Abstract
Pain and anxiety have a complex relationship and pain is known to share neurobiological pathways and neurotransmitters with anxiety. Top-down modulatory pathways of pain have been shown to originate from cortical and subcortical regions, including the dorsolateral prefrontal cortex. In this study, a novel docosahexaenoic acid (DHA)-containing nutraceutical, Souvenaid, was administered to mice with infraorbital nerve ligation-induced neuropathic pain and behavioral responses recorded. Infraorbital nerve ligation resulted in increased face wash strokes of the face upon von Frey hair stimulation, indicating increased nociception. Part of this response involves general pain sensitization that is dependent on the CNS, since increased nociception was also found in the paws during the hot plate test. Mice receiving oral gavage of Souvenaid, a nutraceutical containing DHA; choline; and other cell membrane components, showed significantly reduced pain sensitization. The mechanism of Souvenaid’s activity involves supraspinal antinociception, originating in the prefrontal cortex, since inhibition of the DHA-metabolizing enzyme 15-lipoxygenase (Alox15) in the prefrontal cortex attenuated the antinociceptive effect of Souvenaid. Alox15 inhibition also modulated anxiety behavior associated with pain after infraorbital nerve ligation. The effects of Souvenaid components and Alox15 on reducing central sensitization of pain may be due to strengthening of a known supraspinal antinociceptive pathway from the prefrontal cortex to the periaqueductal gray. Together, results indicate the importance of the prefrontal cortex and DHA/Alox15 in central antinociceptive pathways and suggest that Souvenaid may be a novel therapeutic for neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain refers to a complex chronic pain where nerve fibers may be damaged, dysfunctional, or injured and therefore send improper signals to pain centers [1]. While nociceptive pain is evoked by stimulation of the sensory nerve endings in the tissue, neuropathic pain is caused by injury or disease of neurons in the peripheral or central nervous system (CNS) [2]. The development of neuropathic pain involves sensitization mechanisms. Peripheral sensitization refers to alterations in neuronal excitability restricted to the area of injury itself. After sensitization of pain fibers, normally non-painful stimuli can cause pain [2]. Central sensitization refers to the phenomenon where nociceptor inputs produce a prolonged and reversible increase in the excitability and synaptic efficiency of neurons in central nociceptive pathways, and involves alterations in neuronal signaling in the CNS [3] . The sensitivity of spinal cord neurons is heightened in a way that a neuron is activated by previously subthreshold inputs [2]. Heightened synaptic transmission in pain pathways in the spinal cord and the brain lead to a reduction in pain threshold and a subsequent augmentation of pain responses and spread of pain to non-injured areas [4]. A large brain network, the “pain matrix” is activated during the acute pain experience, as demonstrated by imaging techniques such as functional magnetic resonance imaging (fMRI). The most common areas that are activated during pain include the primary and secondary somatosensory cortices (S1 and S2); the insular, the anterior cingulate cortex and prefrontal cortex (PFC); and the thalamus, demonstrating that these areas are important in pain perception [5]. The descending pain modulatory system is an endogenous pain inhibitory system that originates from multiple cortical and subcortical areas—one of which is the dorsolateral PFC [6]. The PFC sends projections to the periaqueductal gray (PAG) in the midbrain and the rostral ventromedial medulla [7, 8]. In the PAG, ascending pain stimuli are integrated with descending influences from the diencephalon and the limbic forebrain to lead to an overall decrease in pain sensation [9]. The role of the PFC in descending pain modulation has been illustrated in numerous studies—human imaging studies with [11C]-carfentanil revealed that placebo analgesia was related to activation of μ-opioid receptors in the dorsolateral prefrontal cortex [10]. Functional and connectivity analyses also show increased activity in the prefrontal cortex when distraction is used to decrease pain perception, indicating a role for the PFC in descending antinociceptive pathways [11]. Pain is known to share neurobiological pathways and neurotransmitters with anxiety and depression [12, 13]. Pain, anxiety, and depression have a complex relationship, where people with chronic pain often experience anxiety [14, 15].
