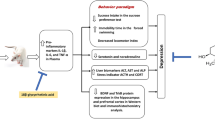
Abstract
The mechanisms by which inflammation affects the different emotional moods are only partially known. Previous works have pointed to stress hormones like glucocorticoids plus the vascular factor endothelin-1 as key factors evoking stressful states especially in relation to endothelial dysfunctions. With this work, it was our intention to establish the role of pro- and anti-inflammatory cytokine expression variations towards depression-like behaviors and consequently the development of neurodegeneration events caused by endothelial damages in the hamster (Mesocricetus auratus). Such a rodent, which is considered a valuable animal model to test depression and anxiety states, exhibited a variety of depression-like behaviors including reduction in sucrose consumption, locomotion, and exploration (p < 0.01) following exposure to unpredictable chronic mild stress. Contextually, a tight correlation between unpredictable chronic mild stress-induced depressive states and expression of the pro-inflammatory cytokines was detected as shown by marked expression levels (p < 0.01) of IL-1β and NF-kB in the hippocampus, amygdala, and prefrontal cortex. Even the anti-inflammatory cytokine IL-10 supplied notably significant (p < 0.001) expression levels in the same areas of resilient hamsters. Application of hemodynamic and endothelial functional studies pointed to altered arterial endothelial activities in depressed with respect to resilient animals. Moreover, evident damaged neuronal fields in the above areas of depressed hamsters allowed us to correlate such a behavioral phenomenon to the upregulation of IL-1β and NF-κB. Overall, the differing roles of pro- and anti-inflammatory cytokines on depressive states, especially in view of brain endothelial damages, may provide novel therapeutic measures against mood disorders linked to neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic stress, which is tightly linked to the rapid pace of our living standards, appears to be a major cause for the development of depression (DEP) [1]. This mental state is widely associated with the activation of body adaptive systems (allostatic), allowing for the introduction and maintenance of a new state of equilibrium (allostasis) in response to destabilizing pathogenic factors. In this case, the immune system appears to exert a key role as pointed out by such a disorder resulting in the alteration of an innate and acquired immunity system associated with inflammatory processes [2]. Despite the numerous reports dealing with the influence of DEP on serum IL-6 levels [3, 4], indications regarding other cytokines such as IL-1β plus IL-10 are relatively few [5, 6].
In the past, impairments of neuronal plasticity have been largely correlated with the pathophysiology of severe mood disorders [7]. This is the case of neurodegenerative disorders such as Huntington’s and Alzheimer’s (AD) diseases [8] characterized by episodes of DEP, thus becoming an important indicator of latent neurodegeneration [9]. Indeed, a specific correlation between DEP in late-life, anhedonia, apathy and evident volume decrease in brain white matter has been traced to subcortical vascular diseases featured in the pathogenesis of elderly mood disorders [10]. Additionally, human imaging studies showing cellular loss in key brain regions such as the prefrontal cortex (PFC) and amygdala (AMY) of patients with mood disorders have attributed reduced brain volume plus microvasculopathy to DEP [11]. Similarly, memory impairments have been also tightly linked to a higher frequency of hippocampal (HIP) atrophy [12].
In order to develop new concepts regarding the onset of such mood disorders as well as new pharmacological therapies, a great amount of attention has been directed to human and animal studies. From these researches, neurovascular unit dysfunctions, featuring blood–brain barrier hyperpermeability, tend to be strongly correlated with oxidative stress and neuroinflammation in neurological disorders such as stroke, epilepsy, and AD [13]. DEP, in contrast to other major psychiatric disorders, is frequently co-morbid with a large number of neurological disorders that are characterized by vascular endothelial dysfunctions, such as cardiovascular diseases and diabetes mellitus [14].
Based on these indications, it was our intention to establish the specific role of pro- and anti-inflammatory cytokines on the development of depressive states, since chronic stressful conditions appear to cause endothelial damages and subsequently increase neurodegenerative events. In the present study, the above key limbic areas were evaluated due to the fact that despite the stressed animals having showed adrenal hypertrophy and elevated circulating epinephrine, only food-deprived stressed animals displayed altered HIP plus hypothalamic cytokine expression levels thus inducing physiological and anhedonic responses [15]. Hence, stressful conditions via elevated brain inflammatory processes in these and other limbic sites may account for high blood pressure of the endothelial wall [16]. For this reason, our attention was focused on establishing if endothelial damages were linked with the maintenance of DEP through neurodegenerative events in AMY, HIP, and PFC, which are largely involved with cognitive decline following the onset of DEP states [17, 18]. Such brain regions were preferred because aside being important sites linked with DEP phenomena, they have recently been associated with oxidative stress and inflammation, which are responsible for the pathophysiology of such an emotional state [19], while other encephalic areas like the raphe nucleus were not contemplated for DEP-related anhedonia processes in the present study. In this context, DEP and resilient (RES) hamsters (Mesocricetus auratus) exposed to unpredictable chronic mild stress (UCMS) protocols were used. It is known that anxiety and DEP in humans are characterized by increased expectations of negative events and interpretation of ambiguous information while a positive outcome is associated with enhanced expectation of a positive interpretation of ambiguous information [20]. Mood-congruent judgment biases for ambiguous information have been demonstrated in a wide range of animal species for which the hamster is considered a valuable species to test DEP and anxiety states [21]. Moreover, it was also our intention to evaluate the expression differences of IL-1β, IL-10, and NF-kB in the above brain regions of hamsters exposed to UCMS and endothelial damages, with the aim of proposing novel molecular therapies for the treatment of mood disorders and neurodegenerative disease such as AD.
