issue and drive the in vivo aggregate-free expression [50] of NpHR. Even eNpHR2.0 showed poor localization at the cell membrane. Further addition of potassium channel (Kir2.1) membrane trafficking and Golgi export signals to eNpHR2.0 (called eNpHR3.0) significantly improved its membrane localization [51], photocurrent amplitude, and safe and reliable expression in freely moving animals [52–54].
Bacteriorhodopsin
Bacteriorhodopsin is yet another ion channel protein found in the plasma membrane of Halobacterium halobium that undergoes a conformational change in response to the absorption of light of a specific wavelength, resulting in the outflow of protons. This efflux of ions generates a life-sustaining transmembrane gradient and electric potential that the bacterial cell then uses to drive adenosine 5′-triphosphate (ATP) synthesis [55].
Archeorhodopsin
Like halorhodposin, archeorhodopsin is also an effective neuronal silencer. Archeorhodopsin is a light-driven outward proton pump.
Microbial Opsin Commodities and Neural Cells
These unique features of microbial opsins can be used in a number of ways to treat different anomalies of human cells. For instance, when ChR2 was expressed in neural tissue, it exhibited same rapid photo-activated kinetics or membrane polarization as demonstrated in culture [56] by giving rise to non-toxic, blue light-driven neuronal stimulation on a millisecond timescale to permit precise quantitative coupling between neuronal activation and optical excitation [7, 40]. In the similar way, NpHR can be used as a component of the natural neuronal process to generate hyperpolarization to overcome or inhibit the membrane potential. So far, several state-of-the-art methods [57] have been established to inactivate or kill affected neuronal networks to treat epilepsy. However, the required rapid reversibility, millisecond precision, and spectral compatibility provided by the simultaneous use of ChR2 and NpHR have been hard to achieve by any other method (Fig. 5).
Opsins Delivery to Brain
Optogenetic techniques have been articulated in detail to ensure the delivery of genetic constructs to the specific regions in brain [7, 40]. They could be delivered either via direct cerebral injection, neural transplantation, or intravenous injection (Fig. 6).
Schematic description of the principal ways opsins can be delivered to the human brain. A direct stereotactic cerebral injection of genetic construct is one approach to deliver the opsins (a). In embryonic stem cells (ESCs) approach, ESCs are extracted from blastocysts grown from few-day-old embryos, proliferated, and differentiated into neural progenitor cells (b). In induced pluripotent stem cells (iPSCs) approach, skin fibroblasts are extracted from patient’s body, developed into iPSCs, reprogrammed to produce NpHR expressing healthy iPSCs, proliferated in large numbers, differentiated into healthy neural progenitor cells (c), and finally grafted into the patient’s brain
Delivery via Stereotactic Cerebral Injection
Briefly, principal neurons (such as cortex, ventrobasal thalamus, dorsal hippocampus, CA1/CA3 pyramidal neurons, RTN) are transfected with a viral vector (typically adeno-associated virus or lentivirus) carrying cell-type specific promoter (such as CamkIIa, glutamic acid decarboxylase (GAD)6), the opsin (such as ChR2, NpHR), optimized with membrane trafficking signal motifs (such as endoplasmic reticulum or Golgi export signals), and a reporter (such as YFP, GFP, mCherry, EYFP, TR) with precise spatiotemporal targeting [58] at any stage of life.
The promoter ensures that only principal neurons of interest are subject to viral transduction [59] and reporter fluorescent proteins help in the detection of opsins using immunochemistry. Cerebral injections are performed stereotactically into the principal neurons of interest (Fig. 6a). Although stereotactic injection can still affect unrelated neuronal population in the injected brain areas, neurons selected by the injected promoter should only be able to synthesize the opsins.
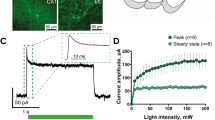
To perform the chronic recordings and stimulation in freely moving animals, a device containing multiple EEG electrodes and chronic multisite optrode (electrode + optics) or optical fiber is implanted into the injected neurons. Detection of magnitude, amplitude, width, rate, and frequency of seizure is achieved via these multiple EEG electrodes using a custom software. A method of detecting and interrupting seizures online has also been devised by routing to a real-time processor capable of determining seizure onset, which then triggers light stimulation and aborts the seizure within 1 s [60]. Similarly, for inhibition of principal neurons with NpHR, light of particular wavelength is delivered via implanted optrode upon seizure activity detection, resulting in decreased epileptiform EEG activity or silencing of epileptic neurons correlated with abortion of behavioral seizures as well. To maximally activate the opsin proteins, lasers of 50 to 200 mW are routinely used in in vivo preparations, whereas 5 to 30 mW lasers perform the job adequately in in vitro experiments. Wavelength-specific light of less than 1 mW is sufficient to maximally activate the characteristic opsin proteins like ChR2. In vitro whole-cell recording is done after slice preparation. Use of a laser to produce light with even higher intensity is considered safe as usually light is lost at almost each connection, such as at splitters, collimators, or connectors and also when it passes through the neural tissue [61].