Previous studies in our lab show that iPLA2 is involved in the antinociceptive effect of tricyclic antidepressants such as nortriptyline and maprotilline. iPLA2 is an enzyme that catalyzes the release of DHA from the sn-2 position of glycerophospholipids in the brain [16]. DHA has been shown to have a role in pain and anxiety, where DHA-deficient diets correspond to decreased pain tolerance in diseases such as rheumatoid arthritis, inflammatory bowel disease, and neuropathy [17]. DHA deficiency leads to an increased incidence of anxiety disorders [18]. DHA inhibits the activation of the transcription factor nuclear factor κB and the release of central regulators of inflammatory pain such as the cytokines interleukin-1 beta (IL-1β) and tumor necrosis factor α [19, 20]. Knockdown of iPLA2 diminished the antinociceptive effect of the antidepressants in a facial carrageenan model of pain (unpublished) and also reduced the endogenous release of DHA in the brain [21]. DHA is critical for maintaining normal brain structure and function and has been shown to be neuroprotective [22]. The possible role of iPLA2 and DHA in pain and anxiety provides a rationale for the development of novel therapeutics aimed at the management of pain and anxiety in humans. Metabolism of PUFAs by lipoxygenases generates fatty acid hydroperoxy products. There are two isoforms of the 15-lipoxygenase enzyme, the type-1 (Alox15) leukocyte type and type-2 (Alox15B) epidermis type [23], both of which regulate the production of fatty acid hydroperoxides [24, 25]. Alox15 metabolizes DHA into 17S-hydroxy-DHA that is then converted to 7S-hydroperoxy,17S-hydroxy-DHA by a 5-lipoxygenase, and thence via epoxy intermediates to resolvin D1 (RvD1 or 7S,8R,17S-trihydroxy docosa-Z,9E,11E,13Z,15E,19Z-hexaenoic acid) and resolvin D2 (RvD2 or 7S,16R,17S trihydroxy-docosa-4Z,8E,10Z,12E,14E,19Z-hexaenoic acid) [26]. The Alox15 product 17S-hydroxy-DHA can also be converted to a 16(17)-epoxide and then to the 10,17-dihydroxy docosatriene termed neuroprotectin D1 [26]. Alox15 catalyzes the metabolism of DHA into docosanoids (resolvins, neuroprotectins, lipoxins, and maresins [27, 28]) that have anti-inflammatory, antiapoptotic, antioxidant, and neuroprotective properties [28]. Neuroprotectin D1, in particular, has been shown to protect against neuropathic pain after traumatic nerve injury in mice [29].
The present study was conducted to elucidate the relationship between pain and anxio-depression, and whether the novel nutraceutical Souvenaid that contains high levels of DHA can alleviate the symptoms of pain as well as pain-related mood disorders. A possible role of Alox15 in plasticity of the prefrontal cortex was also determined.
Methods and Materials
Immunohistochemistry
Six adult male C57BL mice weighing between 20 and 30 g each and about 6–8 weeks old were used in this portion of the study. Mice were deeply anesthetized and perfused through the left cardiac ventricle with a solution of 4 % paraformaldehyde and 0.1 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and sectioned coronally at 100 μm using a vibrating microtome. Sections were washed 30 times for 5 min each with phosphate-buffered saline (PBS) and incubated overnight with a mouse monoclonal antibody to Alox15 (Abcam, Cambridge, U.K.), diluted 1:50 in PBS. They were incubated for 1 h in a 1:200 dilution of biotinylated horse anti-mouse IgG (Vector, Burlingame, CA), reacted for 1 h with avidin-biotinylated horseradish peroxidase complex, and visualized by treatment for 20 min in 0.05 % 3,3-diaminobenzidine tetrahydrochloride solution in Tris-buffer containing 0.05 % hydrogen peroxide. Sections were mounted on glass slides, counterstained with methyl green, and visualized with a light microscope.
Souvenaid Treatment and Partial Infraorbital Nerve Ligation
All procedures involving mice were carried out in accordance to guidelines of the Institutional Animal Care and Use Committee of NUS. Male C57BL mice weighing between 20 and 30 g each and about 6–8 weeks old were used in this portion of the study. They were housed under defined conditions (room temperature 22 °C, relative humidity 65 %, lighting 12 h/day) with free access to food and water. Mice were given daily oral administrations of Souvenaid or the isocaloric control, full cream milk throughout the duration of the study. Souvenaid is a medical nutritional formulation that has been studied for its role in early Alzheimer’s disease [30–33] and contains a high amount of DHA. Souvenaid was developed by the Advanced Medical Nutrition division of Nutricia and contains a patented combination of nutrients, referred to under the trademark Fortasyn Connect™ (Table 1). The total dose of 0.05 ml/10 g body weight chosen for oral gavage was adapted from previous studies on humans [34]. The partial IONL procedure used in this study was adapted from a previous study [35, 36], which established the model and showed that pain induced remained consistent and significantly different from sham-operated animals for 25 days after surgery. The unilateral partial ligation to the right infraorbital nerve (ION) was performed under visual control using a Zeiss microscope (×10–25). Animals were anesthetized with ketamine/medetomidine. They were kept warm with a heating blanket. The mouse was fixed to a sterilized surgical board, after which the skin along the top of the snout was shaved and iodine-sterilized. A midline incision was created to expose the nasal and maxillary bone. All tools were autoclaved prior to surgery and ethanol sterilized for each animal. Blunt dissection with surgical scissors was used to expose the infraorbital part of the right ION, 1–2 mm rostral to infraorbital fissure on the maxillary bone. The ION was delicately isolated using fine forceps, ensuring no damage to facial nerve branches nearby. Approximately 1/2 the diameter of the nerve was tightly ligated with 6–0 silk suture (B. Braun) by passing the suture needle completely under the lateral aspect of the nerve and then up through the middle. The incision was closed using silk sutures (5–0) after confirming hemostasis. For the sham-operated mice, the ION was exposed on the right side using the same procedure, but the ION was not touched or ligated. The operated mice were able to eat and drink unaided soon after waking up.