Materials and Methods
Animals
In the present study, male Syrian golden hamsters (Mesocricetus auratus) weighing 130–160 g (6 months old) were purchased from Charles River (Como, Italy) and brought into the laboratory 2 weeks before starting the experiments. This rodent model was mainly chosen for its valuable ability to supply distinctly specific signs of DEP or anxiety when tested in behavioral analysis such as UCMS protocols [21, 22]. Sixty-one hamsters were randomly chosen for UCMS protocols; 21 served as controls. Room temperature was set at 22 °C, and hamsters were housed one per cage unless grouping was applied during the protocol. A 12-h light/dark cycle was maintained except when a specific condition was needed in the course of stressor schedule. Food and water were freely available except as part of the deprivations during the stressor schedule. Control animals had no contact with stressed animals and were not treated except for water and food deprivation for 18 h prior to sucrose consumption test. Animal maintenance and experimental procedures were carried out in compliance with ethical provisions for Care and Use of Laboratory Animals reported in the legislative law no. 116 (27-01-1992) and authorized by the National Committee of the Italian Ministry of Health. Efforts were made to minimize animal suffering and reduce the number of experiments.
Chronic Mild Stress Protocol
The experimental groups were exposed to a variety of external relevant stressors over a period of 9 weeks. The stressor procedure was performed according to an optimized procedure of UCMS protocol [22, 23] to achieve depressive-like symptoms. At the age of 2 months ±1 week, animals were ear-punched for identification and assigned to one of two treatment groups, controls and UCMS. UCMS treatment started at this point and continued during the behavioral testing phase. Care was taken not to apply stressors just before a behavioral test. The initial tests took place 2 weeks after the beginning of the stress procedure. Because of the nature of UCMS procedure, stressed and control animals were kept in separate but otherwise identical holding rooms. The protocol consisted of a series of mild unpredictable stressors: food deprivation, swimming test, cage tilted, inversion of the light/dark cycle, lights on for a short period of time during the dark phase, and switching cages. Effects of all stressors lasted for at least 14 h with the exception of swimming, which was conducted for 5–10 min. On the average, two of these stressors were applied daily at different times and following a semi-random 2-week schedule. The stress procedure lasted for 9 weeks prior to behavioral tests. Stressors continued to be applied during the testing phase, with at least 6 h of rest being allowed between each testing session.
Sucrose Consumption Test
The sucrose consumption test was used to measure the preferential changes of hamsters in consuming a palatable solution. Animals were first trained to consume a 1.5 % sucrose solution together with water, for 2 weeks. In this same period, a sucrose consumption test was performed twice a week. Subsequently, hamsters had 1 h of free access to the solution following 18 h of food and water deprivation. Sucrose intake was measured by weight changes of bottles at the end of the test. This test was performed once a week during UCMS sessions. Following these sessions, sucrose consumption was handled in hamsters (Fig. 1b) that were defined as DEP anhedonia (n = 20) if there was >25 % decrease in sucrose intake/animal, or stress RES (n = 20) if the decrease in sucrose consumption was <10 % with respect to controls (n = 21).
a A time line of 12 weeks was applied for this study, in which hamsters were given a sucrose solution for 15 days habituation period. These animals were then exposed to an unpredictable chronic mild stress (UCMS; see schema) for 9 weeks in order to establish the depressive states of hamsters. b In this period, the amounts of sucrose consumed by hamsters subjected to 9 weeks of UCMS were established on the basis of >25 % decrease in sucrose intake; hamsters were defined anhedonic DEP while those that displayed <10 % decrease of sucrose consumed were defined as stress-resilient animals with respect to controls (CTRL) that were only exposed to the habituation session. At the end of this period, the hamsters that fitted within the two categories were also exposed to sucrose preference, LDT, EPM, and food consumption tests, for a further indication of the nature of depressive states with respect to their CTRL. Twenty-four hours after the last behavioral test, hamsters were sacrificed and their brains removed for further molecular analyses
Light–Dark Exploration Test
At the end of UCMS sessions, hamsters were exposed to a light–dark exploration test (LDT) apparatus in order to determine the effective DEP states or RES conditions. LDT consists of a box with two compartments: a first arena composed of a small and dark plastic compartment (16 × 16 × 16 cm) and a second arena containing a large translucent and white illuminated compartment (25 × 25 × 30 cm), which was considered the unfamiliar environment. For the different behavioral evaluations, a camera (Logitech QuickCam Pro5000) was positioned 1 m above the apparatus, connected to a computer so that it was possible to record behaviors during the testing sessions. All observations plus behavioral analysis were conducted in the same manner to that previously described [24].
Elevated Plus-Maze Test
Together with LDT, animals were also exposed to an elevated plus-maze test (EPM) apparatus that allowed us to evaluate DEP responses in a more specific manner as previously described [25, 26]. EPM is a wooden maze consisting of four arms arranged in a cross-shaped design. The apparatus was elevated to a height of 50 cm above the floor plus being illuminated by white lamps (4 × 60 W). After the UCMS session, hamsters were tested with LDT and with EPM (on the same day but at different time periods) for seven consecutive days in the morning, noon, and afternoon as indicated in a previous study [27–29].