Delivery via Neural Transplantation
Opsins can also be delivered to the brain via transplanting the opsin-containing neural progenitor cells in the corresponding brain region by employing various neural transplantation techniques outlined in Fig. 6 [62]. In fact, stem cell-based treatment trials are already approved by the US Food and Drug Administration (FDA) for amyotrophic lateral sclerosis and Parkinson’s disease. This method of delivery is particularly considered in epileptic patients. In one approach, embryonic stem cells (ESCs) are extracted from the healthy blastocysts derived from a few-day-old embryos (aborted fetuses or donated embryos left over from in vitro fertilization clinics), differentiated into progenitor cells, and transplanted or grafted into the patient’s brain (Fig. 6b). In another approach, skin fibroblast cells are extracted from patient’s body, developed into induced pluripotent stem cells (iPSCs), reprogrammed to produce healthy NpHR-expressing iPSCs, proliferated in large numbers, and differentiated into healthy progenitor cells (Fig. 6c). The main advantage of iPSCs over ESCs is that they are less prone to rejection by the recipient’s immune system because the transplanted cells come from the recipient. After implantation, these cells begin to form connections with the existing cells, can survive for several years, and produce NpHR with significant improvements in symptoms. Similar to implanted cardiac defibrillators that would confirm the presence of a seizure and respond within milliseconds of onset, the light of a specific wavelength is delivered to the transplanted neuronal cells, either via a cerebrally inserted optical fiber (optrode) or via wireless external delivery to control epileptic seizures before it spreads.
Delivery via Systemic Injection
Systemic (intravenous) injection without the need for cell transplantation [63] is also a possibility for opsin delivery in humans. For a systemic injection, a compound similar to mannitol or Trojan horse therapeutic system is used to assist opsins in crossing the blood–brain barrier (BBB). The Trojan horse therapy is based on fooling the BBB into accepting an endogenous naturally occurring cell such as a macrophage carrying the opsins as self, which then releases the housed opsins at the site of injury into the neurons of interest.
Limitations of Combining Optogenetics and Epilepsy
Thus far, application of this powerful tool has been successful in elucidating normal and pathological mechanisms, and some inquiry has been made into therapeutic applications in different species including rat, mouse, fly, and zebrafish. The development of a nonhuman primate model is one recent achievement that may increase the understanding of how optogenetics may be applied to humans [41, 64].
However, optogenetics is still comparatively a new technique in the sense that the work in this field has not yet been translated into the clinics. There are certain pitfalls and challenges in relation to likely consequences of using optogenetics for modulating the excitability of neuronal networks and related neuropathological conditions. Several unresolved ambiguities are looming over the field regarding its impact on epilepsy study and treatment. Much remains to be learned, and many technical aspects still need to be optimized before application to humans, several of which may prove to be substantial challenges.
Among these aspects are the investigation of the safe and effective optimization of opsin chemistry, delivery, human immune response to these foreign proteins, and the stability of viral vectors in neural tissue.
There are also concerns regarding the monitoring of seizure activity via a non-stationary device that should be capable of detecting seizure algorithms reliably.
Optimization of external light delivery with chronically implanted devices to spatiotemporally activate the opsins is another area of research. Thin, cylindrical optrodes with a tip containing an optical fiber and recording electrode are reminiscent of deep brain stimulation electrodes [65]. Other versions use an optrode implanted in an area, with a separate micro-LED array for recording. Before human use, these systems will need to meet the standards currently used for other implantable devices, with modifications made for patient tolerance and safety.
Parameters of illumination, like the length, frequency, and the effects of a continuous photostimulation over an intermittent stimulation is not completely known. Notably, the millisecond long illumination pulses used to activate ChR2 may overheat and thus damage the nerve tissue [58]. A wireless light delivery method is highly desirable because the implanted electrodes can damage the brain [66]. Non-invasive optical measurement methods, however, have already been in the pipeline, as well as in humans, for the first time, an implantable device was lately experimented to monitor seizure occurrence [9].