Stereotaxic Injection
Mice were anesthetized by i.p. injection of ketamine/medetomidine cocktail and placed on a stereotaxic apparatus (Stoelting, USA). PD146176 Inhibitor (40 μM), dimethyl sulfoxide (DMSO) control, antisense oligonucleotide (200 μM), or sense oligonucleotide (200 μM) was stereotaxically injected into the left and right dorsolateral prefrontal cortex through small craniotomies on the skull (coordinates: 2.5 mm rostral to bregma, 1.5 mm lateral to midline, and 1.5 mm from surface of cortex). A total of 1 μl was injected over 5 min on each side, and the scalp sutured. The inhibitor PD146176 (Cayman Biochemicals, USA) was used to reversibly inhibit the Alox15 enzyme in the PFC. However, as we were unable to verify that PD146176 does not also exert an inhibitory effect on other enzymes, results with PD146176 were verified by antisense oligonucleotide knockdown of Alox15. The antisense oligonucleotide used was a 16-base oligonucleotide (5′-CACATGGTGATGAAGT-3′) which was shown in this study to effectively knock down Alox15 expression in the brain. Scrambled sense oligonucleotide was used as a control (5′-CACGTCTATACACCAC-3′). Both antisense and sense oligonucleotides contained phosphorothioate linkages to prevent nuclease degradation (IBA, Germany). Injections were carried out in a blinded manner to reduce experimenter bias as to the test compound. Solutions (200 μM) were prepared by dissolving lyophilized material in nuclease-free water.
Western Blot Analysis
At the end of behavior studies (see below), mice were deeply anesthetized with a ketamine/medetomidine cocktail and sacrificed by decapitation. The PFC was removed and snap frozen in liquid nitrogen. Tissues were homogenized using a Tissue Tearor ® (Biospec, ITS Science and Medical, Singapore) in ice-cold buffer (T-Per mammalian protein extraction kit, 1 mM EDTA and 0.25 mM DTT). Homogenates were centrifuged at 13,000 rpm for 20 min, and the supernatant was collected. Protein concentration in the supernatant was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, USA). Proteins were resolved in 15 % sodium dodecyl sulfate polyacrylamide gel (SDS–PAGE) under reducing conditions and subsequently electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech, Little Chalfont, U.K.). The molecular weights of the proteins were determined using a Bio-Rad Prestained Protein Ladder (Bio-Rad Laboratories). Non-specific binding sites on the PVDF membrane were blocked by incubation with 5 % non-fat milk in Tris buffered saline–Tween (TBST) for 1 h. The PVDF membrane was then incubated overnight with an affinity-purified mouse monoclonal antibody to Alox15 (Santa Cruz Biotechnology, Santa Cruz, USA, diluted 1:2000) in TBST with 5 % non-fat milk. The membrane was then washed in TBST and incubated with horseradish peroxidase-conjugated horse anti-mouse IgG (ThermoFisher Scientific, Waltham, U.S.A., diluted 1: 2000) for 1 h at room temperature. Immunoreactivity was visualized using an enhanced chemiluminescence kit (Pierce, Rockford, U.S.A.) according to the manufacturer’s instructions. Band intensities were quantified by densitometric analysis.
Pain Behavioral Assay
Mice were tested for baseline behavioral responses before IONL. Mice were tested once daily for the first 7 days, then once every 2 days until the 28-day time point or until the study was ceased. Testing was carried out individually in a deep rectangular plastic tank, sized (L × W × H) 60 × 40 × 25 cm [37]. A von Frey hair filament (Touch-Test Sensory Evaluator, North Coast Medical, Morgan Hill, USA) delivering 1 g of force (4.08 log units) was first reached into the tank for 5–10 min to ensure that the mice are familiarized with the reaching movements before testing. Mice were observed to ensure that they were able to move freely during this time. Test stimuli using the von Frey hair was administered when the mice are neither moving nor freezing and with all four paws placed on the ground while exhibiting sniffing behavior. The operated area of the face was probed 20 times with the von Frey hair to obtain a sufficient number of responses to reduce variability among individual animals in each group. Each new stimulus was applied at least 30 s after the previous stimulation. Directed facial grooming (a continuous series of facial wash strokes directed to the stimulated facial area by the mice) was used as an indicator of unilateral facial pain in freely moving mice. The number of immediate asymmetric facial grooming/scratching strokes was summed to calculate the total number of facial strokes exhibited by an animal after 20 stimulations using the von Frey hair filament. After each trial, all chambers were cleaned with a hypochlorous solution to prevent a bias based on olfactory cues. The observer was blind to experimental conditions.
Paw Withdrawal Test from a Hot Plate
The hot plate test is a test of the pain response in animals. Hot plate test was carried out on days 3, 12, 21, and 28 after IONL. The latency response to the thermal stimuli consists of licking of the hind paw or jumping. The surface of the hot plate was heated to a consistent temperature of 55 °C. The mouse was placed in the testing apparatus and the timer started. The latency to show a nociceptive response (hind paw lick, hind paw flick, or a jump) was measured with a foot-pedal timer. The mouse was immediately removed when a response was observed. If there is no response within 30 s, the test was terminated and the mouse removed from the hotplate. After each trial, all chambers were cleaned with a hypochlorous solution to prevent a bias based on olfactory cues. The observer was blind to experimental conditions.