RNA Extaction and Semi-quantitative RT-PCR
PFC, AMY, and HIP of DEP, RES, and controls were removed and processed in TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. We confirmed purity of our samples by spectroscopy at 260/280 and 260/230. RNA concentration was determined from the optical density at a wavelength of 260 nm (using an OD260 unit equivalent to 40 μg/ml of RNA). One microgram of RNA was reverse transcribed (cDNA) using a reverse transcriptase system kit (Applied Biosystems, Foster City, CA). Semi-quantitative real-time PCR was performed using SYBR Green universal PCR master mix (Bio-Rad Laboratories, Hercules, CA). The thermal profile for PCR included an initial denaturation at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 45 s, annealing at 57 °C for 30 s, and extension at 72 °C for 1 min. Reactions were done in triplicate and analyzed using the ΔΔCt method with GAPDH as a normalization control. Briefly, fold increases or decreases were determined with respect to controls after normalizing with a housekeeping gene − ΔΔCT, where ΔCT is (gene of interest CT) − (GAPDH CT) and ΔΔCT is (ΔCT treated) − (ΔCT control).
The primer sequences were as follows: hamster GAPDH, 5′-CCGAGTATGTTGTGGAGTCTA-3′ and 5′-GCTGACAATCTTGAGGGA-3′; hamster IL-1β, 5′-ATCTTCTGTGACTCCTGG-3′ and 5′-GGTTTATGTTCTGTCCGT-3′; hamster IL-10, 5′-AGACAAAGCCAGAGTCATT-3′ and 5′-TCGGTATGCTAAGGCACAG-3′; and hamster NF-κB, 5′-GGAGTCTGTGGACCTGTGCG-3′ and 5′-TGAACCATTCGGAGGCTGTG-3′.
Western Blotting
Hamsters belonging to the above groups were sacrificed after the last behavioral session. Their brains were removed and AMY, PFC, and HIP were dissected out and homogenized on ice in a lysis buffer [150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM EDTA, 0.5 % sodium deoxycholate, 0.1 % SDS, plus 1 % nonidet P-40] containing a cocktail of proteinase inhibitors (Roche) and a phosphatase inhibitor (Sigma). Samples were centrifuged at 12,500 rpm at 4 °C for 30 min, and the supernatant was collected and stored at −80 °C for future immunoblotting analyses. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein per sample (20 μg) were separated by electrophoresis on 8 and 10 % Tris-glycine gels with 4 % stacking gels, then they were transferred to PVDF membranes and the membranes were blocked with 5 % serum albumin for 1 h. The primary antibodies anti-IL-1β, IL-10, NF-kB, and anti-β-actin were used for all brain regions (1:1000; Cell Signaling Technology, Danvers, MA). The secondary antibodies (all 1:7000 diluted in blocking solution) were horseradish peroxidase conjugated anti-rabbit IgG (Chemicon International, Inc., Temecula). A horseradish peroxidase chemiluminescence kit with enhanced luminol and oxidizing reagents (Bio-Rad) were used to visualize chemiluminescent signals. These enhanced blot signals were photographed using a CCD camera (Molecular Imager Gel Doc XR System; Bio-Rad) in a darkroom. The volume of the bands (i.e., area intensity) was quantified using Quantity One Software (Bio-Rad).
Vessel Dissection and Pulse Wave Analysis
Using a dissecting microscope, brain arteries were obtained from DEP, RES, and control hamster brains and a 1- to 2-mm segment was cannulated on a buffer-filled, pulled-glass pipette in a custom-built arteriograph (Instrumentation and Model Facility, UVM). Prior to securing the distal end of the arterial segment onto a second pipette, luminal contents were flushed at 10 mmHg. The intraluminal pressure was measured and maintained using a pressure-servo system (Living Systems Instrumentation) with an in-line transducer. Vessels were then tested for leakage, and those unable to maintain a pressure of 50 mmHg were discarded. All experiments were performed under non-flow conditions. The arteriograph was positioned onto the stage of an inverted microscope (Nikon TMS; MVI), which was equipped with a video dimension analyzer attached to computerized software (Win Daq DI-720; DATAQ Instruments, Inc). Vessels were continuously superfused with PSS maintained at 37 °C and pH 7.4 and monitored with a micro-pH probe placed in the superfused bath (Microelectrodes Inc). To avoid possible confounding effects of myogenic tone at higher transmural pressures, vessels were equilibrated for 10 min at 10 mmHg followed by 40 min at 50 mmHg. The transmural pressure was maintained at 50 mmHg for the remainder of the procedure. After equilibration, vessels were exposed to increasing concentrations of serotonin (10−8 to 10−5 M) and acetylcholine (10−9 to 10−4 M) and immediately recorded at each concentration until a maximal response was achieved. Pharmacological sensitivity was determined by standard curve analysis of data imported into the Sigma Plot program (SigmaPlot version 9.0; Systat Software Inc), and the acetylcholine concentration required to produce the EC50 value of the maximal vasodilatory response was determined.
Analysis of Neurodegeneration
The possibility that endothelial damages evoked enhanced neurodegeneration events was established using the amino cupric silver stain (ACS) method, which is applied for detecting both necrosis and apoptotic degeneration [30]. This method has proven to be a valuable tool since it guaranteed a selective analysis of early and semi-acute neurodegeneration events not only in advanced damaged cell bodies, dendrites, axons, and terminals but also the recruitment of new structures in progressive pathologic cases. For this part, a serial set of representative coronal sections (30 μm) from hamsters belonging to the same above groups was obtained at a cryostat (Microm-HM505E; Zeiss, Wallford, Germany) and selected at an interval of 240 μm for ACS procedures as described in previous studies [26]. Counterstained sections with 0.5 % neutral red solution (Carlo Erba, Milan, Italy) were then mounted with DPX (Sigma, Milano, Italy) and observed using a bright-field Dialux EB 20 microscope (Leitz, Stuttgart, Germany). For the estimation of damaged fields, it was necessary to calculate the neuronal volumes (defined as Vref) in different brain sites using the following formula:
Statistical Analysis
Data expressed as mean ± SEM were evaluated and compared among the different conditions by using a one-way ANOVA followed by Bonferroni’s post hoc test (GraphPad InStat 3.0 for MacIntosh, La Jolla, CA). Statistical significance was considered when p < 0.05.