It is also important to quantify the effect of the light on neuronal firing. For example, the use of too strong light intensity to activate ChR2 may lead to opposite results, i.e., induction of a depolarization block and resultant cessation in firing activity. Without optimizing the effect of light intensity on neuronal firing, one might assume that the target cell is activated when, in reality, its firing is inhibited which may lead to false positive results.
Similarly, when using the inhibitory opsins, it is important to ensure that the light protocol is effective at reducing the firing rate of the opsin-expressing cells. These firing characterization studies can also lead to better quantification and understanding of the degree of change in firing required to produce the desired network/behavioral effect. In some cases, a change in the firing pattern might be desired and should be tested. For example, a change from bursting to tonic firing or vice versa might produce a robust effect on seizures even in the absence of a change in the mean firing rate. Therefore, fine quantification of the spiking is required to develop the minimal intervention methods to stop seizures without unwanted effects. This might be obtained with a relatively weak light intensity that would switch firing patterns (by mildly altering membrane potential) without dramatically modulating neuronal firing.
Conclusions
Microorganisms are again gaining momentum due to their unique cell architecture, biology, and metabolism as a potential platform [67–72] in biotechnology industry for the manufacturing of value-added products that may be worth millions of dollar. The development of N. pharaonis, as an industrial biotechnology host, would undeniably benefit the entire archeal genus which is less developed as compared to the rest of the microbes and pave the way to develop more productive and cost-effective alternate microbial platforms of biotechnological significance. Optogenetics is a relatively new neuroscience research tool to control neural activity of genetically modified neurons and circuits in bidirectional way with great temporal and spatial resolution using light. Thus, dissection and targeting of critical players in epilepsy and other related disorders for responsive treatments could be possible. Additionally, advances in wireless light delivery and to implant a device to monitor seizure activity in humans have already been made. Optogenetics has now been applied to various neuronal circuits in deducing the role of these circuits in numerous other neurological diseases, such as dopaminergic neuronal cells of the midbrain ventral tegmental area in depression [73] and medium spiny neurons of the basal ganglia in Parkinson’s disease [74]. Similarly, optogenetics could also be used in finding the role of dopamine D1-expressing neurons of the prefrontal cortex in examining a circuit underlying temporal control of behavior [75] as well as in post-stroke epileptic seizures, investigating the role of ventral surface astrocytes in breathing control [76] and stimulation of the heart muscle [77]. Last but not least, this review will pave the way for exploiting opsins more efficiently both to explore the pathophysiology of epilepsy and industrial biotechnology hosts in metabolic engineering to treat epilepsy.
References
Goldberg EM, Coulter DA (2013) Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci 14:337–349
Guillery RW, Harting JK (2003) Structure and connections of the thalamic reticular nucleus: advancing views over half a century. J Comp Neurol 463:360–371
Sherman SM, Guillery RW (2002) The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond Ser B Biol Sci 357:1695–1708
de Biasi S, Frassoni C, Spreafico R (1986) GABA immunoreactivity in the thalamic reticular nucleus of the rat. A light and electron microscopical study. Brain Res 399:143–147
Spreafico R, Battaglia G, Frassoni C (1991) The reticular thalamic nucleus (RTN) of the rat: cytoarchitectural, Golgi, immunocytochemical, and horseradish peroxidase study. J Comp Neurol 304:478–490
Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW (2002) Electrical synapses in the thalamic reticular nucleus. J Neurosci 22:1002–1009
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268
Kokaia M, Andersson M, Ledri M (2013) An optogenetic approach in epilepsy. Neuropharmacology 69:89–95
Krook-Magnuson E, Soltesz I (2015) Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat Neurosci 18:331–338
Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (2012) Jasper’s basic mechanisms of the epilepsies. Oxford University Press, New York
Pittau F, Megevand P, Sheybani L, Abela E, Grouiller F, Spinelli L, Michel CM, Seeck M et al (2014) Mapping epileptic activity: sources or networks for the clinicians? Front Neurol 5:218