Light/Dark Box
Light/dark box is a characteristic tool used in the assessment of anxiety [38] and is based on the innate aversion of rodents to brightly illuminated areas and on the spontaneous exploratory behavior of rodents in response to mild stressors, that is, novel environment and light. Light/dark box behavioral testing was carried out on days 3, 12, 21, and 28 after IONL. The apparatus used for the light/dark transition test consisted of an arena (40 × 40 × 40 cm) divided into two sections of equal size by a partition with an opening. All the home cages containing mice were transferred to the behavior testing room 30 min before the first trial begins for acclimatization. The “light” side of the chamber is brightly illuminated by white diodes (390 lx), whereas the other side is dark (2 lx). Mice were placed into the dark side and the camera tracking software started. Mice were allowed to move freely between the two chambers for 10 min, with time spent in each chamber tracked. After each trial, all chambers were cleaned with a hypochlorous solution to prevent a bias based on olfactory cues. The observer was blind to experimental conditions.
Elevated Zero Maze
The elevated zero maze is another commonly used paradigm to test anxiety in animals [13]. The test is based on the rodents’ innate aversion to open exposed spaces. Elevated zero maze behavioral testing was carried out on days 3, 12, 21, and 28 after IONL. The apparatus consisted of an elevated zero-shaped maze with two closed arms and two open arms. All the home cages containing mice were transferred to the behavior testing room 30 min before the first trial begins for acclimatization. Mice are placed in the open arm of the maze, and their behavior observed for 10 min, including head dips, stretch attempts, transitions from open to closed arms, and entries into open arms without transitions. After each trial, all chambers were cleaned with a hypochlorous solution to prevent a bias based on olfactory cues. The observer was blind to experimental conditions.
Forced Swim Test
The forced swim test (FST) is a test of behavioral despair and is centered on a rodent’s response to the threat of drowning, which has been interpreted in the literature as measuring susceptibility to depression. FST behavioral testing was carried out on days 3, 12, 21, and 28 after ION ligation. FSTs were carried out using a procedure modified from the original report by Porsolt [39]. The mice were individually placed into glass cylinders (25-cm height; 10-cm diameter) containing 10 cm of water maintained at 22–24 °C for a total of 6 min in a random order. Four mice at a time were videotaped from the side, and an opaque cardboard divider separated the cylinders so that the mice could not see each other during the trials. The predominant behavior which each mouse exhibited was recorded every 5 s during the last 4 min of the 6-min trial period, allowing 2 min of acclimatization. The behaviors included were immobility, climbing, and swimming. Swimming behavior is defined as movement (usually horizontal) throughout the cylinder that also includes crossing into another quadrant; climbing movement is defined as upward-directed movements of the forepaws along the side of the cylinder; immobility is defined as the absence of active, escape-oriented behaviors such as swimming, jumping, rearing, sniffing, or diving. After the trials, the mice were dried and placed in a cage surrounded by heat pad for 15–20 min. The observer was blind to the experimental conditions.
Results
Immunohistochemistry
Moderately dense immunolabeling for Alox15 was observed in the normal mouse prefrontal cortex. Label was mostly present in punctate profiles in the neuropil and occasional large-diameter dendrites but not in cell bodies (Fig. 1).
Western Blot Analysis
The Alox15 antibody detected a single 75-kDa band in the adult mouse brain (Fig. 2a), consistent with the predicted size of Alox15 protein. Analyses of the density of Alox15 bands normalized to β-actin showed a significant (P < 0.001) decrease in Alox15 protein levels in antisense-injected mice, indicating effective knockdown by Alox15 antisense oligonucleotide (Fig.2b).
Souvenaid Vs. Milk, after IONL
Animals in this portion of the study were either subjected to the IONL, or a sham surgery, and then administered with either Souvenaid or the isocaloric control milk.
Pain Behavior
There was an immediate increase in responses to stimulation with the von Frey filament from day 1 of testing after IONL for the ION-ligated groups, with no significant difference from baseline for the sham-operated groups (P < 0.001). Pain behavior was high and consistent until day 9, after which there was a gradual decline in asymmetrical grooming behavior (Fig. 3a). The milk IONL and milk sham groups showed a similar trend, whereas Souvenaid IONL mice exhibited significantly (P < 0.05) less pain responsiveness compared to the milk IONL group.
Paw Withdrawal Test from a Hot Plate
There was a significant difference in the paw withdrawal latency on a heat pad between the milk IONL and Souvenaid IONL groups on days 12 and 21 (P < 0.05) (Fig. 3b), where Souvenaid IONL mice exhibited significantly greater latency to withdrawal. Sham-operated groups had significantly greater latency to withdrawal than did IONL groups at all time points, except day 28.
Light/Dark Box
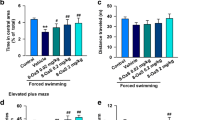
Souvenaid IONL mice spent significantly more time in the light side of the light/dark box than did milk IONL mice on days 12 and 21 after IONL surgery (P < 0.05), whereas sham-operated mice spent significantly more time in the light side of the light/dark box than did IONL-operated mice, regardless of treatment group (P < 0.05). No significant difference was observed between the Souvenaid IONL and milk IONL groups on days 3 and 28 after surgery. Only data from day 12 is shown (Fig. 4a).