Results
UCMS Induces a Depression-Like State in Hamsters
Of the 61 hamsters in the experimental groups, 20 were established to be DEP anhedonia and 20 were stress RES according to the sucrose consumption results; 21 were used as control (Fig. 1). When these animals were subjected to a UCMS protocol, they exhibited differing DEP-like behaviors. In a first case, hamsters identified as DEP showed a reduced desire of executing open arms entries (OAE) [F (2;12) = 4.02, p < 0.05] with respect to controls (−75 %; Fig. 2a). Rather, the former animals exhibited evident explorative activities [F (2;12) = 4.07, p < 0.05] despite hamsters spending a moderately greater amount of time (40 %) in the dark box (Fig. 2d). It was interesting to note that DEP-like states were notably attenuated in RES hamsters for all behavioral tests with respect to controls. Indeed, RES hamsters spent most of their time (78 %) in open arms (OAT) [F (3;16) = 3.23, p < 0.05] with respect to DEP hamsters that seemed to remain for a greater amount of time in closed arms (Fig. 2b). In this context, it was clear that RES hamsters preferentially executed motor activities [F (2;12) = 3.91; p < 0.05] as indicated by hamsters spending a greater (90 %) amount of time moving from open to closed arms (Fig. 2c).
For this part, animals were tested in both EPM (a–c) and LDT (d) to evaluate DEP. The test was performed after UCMS protocol. Each bar represents percent mean ± S.E.M. of OAE (a), OAT (b), backward and forward locomotor activity (c), or time spent in the dark box (d) with respect to RES and CTRL. The differences were evaluated by ANOVA plus a post hoc Newman-Keuls test when p < 0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
IL-1β, IL-10, and NF-kB mRNA Levels in DEP and Resilient Hamsters
From the evaluation of the three cytokines in PFC, AMY, and HIP, it appeared that mainly IL-1β and NF-kB were notably activated in AMY (95 %, p < 0.001) and HIP (125 %, p < 0.001) of DEP with respect to controls (Fig. 3d–i). The same relationship was detected in RES animals despite being less evident for both brain areas (66 %; 62 %, respectively, p < 0.01). This difference was of an even greater nature when expression levels of IL-1β in AMY (79 %; p < 0.001) and HIP (96 %; p < 0.001) of DEP were compared to RES hamsters (Fig. 3d, g), thus strengthening the importance of neuroinflammatory events maintaining DEP states. Surprisingly, such a difference was not detected in PFC of both DEP and RES hamsters (Fig. 3a, c). In contrast, the anti-inflammatory cytokine (IL-10) notably increased in HIP (118 %; p < 0.001) and even in PFC (120 %; p < 0.001) of the latter hamsters, while a weaker enhancement occurred in AMY (68 %; p < 0.01) of this same group (Fig. 3b–h), thus pointing to a probable protective role of anti-inflammatory molecules on DEP.
Expression levels of IL-1β, IL-10, and NF-kB in PFC, AMY, and HIP of DEP, RES, and CTRL hamsters. IL-1β, IL-10, and NF-kB mRNA levels in PFC (a–c), AMY (d–f), and HIP (g–i), respectively, of DEP and RES with respect to CTRL were determined by qPCR methods. Data were expressed as fold increase by ANOVA plus a post hoc Newman-Keuls test when p < 0.05; one asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
IL-1β, IL-10, and NF-kB Signaling in DEP and RES Hamsters
In order to further delineate the downstream signaling involved with the neuroinflammatory effects of the different groups, protein expression differences in the same brain regions of both DEP and RES hamsters demonstrated overlapping patterns with mRNA expression differences. In the case of IL-1β protein, this cytokine resulted to be extremely increased in HIP (91 %; p < 0.001) of the former hamsters with respect to controls (Fig. 4c) while only a significant amount of protein was obtained in PFC (40 %; p < 0.05) and AMY (71 %; p < 0.01), despite being of a less evident nature (Fig. 4a, c). In RES hamsters, this protein resulted to be greatly (65 %; p < 0.01) and moderately (43 %; p < 0.05) expressed in HIP (Fig. 4c) and AMY (Fig. 4b), respectively, while similar levels (as controls) were reported for PFC (Fig. 4a). Interestingly, exceedingly greater quantities of IL-1β protein (90 %; p < 0.001) were expressed in PFC of DEP hamsters with respect to RES, whereas the other two areas expressed only moderate amounts (38 %; p < 0.05). Contextually, the expression capacities of IL-10 resulted to be increased in PFC (84 %; p < 0.01), AMY (90 %; p < 0.001), and HIP (88 %; p < 0.01), of RES with respect to controls, which, in accordance with PCR results, appeared to partially evoke a neuroprotective role (Fig. 5a–c). In the case of NF-kB protein, it seemed to be moderately increased in PFC of DEP (34 %; p < 0.05) as compared to RES and control groups (Fig. 6a). In addition, a significant increment of NF-kB protein was also observed in AMY (78 %; p < 0.01) and HIP (86 %; p < 0.01) of DEP animals with respect to controls despite being of a less evident nature in RES hamsters (39 %; Fig. 6b, c).