Salin P, Tseng GF, Hoffman S, Parada I, Prince DA (1995) Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci 15:8234–8245
Zhang L, Jones EG (2004) Corticothalamic inhibition in the thalamic reticular nucleus. J Neurophysiol 91:759–766
Jin CL, Zhuge ZB, Wu DC, Zhu YY, Wang S, Luo JH, Chen Z (2007) Lesion of the tuberomammillary nucleus E2-region attenuates postictal seizure protection in rats. Epilepsy Res 73:250–258
Ramcharan EJ, Gnadt JW, Sherman SM (2000) Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci 17:55–62
Amtul Z, Rahman AU (2015) Neural plasticity and memory: molecular mechanism. Rev Neurosci 26:253–268
Amtul Z, Rahman AU (2016) Neural plasticity and memory: is memory encoded in hydrogen bonding patterns? Neuroscientist 22:9–18
Purves D (2001) Voltage-gated ion channels. In: Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM (eds) Neuroscience. Sinauer Associates, Sunderland (MA)
Junge D (1981) Nerve and Muscle Excitation. Sunderland, Mass.: Sinauer Associates
Zaman T, Lee K, Park C, Paydar A, Choi JH, Cheong E, Lee CJ, Shin HS (2011) Cav2.3 channels are critical for oscillatory burst discharges in the reticular thalamus and absence epilepsy. Neuron 70:95–108
Guido W, Weyand T (1995) Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol 74:1782–1786
Destexhe A, Sejnowski TJ (2003) Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev 83:1401–1453
Steriade M, McCormick DA, Sejnowski TJ (1993) Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685
Ji GJ, Zhang Z, Xu Q, Zang YF, Liao W, Lu G (2014) Generalized tonic-clonic seizures: aberrant interhemispheric functional and anatomical connectivity. Radiology 271:839–847
Jefferys JG (2010) Advances in understanding basic mechanisms of epilepsy and seizures. Seizure 19:638–646
Sakmann B, Neher E (1984) Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 46:455–472
Magiorkinis E, Sidiropoulou K, Diamantis A (2010) Hallmarks in the history of epilepsy: epilepsy in antiquity. Epilepsy Behav 17:103–108
Hosford DA, Clark S, Cao Z, Wilson WA Jr, Lin FH, Morrisett RA, Huin A (1992) The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science 257:398–401
Snead OC III (1995) Basic mechanisms of generalized absence seizures. Ann Neurol 37:146–157
Fong GC, Shah PU, Gee MN, Serratosa JM, Castroviejo IP, Khan S, Ravat SH, Mani J et al (1998) Pineda G, gado-Escueta AV: childhood absence epilepsy with tonic-clonic seizures and electroencephalogram 3-4-Hz spike and multispike-slow wave complexes: linkage to chromosome 8q24. Am J Hum Genet 63:1117–1129
Destexhe A (1999) Can GABAA conductances explain the fast oscillation frequency of absence seizures in rodents? Eur J Neurosci 11:2175–2181
Mayville C (2000) Fakhoury T, bou-Khalil B: absence seizures with evolution into generalized tonic-clonic activity: clinical and EEG features. Epilepsia 41:391–394
Shih TT, Hirsch LJ (2003) Tonic-absence seizures: an underrecognized seizure type. Epilepsia 44:461–465
Coulter DA, Huguenard JR, Prince DA (1989) Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol 25:582–593
Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Adamson PC (2013) Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 54:141–155
Sagher O, Thawani JP, Etame AB, Gomez-Hassan DM (2012) Seizure outcomes and mesial resection volumes following selective amygdalohippocampectomy and temporal lobectomy. Neurosurg Focus 32:E8
Amtul Z (2017) Nature’s medicines to treat epileptic seizures in studies in natural product chemistry, 56. Rahman AU (ed). Elsevier Science Publishers, Amsterdam (in print)
Loscher W (2011) Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20:359–368
Tye KM, Deisseroth K (2012) Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13:251–266
Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E et al (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446:633–639
Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K et al (2011) An optogenetic toolbox designed for primates. Nat Neurosci 14:387–397
Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD (2007) Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17:2105–2116
Wagner FB, Truccolo W, Wang J, Nurmikko AV (2015) Spatiotemporal dynamics of optogenetically induced and spontaneous seizure transitions in primary generalized epilepsy. J Neurophysiol 113:2321–2341
Buchholz K, Collins J (2013) The roots—a short history of industrial microbiology and biotechnology. Appl Microbiol Biotechnol 97:3747–3762
Baltz RH (2014) Combinatorial biosynthesis of cyclic lipopeptide antibiotics: a model for synthetic biology to accelerate the evolution of secondary metabolite biosynthetic pathways. ACS Synth Biol 3:748–758