Behavior experiments were conducted on day 12 after IONL or sham surgery, with administration of Souvenaid or milk. a Time spent in the light side of the light/dark box. b Number of stretch attempts exhibited in an elevated zero maze. c Number of head dips exhibited in an elevated zero maze. d Time spent immobile during forced swim test. e Time spent climbing during FST. f Time spent swimming during FST. Asterisks (*) indicate significant differences at P < 0.05
Elevated Zero Maze
Souvenaid IONL mice exhibited a trend toward more stretch attempts on the elevated zero maze than did milk IONL mice on days 12 and 21 after IONL surgery, whereas sham-operated mice exhibited more stretch attempts than did IONL-operated mice, regardless of treatment group. No significant difference was observed between Souvenaid IONL and milk IONL groups on day 28 after surgery. Only data from day 12 is shown (Fig. 4b). Mice showed a similar trend in exhibiting head dips on the maze (Fig. 4c).
Forced Swim Test
There were no significant differences in the swimming, climbing, or immobility behaviors exhibited by mice in all four groups on all days of testing. Only data from day 12 is shown (Fig. 4d–f).
Souvenaid Administration with Inhibition of PFC Alox15
Animals in this portion of the study were subjected to the IONL, and then administered with either Souvenaid or the isocaloric control. They underwent an intracortical injection of the inhibitor to Alox15 or vehicle (DMSO) control on day 11.
Pain Behavior
There was an immediate increase in responses to stimulations with the von Frey filament from day 1 of testing after IONL. Pain behavior was high and consistent until day 9, after which there was a gradual decline in asymmetrical grooming behavior (Fig. 5a). After Alox15 inhibitor injection, there was an immediate and significant increase in pain sensitivity observed in the Souvenaid/Alox15 inhibitor group, which was not observed in the Souvenaid/DMSO control group (P < 0.001).
a Pain behavior exhibited by mice after infraorbital nerve ligation, with administration of Souvenaid or the isocaloric control, milk, with intracortical injection of Alox15 inhibitor or vehicle control to the prefrontal cortex. Asterisks (*) indicate significant differences at P < 0.001. b Pain behavior exhibited by mice showing latency of withdrawal of paw on a hot plate. Asterisks (*) indicate significant differences at P < 0.05
Paw Withdrawal Test from a Hot Plate
Souvenaid/DMSO control mice exhibited significantly increased latency to paw withdrawal on the heat pad on day 12 after IONL surgery compared to milk/DMSO control, milk/Alox15 inhibitor, and Souvenaid/Alox15 inhibitor groups (P < 0.05) (Fig. 5b).
Light/Dark Box
Souvenaid/DMSO control mice spent significantly more time in the light side of the light/dark box on day 12 after IONL surgery compared to milk/DMSO control and milk/Alox15 inhibitor groups (P < 0.01) and a trend toward more time in the light side compared to the Souvenaid/Alox15 inhibitor group (P = 0.065) (Fig.6a).
Behavior experiments were conducted on day 12 after IONL, with administration of Souvenaid or the isocaloric control, milk, with intracortical injection of Alox15 inhibitor or vehicle control to the PFC. a Time spent in the light side of the light/dark box. b Number of stretch attempts exhibited in an elevated zero maze. c Number of head dips exhibited in an elevated zero maze. d Time spent immobile during FST. e Time spent climbing during FST. f Time spent swimming during FST. Asterisks (*) indicate significant differences at P < 0.05
Elevated zero Maze
Souvenaid/DMSO control mice exhibited a trend toward more stretch attempts and significantly more head dips on day 12 after IONL surgery compared to milk/DMSO control, milk/Alox15 inhibitor, and Souvenaid/Alox15 inhibitor groups (P < 0.001) (Fig. 6b, c).
Forced Swim Test
There were no significant differences in the swimming, climbing, or immobility behaviors exhibited by mice in all four groups on day 12 after IONL surgery (Fig. 6d–f).
Souvenaid Administration with Antisense Knockdown of PFC Alox15
Animals in this portion of the study were subjected to the IONL, and then administered with either Souvenaid or the isocaloric control. They underwent an intracortical injection of the Alox15 antisense or scrambled sense control on day 8 and were tested for behavioral differences on day 12.
Pain Behavior
There was an immediate increase in responses to stimulations with the von Frey filament from day 1 of testing after IONL. Pain behavior was high and consistent until day 9, after which there was a gradual decline in asymmetrical grooming behavior (Fig. 7a). After Alox15 antisense injection, there was a significant increase in pain sensitivity in the Souvenaid/antisense group, which was not observed in the Souvenaid/sense control group or the milk/antisense group (P < 0.001).
a Pain behavior exhibited by mice after IONL, with administration of Souvenaid or the isocaloric control, milk, with intracortical injection of Alox15 antisense or scrambled sense control to the PFC. Asterisks (*) indicate significant differences at P < 0.001. b Pain behavior exhibited by mice showing latency of withdrawal of paw on a hot plate. Asterisks (*) indicate significant differences at P < 0.001
Paw Withdrawal Test from a Hot Plate
Souvenaid/sense mice exhibited significantly increased latency to paw withdrawal on the heat pad on day 12 after IONL surgery compared to milk/sense, milk/antisense, and Souvenaid/antisense groups (P < 0.01) (Fig. 7b).
Light/Dark Box
Souvenaid/sense mice spent significantly more time in the light side of the light/dark box on day 12 after IONL surgery compared to milk/sense, milk/antisense, and Souvenaid/antisense groups (P < 0.01) (Fig. 8a).