Immunoblotting was used to detect IL-1β expression in PFC (a), AMY (b), and HIP (c) of the same groups reported in Fig. 3 after UCMS. Quantification of immunoblotting results was expressed as either a ratio of IL-1β/β-Actin (fold increase ± S.E.M.). Immunoblotting differences in DEP, RES, and CTRL animal groups were evaluated using a two-way ANOVA followed by a post hoc Newman-Keuls multiple range when p value ≤0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
Immunoblotting was used to detect IL-10 expression in PFC (a), AMY (b), and HIP (c) of the same groups reported in Fig. 3, after UCMS. Quantification of immunoblotting results was expressed as either a ratio of IL-10/β-Actin (fold increase ± S.E.M.). Immunoblotting differences in DEP, RES, and CTRL animal groups were evaluated using a two-way ANOVA followed by a post hoc Newman-Keuls multiple range when p value ≤0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
Immunoblotting was used to detect NF-kB expression in PFC (a), AMY (b), and HIP (c) after UCMS. Quantification of immunoblotting results was expressed as either a ratio of NF-kB/β-Actin (fold increase ± S.E.M.). Immunoblotting differences in DEP, RES, and CTRL animal groups were evaluated using a two-way ANOVA followed by a post hoc Newman-Keuls multiple range when p value ≤0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
Effects of DEP on Endothelial Function
Acetylcholine is a potent vasodilator of brain arteries of DEP and RES hamsters with respect to controls (Fig. 7a). This neuromediator assures a notable stability during vascular constriction, which is considered to be appropriate for observing the effects of DEP on vascular morpho-functional features. In particular, we observed evident increases in vasodilation of these animals with respect to controls (96 %) and RES (56 %) hamsters (Fig. 7a). From the different experiments, it seemed that such a neuromediator induced vasodilation in a concentration-dependent (10−8–10−5 M) manner. Vasodilation was significantly greater in vessels of RES animals with respect to DEP (48 %) especially at 10−8 M (Fig. 7c), thus suggesting either less responsiveness of vessels in the brain of DEP hamsters or that they are greatly damaged. Suspected endothelial damages were confirmed by myogenic tone graphs in which it was possible to register a great increase of myogenic tone in DEP animals especially at 20 and 30 mmHg (Fig. 7b). However, this tone resulted to be significantly reduced when vessel pressure increased from 50 to 110 mmHg.
a Concentration response to serotonin vasoconstriction in pressurized brain arteries from DEP, RES, and CTRL hamsters. b Measurement of myogenic tone of brain arteries from the same groups was handled at different levels of pressure P (mmHg). c Brain arteries were dilated with acetylcholine at different concentrations. d An inverted microscope was used to show the conditions of the dissected brain arteries. Data are reported as mean ± S.E.M. when p value ≤0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
Neurodegeneration Analysis
The varying behavioral events of both DEP and RES hamsters seemed to be strongly correlated to altered neuronal responses as supported by evident neurodegeneration activities in these brain areas noted for their emotional and cognitive roles. Indeed, by using ACS approaches, it was possible to highlight distinct damages of neuronal fields, via argyrophilic reactions especially in the former animal group (Fig. 8). In particular, consistently dark perikarya, dendrites, and axons were detected in HIP, AMY, and PFC of DEP with respect to controls and RES hamsters (Fig. 8(b, e, h)). Interestingly, a significantly evident neurodegeneration response was obtained in PFC as shown by a notable level of damaged neurons in DEP animals (Fig. 8(h); 168 %) while a moderate level was detected in AMY (Fig. 8(e); 68 %) with respect to the few if any signals in controls (Fig. 8(d, g)). In contrast to DEP, RES hamsters supplied decreased neurodegeneration signals in all brain areas, especially in PFC (−105 %) and HIP (−90 %) with respect to DEP hamsters (Fig. 8(c, i)).
A Neurodegeneration processes in PFC, AMY, and HIP of DEP, RES, and CTRL hamsters. Representative photograms of ACS (a–i) in which arrows pointing to dark neuronal perikarya indicate damaged neurons showing the different levels of neurodegeneration/neuroprotection as reported for brain regions after UCMS. Scale bar 60 μm. The data were expressed as number of degenerated neurons ± S.E.M. Evaluations were conducted using ANOVA plus a post hoc Newman-Keuls test when p value ≤0.05. One asterisk p < 0.05, two asterisks p < 0.01, three asterisks p < 0.001
Discussion
The results of the present study support the tight relationship between neuroinflammatory and persistent DEP conditions that are commonly typical of neuropsychiatric states like anorexia, malaise, and decreased physical capabilities [31]. It has already been shown that the removal or inactivation of potentially damaging agents is primarily mediated via one of two cell systems: glia of the CNS or lymphocytes, monocytes, and macrophages of the hematopoietic system [32]. Indeed, patients who have major DEP disorders exhibit altered immunologic markers such as pro-inflammatory cytokine activities [33, 34]. Even indications deriving from other works point to chronic stress as a main factor exacerbating the release of pro-inflammatory cytokines and hence evoking increased episodes of DEP [35]. Moreover, recent studies have emphasized that stress by interacting with the immune system may increase the levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6, which are involved with the activation of anxiety and DEP [36]. These indications are in line with the activating role(s) of neuroinflammatory events on neurological disorders like AD [37, 38], amyotrophic lateral sclerosis, epilepsy, Huntington’s disease, multiple sclerosis, and Parkinson’s disease [39], which often display co-morbid events with DEP [31]. The increased expression levels of IL-1β and NF-kB being prevalently detected in DEP hamsters while IL-10 increased only in RES hamsters tend to strengthen the bidirectional relationship of pro- and anti-inflammatory factors even for such emotive states in other rodent models plus human patients [40]. Indications deriving from these and similar results tend to favor the induction of DEP states in hamsters, which consumed less sucrose, being correlated to elevated levels of the same above cytokine levels [41], and such an expression condition, in turn, might account for increased levels of the protective cytokine IL-10 in non-DEP patients [40, 42] like that of our RES hamsters. In this context, such a relationship tends to propose new conditions for curing DEP and in particular by simply varying the switching on/off levels of NF-kB, IL-1β, caspase-3, and substance P of the cortex and HIP like that reported for pain plus DEP co-morbid disorders [43, 44].