Xu Y, Jiang Y, Chen Y, Zhu S, Shen S (2014) Hydrogen production and wastewater treatment in a microbial electrolysis cell with a biocathode. Water Environ Res 86:649–653
Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P et al (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 100:13940–13945
Schobert B, Lanyi JK (1982) Halorhodopsin is a light-driven chloride pump. J Biol Chem 257:10306–10313
Lanyi JK, Oesterhelt D (1982) Identification of the retinal-binding protein in halorhodopsin. J Biol Chem 257:2674–2677
Gradinaru V, Thompson KR, Deisseroth K (2008) eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol 36:129–139
Oesterhelt D, Tittor J (1989) Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci 14:57–61
Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR et al (2010) Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141:154–165
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V et al (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C et al (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330:1677–1681
Oesterhelt D, Stoeckenius W (1973) Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A 70:2853–2857
Zhang F, Wang LP, Boyden ES, Deisseroth K (2006) Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3:785–792
Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT et al (2005) Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A 102:17816–17821
Yizhar O, Fenno L, Zhang F, Hegemann P, Diesseroth K (2011) Microbial opsins: a family of single-component tools for optical control of neural activity. Cold Spring Harb Protoc 2011:top102
Betley JN, Sternson SM (2011) Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum Gene Ther 22:669–677
Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR (2013) Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci 16:64–70
Britt JP, McDevitt RA, Bonci A (2012) Use of channelrhodopsin for activation of CNS neurons. Curr Protoc Neurosci Chapter 2:Unit2
Amtul Z (2017) Regenerative cell-based therapies to combat neurodegenerative disorders in Frontiers in Stem Cell and Regenerative Medicine Research, Rahman AU (ed), Elsevier Science Publishers, Amsterdam. in press:1–21
Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA, Forsayeth JR, Cunningham J et al (2005) AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol 194:476–483
Ruiz O, Lustig BR, Nassi JJ, Cetin A, Reynolds JH, Albright TD, Callaway EM, Stoner GR et al (2013) Optogenetics through windows on the brain in the nonhuman primate. J Neurophysiol 110:1455–1467
Lin GX, Luo YM, Cheng A, Yang SY, Wang JS, Goldman RD (2012) Personal experience in pediatric emergency medicine training in Canada and China. Chin Med J 125:3747–3749
Cox MP, Ma H, Bahlke ME, Beck JH, Schwartz TH, Kymissis I (2010) LED-based optical device for chronic in vivo cerebral blood volume measurement. IEEE Trans Electron Devices 57:174–177
Amtul Z (2003) Studies into urease enzyme and Alzheimer’s disease, 106, Karachi University. Ref Type: Thesis/Dissertation
Amtul Z, Follmer C, Mahboob S, Rahman AU, Mazhar M, Khan KM, Siddiqui RA, Muhammad S et al (2007) Germa-gamma-lactones as novel inhibitors of bacterial urease activity. Biochem Biophys Res Commun 356:457–463
Amtul Z, Kausar N, Follmer C, Rozmahel RF, Rahman AU, Kazmi SA, Shekhani MS, Eriksen JL et al (2006) Cysteine based novel noncompetitive inhibitors of urease(s)—distinctive inhibition susceptibility of microbial and plant ureases. Bioorg Med Chem 14:6737–6744
Amtul Z, Rahman AU, Siddiqui RA, Choudhary MI (2002) Chemistry and mechanism of urease inhibition. Curr Med Chem 9:1323–1348
Amtul Z, Rasheed M, Choudhary MI, Supino R, Khan KM, Atta UR (2004) Kinetics of novel competitive inhibitors of urease enzymes by a focused library of oxadiazoles/thiadiazoles and triazoles. Biochem Biophys Res Commun 319:1053–1063
Rasheed M, Khan KM, Ateeq HS, Choudhary MI, Rahman AU, Perveen Z, Zia-Ullah, Amtul Z (2002) Synthesis of Biologically Active Compounds, Karachi, Pakistan, Print Arts, pp 235–241
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC et al (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536
Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626
Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ (2012) Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci U S A 109:20726–20731
Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM et al (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575
Arrenberg AB, Stainier DY, Baier H, Huisken J (2010) Optogenetic control of cardiac function. Science 330:971–974
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Amtul, Z., Aziz, A.A. Microbial Proteins as Novel Industrial Biotechnology Hosts to Treat Epilepsy. Mol Neurobiol 54, 8211–8224 (2017). https://doi.org/10.1007/s12035-016-0279-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0279-3