Behavior experiments were conducted on day 12 after IONL, with administration of Souvenaid or the isocaloric control, milk, with intracortical injection of Alox15 antisense or scrambled sense control to the PFC. a Time spent in the light side of the light/dark box. b Number of stretch attempts exhibited in an elevated zero maze. c Number of head dips exhibited in an elevated zero maze. d Time spent immobile during FST. e Time spent climbing during FST. f Time spent swimming during FST. Asterisks (*) indicate significant differences at P < 0.05
Elevated zero Maze
Souvenaid/sense mice exhibited significantly more stretch attempts (P < 0.05) and head dips (P < 0.001) on day 12 after IONL surgery compared to milk/sense, milk/antisense, and Souvenaid/antisense groups (Fig. 8b, c).
Forced Swim Test
There were no significant differences in the swimming, climbing, or immobility behaviors exhibited by mice in all four groups on day 12 after IONL surgery (Fig. 8d–f).
Discussion
The present study was conducted to elucidate the relationship between pain and anxio-depression and whether Souvenaid, which contains high levels of DHA, can alleviate the symptoms of pain as well as pain-related mood disorders. The results of this experiment show that IONL induces long-lasting orofacial neuropathic pain. Interestingly, animals that underwent the IONL procedure showed an increased sensitivity to the hot plate, indicating that a degree of central sensitization took place. Central sensitization refers to a mechanism in neuropathic pain where spinothalamic track neurons develop increased background activity, enlarged receptive fields, and increased responses to afferent impulses, including normally innocuous stimuli [4]. The spread of sensitivity to non-injured areas such as the high paw is indicative of the fact that there were changes to somatosensory neurons in the dorsal horn of the spinal cord following peripheral stimulation via IONL, causing changes in synaptic transmission in the central pain pathways. Animals that underwent the IONL procedure also showed increased anxiety in the light/dark box and elevated zero maze assays but did not exhibit increased signs of depression. The relationship between chronic pain and anxiety has been well documented in the literature, where pain increases the potential for anxiety and anxiety may alter the perception of pain. Pain is more than simply a sensation (nociception) and is instead a complex perceptual experience combining a sensory element with psychological and social influences [40, 41]. Studies show that patients with chronic pain conditions such as fibromyalgia are four to five times more likely to have had a lifetime diagnosis of anxiety disorders such as obsessive-compulsive disorder, generalized anxiety disorder, or post-traumatic stress disorder [42]. Results of this study are consistent with the literature in demonstrating that mice that are experiencing neuropathic orofacial pain show increased anxiety, whereas sham-operated mice did not exhibit symptoms of anxiety.
We next investigated whether Souvenaid, containing high levels of DHA, could be used as a novel nutraceutical analgesic. This is in view of previous studies that have shown prefrontal cortical iPLA2 and DHA to have a role in antinociception. The result of this experiment shows that Souvenaid effectively decreases neuropathic pain in an orofacial pain model in mice, as we can see from Fig. 3a where, after Souvenaid administration, mice showed decreased behavioral response to probing with a von Frey filament. Souvenaid administration was also shown to increase the tolerance of IONL mice to the hot plate, indicating that the mechanism of action of Souvenaid is within the CNS, either via a descending inhibitory pathway from the prefrontal cortex or by diminishing sensitization of ascending spinothalamic track neurons. Supraspinal antinociception refers to a top-down pain modulatory pathway that originates in higher brain regions such as the hypothalamus, the amygdala, and the prefrontal cortex [10, 43]. These regions send projections to the midbrain periaqueductal gray region, which then projects to the medulla. Neurons within the nucleus raphe magnus and nucleus reticularis gigantocellularis within the rostral ventromedial medulla have been shown to project to the spinal or medullary dorsal horns to directly or indirectly enhance or reduce nociceptive traffic, changing the perception of pain [44]. Souvenaid also has a significant effect on pain-related anxiety, and animals that received Souvenaid treatment exhibited less signs of anxiety in the light/dark box as well as the elevated zero maze (Fig. 4). The IONL procedure did not appear to induce depression in mice, nor did Souvenaid administration alter the depression-like response of mice in the forced swim test. The ability of Souvenaid, which contains a high amount of DHA (1200 mg/125 ml), to alleviate pain and anxiety alludes to the possibility that DHA is an important mediator involved in the relief of pain, which has been richly substantiated in the literature. DHA has been shown to have antinociceptive effects in mice, where exogenous dietary administration of DHA has been found to dose-dependently exert antinociceptive effects against thermal and chemical stimulation compared to control [45], and both basic and clinical studies have shown that a dietary intake of DHA results in the reduction of pain associated with disorders such as rheumatoid arthritis, dysmenorrhea, inflammatory bowel disease, and neuropathy [17]. Apart from its role in pain, DHA has also been shown to affect stress responses in rats, where rats that were fed a DHA-deficient diet from birth showed increased psychological stress and conditioned-fear stress in anxiety tasks [46], and a decreased dietary intake of fish and polyunsaturated fatty acids are shown to correlate with an increased incidence of anxiety disorders [18]. Fortasyn™ connect formulation used in Souvenaid has shown efficacy in the treatment of behavioral disturbances and social cognition skills [47], and this study is novel in having shown a link to pain/central sensitization and anxiety.