In accordance with other works, it appears that DEP is not only an indicator of emotional state-related neurodegeneration processes; rather, symptoms leading to the development of this emotional state, particularly in a later biological period, may also become a reliable indicator of latent neurodegeneration [45, 46]. Indeed, human imaging studies showing cellular loss in key brain regions such as PFC and AMY of patients with mood disorders attribute DEP to a selective disruption of brain volume rather than to altered interhemispheric connections [47, 48]. Still other studies carried out on animal models like WKY rats, a putative model of this emotional state, point to a consistent loss of neuronal volume in another telencephalic region such as the HIP with respect to their control in which the activation of apoptosis in DEP patients constitutes a main cause of neurodegeneration [49]. As a consequence, it appears that apoptosis and cell death, which constantly occur under both physiological and pathological conditions, are largely characterized by cell debris being mostly cleared by immune cells without the induction of chronic inflammation [50]. However, during systemic inflammation, apoptosis of stressed cells might further exacerbate the underlying pathology by directly or indirectly activating caspases [51]. Conversely, the increased expression of the anti-inflammatory molecule IL-10 and decreased IL-1β levels in RES hamsters tend to underlie a neuroprotective effect of the latter cytokine, very probably via the deregulated NLRP assembly and above all the inhibition of microglial formation [52, 53]. This relationship seems to go in the same direction of increased IL-10 levels in HIP plus PFC of RES hamsters thus further supporting diminished damaged neuronal cells in such brain areas. It appears that IL-10, deriving from astrocytes and/or microglia, is exerting a neuroprotective role via either the activation of microglia TGF-β [54] or perhaps through the reduction of gluatamatergic signaling resulting in an impairment of NMDAR-dependent ROS formation [55]. In this scenario, the fact that PFC showed the highest neurodegeneration effects despite the least inflammation in DEP state may be initially due to reduced levels of phosphorylated cAMP response-element binding protein/brain-derived neurotrophic factor (BDNF) in PFC plus HIP that normally exert protective measures against neuroinflammatory events [56]. In addition, such a notable damaged condition of PFC may also very likely be attributed to the elevated amounts of hypermethylated ankyrin gene, which is typical of neuropathological conditions like that of AD [57] together with low BDNF levels subsequently leading to the activation of the serotoninergic signals [58] that in turn may very probably be responsible for the activation of neuroprotective mechanisms via the blockade of the glutamatergic pathway [59].
As far as the neurovascular unit, consisting of cerebral microvessels, glial cells (astroglia, microglia, oligodendroglia) plus neurons is concerned, this structure appears to be the epicenter of several tightly controlled, dynamic, and complex cellular interactions between glia and neurons, and the coupling of neuronal activity with endothelium-dependent cerebral blood flow [60]. Evidences of an association between DEP and neurovascular dysfunctions have been shown to mostly derive from the assessment of altered peripheral vascular endothelial of DEP cases along with epidemiological data associating mood disorders with vascular impairment due to massive transmembrane ionic and water shifts plus oxidative stress [31]. It is known that the latter cause of vascular impairment along with neuroinflammation is implicated in the neurobiology of DEP [61, 62] and is mechanistically linked to the presence of altered neurovascular units with blood–brain barrier hyperpermeability in selected neurological disorders, such as stroke, epilepsy, traumatic brain injury, and AD [63]. In contrast to other psychiatric disorders, DEP is frequently co-morbid with the above neurological disorders and constitutes an independent risk factor for morbidity and mortality in patients featuring vascular endothelial dysfunction such as diabetes mellitus [63–65].
Overall, results of the present work point to a switching on/off mechanism of the pro- (IL-1β plus NF-kB) and anti-inflammatory (IL-10) cytokines as key factors on the development of DEP. The high levels of IL-10 together with low levels of IL-1β and NF-kB in RES hamsters tend to suggest a neuroprotective role against neurodegeneration, via very likely a reduction of neurotrophic factors such as BDNF or neurotrophin [53, 66]. On the other hand, elevated levels of IL-1β and NF-kB in DEP hamsters appear to be consistent with the maintenance of this emotional state. Moreover, altered vascular functions were, instead, related to elevated secretion of pro-inflammatory cytokines. In particular, elevated IL-10 levels coinciding with the absence of damaged blood vessels in RES hamsters may supply us with further indications on how anti-inflammatory substances like the polyphenol, resveratrol, and the Chinese herbal medicine Kaixinjieyu are able to exert a protective role against vascular damage linked to neurodegenerative disorders [44]. Nonetheless, the neuronal mechanisms involved with inflammatory and neurodegenerative conditions still remain to be elucidated; the differentiated pro- and anti-inflammatory cytokine roles within DEP-prone brain areas may represent novel therapeutic targets for psychiatric and mood disorders.
Abbreviations
- DEP:
-
Depression
- RES:
-
Resilient
- AMY:
-
Amygdala
- PFC:
-
Prefrontal cortex
- UCMS:
-
Unpredictable chronic mild stress
References
Lam RW, Malhi GS, McIntyre RS, Demyttenaere K, Gorwood P, Michalak EE et al (2013) Fatigue and occupational functioning in major depressive disorder. Aust NZJ Psychiatry 47:989–991
Krishnadas R, Cavanagh J (2012) Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry 83:495–502
Gorska-Ciebiada, M., Saryusz-Wolska, M., Borkowska, A., Ciebiada, M., Loba, J. (2015) Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab Brain Dis [Epub ahead of print]
Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhães PV, Kapczinski F, Kauer-Sant’Anna M (2015) Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 11:1002–1012
Hocaoglu C, Kural B, Aliyazicioglu R, Deger O, Cengiz S (2012) IL-1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α and its relationship with lipid parameters in patients with major depression. Metab Brain Dis 4:425–430
Peter J, Frey O, Stallmach A, Bruns T (2013) Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol 27:13–37
Manji HK, Duman RS (2001) Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull 2:5–49
De Souza J, Jones LA, Rickards H (2010) Validation of self-report depression rating scales in Huntington’s disease. Mov Disord 1:91–96
Burgut FT, Benaur M, Hencliffe C (2006) Late-life depression: a neuropsychiatric approach. Expert Rev Neurother 1:65–72
Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, Jagust W, Chui H, Mack WJ (2008) The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry 10:1040–1050
Sakkas LI, Bogdanos DP (2016) Systemic sclerosis: new evidence re-enforces the role of B cells. Autoimmun Rev 2:155–161
Appenzeller S, Carnevalle AD, Li LM, Costallat LT, Cendes F (2006) Hippocampal atrophy in systemic lupus erythematosus. Ann Rheum Dis 12:1585–1589
Wichers M, Maes M (2002) The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Inter J Neuropsychoparmacol 5(4):375–388
Domingo AK, Asmal L, Seedat S, Esterhuizen TM, Laurence C, Volmink J (2015) Investigating the association between diabetes mellitus, depression and psychological distress in a cohort of South African teachers. S Afr Med J 12:1057–1060
Remus JL, Stewart LT, Camp RM, Novak CM, Johnson JD (2015) Interaction of metabolic stress with chronic mild stress in altering brain cytokines and sucrose preference. Behav Neurosci 129:321–330
Ji BS, Cen J, Liu L, He L (2013) In vitro and in vivo study of dolichyl phosphate on the efflux activity of P-glycoprotein at the blood-brain barrier. Int J Dev Neurosci 31:828–835
Han P, Caselli RJ, Baxter L, Serrano G, Yin J, Beach TG, Reiman EM, Shi J (2015) Association of pituitary adenylate cyclase-activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol 3:333–339
Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang Y, Li W, Li W (2016) Chronic glucocorticoids exposure enhances neurodegeneration in the frontal cortex and hippocampus via NLRP-1 inflammasome activation in male mice. Brain Behav Immun 52:58–70
Abelaira, H.M., Réus, G.Z., Ignácio, Z.M., Dos Santos, M.A., de Moura, A.B., Matos, D., Demo, J.P., da Silva, J.B., Danielski, L.G., Petronilho, F., Carvalho, A.F., Quevedo, J. (2016) Ketamine exhibits different neuroanatomical profile after mammalian target of rapamycin inhibition in the prefrontal cortex: the role of inflammation and oxidative stress. Mol Neurobiol (In press).
Lackner RJ, Moore MT, Minerovic J, Fresco DM (2015) Explanatory flexibility and explanatory style in treatment-seeking clients with axis I psychopathology. Cognit Ther Res 6:736–743
Bethell KD, Koyama NF (2015) Happy hamsters? Enrichment induces positive judgement bias for mildly (but not truly) ambiguous cues to reward and punishment in Mesocricetus auratus. R Soc Open Sci 7:140399
Frisbee JC, Brooks SD, Stanley SC, d’Audiffret AC (2015) An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. J Vis Exp 2:106
Jayatissa MN, Henningsen K, West MJ, Wilborg O (2009) Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res 1290:133–141
Bourin M, Hascoet M (2003) The mouse light/dark box test. Eur J Pharmacol 463:55–65
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Avolio E, Alò R, Carelli A, Canonaco M (2011) Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav Brain Res 218:288–295
Alò R, Mele M, Fazzari G, Avolio E, Canonaco M (2015b) Exposure to sub-chronic unpredictable stress accounts for antidepressant-like effects in hamsters treated with BDNF and CNQX. Brain Res Bull 118:65–77
Alò R, Mele M, Avolio E, Fazzari G, Canonaco M (2015a) Distinct amygdalar AMPAergic/GABAergic mechanisms promote anxiolitic-like effects in an unpredictable stress model of the hamster. J Mol Neurosci 55:541–551
Alò R, Avolio E, Mele M, Storino F, Canonaco A, Carelli A, Canonaco M (2014) Excitatory/inhibitory equilibrium of the central amygdala nucleus gates anti-depressive and anxiolytic states in the hamster. Pharmacol Biochem Behav 118:79–86
Bueno A, De Olmos S, Heimer L, De Olmos J (2003) NMDA-antagonist MK-801- induced neuronal degeneration in Wistar rat brain detected by the amino-cupric-silver method. Exp Toxicol Pathol 54:319–334
Lasselin, J., Elsenbruch, S., Lekander, M., Axelsson, J., Karshikoff, B., Grigoleit, J.S., Engler, H., Schedlowski, M., Benson, S. (2016) Mood disturbance during experimental endotoxemia: predictors of state anxiety as a psychological component of sickness behavior. Brain Behav Imm [Epub ahead of print].