This study provides new insight into the possible mechanisms that underlie the efficacy of Souvenaid. Since Souvenaid is enriched in DHA which is an anti-inflammatory fatty acid, one possibility is that it could act an anti-inflammatory agent in the periphery to reduce pain after IONL. However, a central sensitization effect was also observed where mice exhibited decreased pain tolerance in the hindlimb during the heat pad test, after infraorbital nerve ligation in the face, indicating decreased pain tolerance at a location away from the site of injury. Souvenaid was shown to improve the tolerance of mice to the heat pad, and interestingly, Alox15 inhibition/knockdown in the prefrontal cortex abolished this antinociceptive effect, confirming that part of the antinociceptive effect of Souvenaid is via activation of central antinociceptive pathways originating from the prefrontal cortex. Together, results indicate the ability of DHA to act via a central mechanism to alter plasticity in the central pain pathways to affect antinociception, and this effect is dependent on Alox15 to metabolize DHA into its products such as resolvins, neuroprotectins, maresins, and lipoxins. It is postulated that these biomolecules may facilitate the descending pain inhibitory pathway originating from the PFC.
In conclusion, results of this study indicate that it may be possible to use novel pharmaceuticals, or nutraceuticals such as Souvenaid that have less of a direct effect on neurotransmitters in the brain compared to antidepressants, to treat neuropathic pain and associated anxiety. This is beneficial as it would lead to fewer side effects and therefore greater compliance, as one of the major issues facing mental healthcare professionals is the lack of compliance of patients with their drugs due to side effects and discomfort faced.
Abbreviations
- AA:
-
Arachidonic acid
- AD:
-
Alzheimer ’s disease
- Alox15:
-
Arachidonate 15-lipoxygenase
- Alox15B:
-
Epidermis type 15-lipoxygenase
- CNS:
-
Central nervous system
- DHA:
-
Docosahexanoic acid
- DMSO:
-
Dimethyl sulfoxide
- fMRI:
-
Functional magnetic resonance imaging
- FST:
-
Forced swim test
- HFS:
-
High frequency stimulation
- ION:
-
Infraorbital nerve
- IONL:
-
Infraorbital nerve ligation
- IL-1β:
-
Interleukin-1 beta
- iPLA2 :
-
Calcium independent Phospholipase A2
- NPD1:
-
Neuroprotectin D1
- PAG:
-
Periaqueductal gray
- PFC:
-
Prefrontal cortex
- PLA2 :
-
Phospholipase A2
- PUFA:
-
Polyunsaturated Fatty Acid
- PVDF:
-
Polyvinylidene difluoride
- RvD1:
-
Resolvin D1
- RvD2:
-
Resolvin D2
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel
- TBST:
-
Tris buffered saline–Tween
References
Koltzenburg M, Scadding J (2001) Neuropathic pain. Curr Opin Neurol 14(5):641–647
Schaible H-G (2006) Peripheral and central mechanisms of pain generation. In: Analgesia. Springer, pp 3–28
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–15. doi:10.1016/j.pain.2010.09.030
Ji RR, Kohno T, Moore KA, Woolf CJ (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26(12):696–705. doi:10.1016/j.tins.2003.09.017
Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011) The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93(1):111–124. doi:10.1016/j.pneurobio.2010.10.005
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain: A Journal of Neurology 126(Pt 5):1079–1091
Bingel U, Tracey I (2008) Imaging CNS modulation of pain in humans. Physiology 23:371–380. doi:10.1152/physiol.00024.2008
Bingel U, Schoell E, Buchel C (2007) Imaging pain modulation in health and disease. Curr Opin Neurol 20(4):424–431. doi:10.1097/WCO.0b013e328259c34d
Almeida TF, Roizenblatt S, Tufik S (2004) Afferent pain pathways: a neuroanatomical review. Brain Res 1000(1–2):40–56. doi:10.1016/j.brainres.2003.10.073
Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS (2005) Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(34):7754–7762. doi:10.1523/JNEUROSCI.0439-05.2005
Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR (2004) Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain 109(3):399–408. doi:10.1016/j.pain.2004.02.033
Delgado PL (2004) Common pathways of depression and pain. The Journal of clinical psychiatry 65(Suppl 12):16–19
Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W (2009) Depression and pain. Front Biosci (Landmark Ed) 14:5031–5051
Katon W, Lin EH, Kroenke K (2007) The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 29(2):147–155. doi:10.1016/j.genhosppsych.2006.11.005
McCracken LM, Spertus IL, Janeck AS, Sinclair D, Wetzel FT (1999) Behavioral dimensions of adjustment in persons with chronic pain: pain-related anxiety and acceptance. Pain 80(1–2):283–289
Fujita S, Ikegaya Y, Nishikawa M, Nishiyama N, Matsuki N (2001) Docosahexaenoic acid improves long-term potentiation attenuated by phospholipase A(2) inhibitor in rat hippocampal slices. Br J Pharmacol 132(7):1417–1422. doi:10.1038/sj.bjp.0703970
Tokuyama S, Nakamoto K (2011) Unsaturated fatty acids and pain. Biol Pharm Bull 34(8):1174–1178
Jacka FN, Pasco JA, Williams LJ, Meyer BJ, Digger R, Berk M (2013) Dietary intake of fish and PUFA, and clinical depressive and anxiety disorders in women. Br J Nutr 109(11):2059–2066. doi:10.1017/S0007114512004102