Relja B, Horstmann JP, Kontradowitz K, Jurida K, Schaible A, Neunaber C, Oppermann E, Marzi I (2015) Nlrp1 inflammasome is downregulated in trauma patients. J Mol Med 12:1391–1400
Gold SM, Irwin MR (2009) Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin N Am 2:309–320
Momeni M, Ghorban K, Dadmanesh M, Khodadadi H, Bidaki R, Kazemi Arababadi M, Kennedy D (2016) ASC provides a potential link between depression and inflammatory disorders: a clinical study of depressed Iranian medical students. Nord J Psychiatry 11:1–5
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. az Metab Brain Dis 1:27–53
Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, Thuret S, Pariante CM, Zunszain PA (2014) Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int J Neuropsychopharmacol 31:18
Wuwongse S, Chang RC, Law AC (2010) The putative neurodegenerative links between depression and Alzheimer’s disease. Prog Neurobiol 4:362–375
Murphy N, Grehan B, Lynch MA (2014) Glial uptake of amyloid beta induces NLRP3 inflammasome formation via cathepsin-dependent degradation of NLRP10. Neruomol Med 1:205–215
Hemmerle AM, Herman JP, Seroogy KB (2012) Stress, depression and Parkinson’s disease. Exp Neurol 1:79–86
Liu, C.S., Adibfar, A., Herrmann, N., Gallagher, D., Lanctôt, K.L. (2016) Evidence for inflammation-associated depression. Curr Top Behav Neurosci (In press).
Rossetti AC, Papp M, Gruca P, Paladini MS, Racagni G, Riva MA, Molteni R (2016) Stress-induced anhedonia is associated with activation of the inflammatory system in the rat brain: restorative effect of pharmacological intervention. Pharmacol Res 103:1–12
Daniele S, Da Pozzo E, Zappelli E, Martini C (2015) Trazodone treatment protects neuronal-like cells from inflammatory insult by inhibiting NF-κB, p38 and JNK. Cell Signal 27:1609–1629
Arora V, Chopra K (2013) Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J Affect Disord 3:1041–1052
Pan J, Lei X, Wang J, Huang S, Wang Y, Zhang Y, Chen W, Li D, Zheng J, Cui H, Liu Q (2015) Effects of Kaixinjieyu, a Chinese herbal medicine preparation, on neurovascular unit dysfunction in rats with vascular depression. BMC Complement Altern Med 15:291
Dowling NM, Johnson SC, Gleason CE, Jagust WJ (2015) The mediational effects of FDG hypometabolism on the association between cerebrospinal fluid biomarkers and neurocognitive function. NeuroImage 105:357–368
Tizabi, Y. (2015) Duality of antidepressants and neuroprotectants. Neurotox Res [Epub ahead of print].
Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA (2005) Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 1:21–26
Ozalay O, Calli C, Kitis O, Cagdas Eker M, Donat Eker O, Ozan E, Coburn K, Saffet Gonul A (2013) The relationship between the anterior corpus callosum size and prefrontal cortex volume in drug-free depressed patients. J Affect Disord 2:281–285
Hurley LL, Tizabi Y (2013) Neuroinflammation, neurodegeneration, and depression. Neurotox Res 2:131–144
Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Wang W, Wang YX, Jiang CL (2015) NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol 18:8
Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G (2010) Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res 12:3100–3104
Brown GC, Vilalta A (2015) How microglia kill neurons. Brain Res 1628:288–297
Zhu Y, Chen X, Liu Z, Peng YP, Qiu YH (2015) Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int J Mol Sci 28:17
Norden DM, Fenn AM, Dugan A, Godbout JP (2014) TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62:881–895
Koriauli S, Natsvlishvili N, Barbakadze T, Mikeladze D (2015) Knockdown of interleukin-10 induces the redistribution of sigma1-receptor and increases the glutamate-dependent NADPH-oxidase activity in mouse brain neurons. Biol Res 9:48–55
Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z, Liu D (2015) Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol 768:49–57
Lunnon K, Smith R, Hannon E, De Jager PL, Srivastava G, Volta M, Troakes C, Al-Sarraj S, Burrage J, Macdonald R, Condliffe D, Harries LW, Katsel P, Haroutunian V, Kaminsky Z, Joachim C, Powell J, Lovestone S, Bennett DA, Schalkwyk LC, Mill J (2014) Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat Neurosci 17:1164–1170
Homberg JR, Molteni R, Calabrese F, Riva MA (2014) The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci Biobehav 43:35–47
Pham, T.H., Mendez-David, I., Defaix, C., Guiard, B.P., Tritschler, L., David, D.J., Gardier, A.M. (2016) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacol. (In press).
Xue Q, Liu Y, Qi H, Ma Q, Xu L, Chen W, Chen G, Xu X (2013) A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int J Biol Sci 9:174–189
Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O (2004) Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol 19:89–95
Gibson SA, Korade Z, Shelton RC (2012) Oxidative stress and glutathione response in tissue cultures from persons with major depression. J Psychiatr Res 46:1326–1332
Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC, Tan L (2014) Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 42(4):1295–1310
Barnard KD, Skinner TC, Peveler R (2006) The prevalence of co-morbid depression in adults with type 1 diabetes: systematic literature review. Diabet Med 4:445–448
Chen C, Wang Y, Zhang J, Ma L, Gu J, Ho G (2014) Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis Model Mech 6:723–730
Hamamcioglu K, Reder AT (2007) Interferon-beta regulates cytokines and BDNF: greater effect in relapsing than in progressive multiple sclerosis. Mult Scler 4:459–470
Acknowledgments
We thank the Italian University Research Ministry (MIUR), Regione of Calabria (POR, FSE-2007/2013) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avolio, E., Fazzari, G., Mele, M. et al. Unpredictable Chronic Mild Stress Paradigm Established Effects of Pro- and Anti-inflammatory Cytokine on Neurodegeneration-Linked Depressive States in Hamsters with Brain Endothelial Damages. Mol Neurobiol 54, 6446–6458 (2017). https://doi.org/10.1007/s12035-016-0171-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0171-1