Calder PC (2006) N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83(6 Suppl):1505S–1519S
Goldberg RJ, Katz J (2007) A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 129(1–2):210–223. doi:10.1016/j.pain.2007.01.020
Lee LH, Tan CH, Shui G, Wenk MR, Ong WY (2012) Role of prefrontal cortical calcium independent phospholipase A(2) in antidepressant-like effect of maprotiline. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 15(8):1087–1098. doi:10.1017/S1461145711001234
Rapoport SI, Ramadan E, Basselin M (2011) Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins & other lipid mediators 96(1–4):109–113. doi:10.1016/j.prostaglandins.2011.06.003
Jiang WG, Watkins G, Douglas-Jones A, Mansel RE (2006) Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot Essent Fat Acids 74(4):235–245. doi:10.1016/j.plefa.2006.01.009
Sigal E, Craik CS, Highland E, Grunberger D, Costello LL, Dixon RA, Nadel JA (1988) Molecular cloning and primary structure of human 15-lipoxygenase. Biochem Biophys Res Commun 157(2):457–464
Brash AR, Boeglin WE, Chang MS (1997) Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A 94(12):6148–6152
Kohli P, Levy BD (2009) Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 158(4):960–971. doi:10.1111/j.1476-5381.2009.00290.x
Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 1851(4):397–413. doi:10.1016/j.bbalip.2014.08.006
Dobson EP, Barrow CJ, Kralovec JA, Adcock JL (2013) Controlled formation of mono- and dihydroxy-resolvins from EPA and DHA using soybean 15-lipoxygenase. J Lipid Res 54(5):1439–1447. doi:10.1194/jlr.M036186
Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, Ji RR (2013) Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol 74(3):490–495. doi:10.1002/ana.23928
Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikkert MG, Wurtman RJ, Wilkinson D, Twisk JW, Kurz A (2010) Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association 6(1):1–10 e11. doi:10.1016/j.jalz.2009.10.003
Rijpma A, Meulenbroek O, van Hees AM, Sijben JW, Vellas B, Shah RC, Bennett DA, Scheltens P, Olde Rikkert MG (2015) Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther 7(1):51. doi:10.1186/s13195-015-0134-1
Ritchie CW, Bajwa J, Coleman G, Hope K, Jones RW, Lawton M, Marven M, Passmore P (2014) Souvenaid(R): a new approach to management of early Alzheimer’s disease. J Nutr Health Aging 18(3):291–299. doi:10.1007/s12603-013-0411-2
Shah RC, Kamphuis PJ, Leurgans S, Swinkels SH, Sadowsky CH, Bongers A, Rappaport SA, Quinn JF, Wieggers RL, Scheltens P, Bennett DA (2013) The S-connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther 5(6):59. doi:10.1186/alzrt224
Scheltens P, Twisk JW, Blesa R, Scarpini E, von Arnim CA, Bongers A, Harrison J, Swinkels SH, Stam CJ, de Waal H, Wurtman RJ, Wieggers RL, Vellas B, Kamphuis PJ (2012) Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. Journal of Alzheimer’s disease : JAD 31(1):225–236. doi:10.3233/JAD-2012-121189
Dominguez CA, Kouya PF, Wu WP, Hao JX, Xu XJ, Wiesenfeld-Hallin Z (2009) Sex differences in the development of localized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neuropathic pain. Gender medicine 6(Suppl 2):225–234. doi:10.1016/j.genm.2009.01.003
Xu M, Aita M, Chavkin C (2008) Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. The journal of pain : official journal of the American Pain Society 9(11):1036–1048. doi:10.1016/j.jpain.2008.06.006
Vos BP, Strassman AM, Maciewicz RJ (1994) Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience 14(5 Pt 1):2708–2723
Bourin M, Hascoet M (2003) The mouse light/dark box test. Eur J Pharmacol 463(1–3):55–65
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie 229(2):327–336
Asmundson GJ, Wright KD, Stein MB (2004) Pain and PTSD symptoms in female veterans. Eur J Pain 8(4):345–350. doi:10.1016/j.ejpain.2003.10.008
Melzack R, Katz J (2004) The gate control theory: reaching for the brain. Pain: Psychological perspectives:13–34
Raphael KG, Janal MN, Nayak S, Schwartz JE, Gallagher RM (2006) Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain 124(1–2):117–125. doi:10.1016/j.pain.2006.04.004
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia—imaging a shared neuronal network. Science 295(5560):1737–1740. doi:10.1126/science.1067176
Willis WD Jr (1985) Central nervous system mechanisms for pain modulation. Applied neurophysiology 48(1–6):153–165
Nakamoto K, Nishinaka T, Mankura M, Fujita-Hamabe W, Tokuyama S (2010) Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biol Pharm Bull 33(6):1070–1072
Takeuchi T, Iwanaga M, Harada E (2003) Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res 964(1):136–143
Pardini M, Serrati C, Guida S, Mattei C, Abate L, Massucco D, Sassos D, Amore M, Krueger F, Cocito L, Emberti Gialloreti L (2015) Souvenaid reduces behavioral deficits and improves social cognition skills in frontotemporal dementia: a proof-of-concept study. Neurodegener Dis 15(1):58–62. doi:10.1159/000369811
Acknowledgments
This work was supported by grants from the National Medical Research Council and the National University Health System of Singapore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shalini, SM., Herr, D.R. & Ong, WY. The Analgesic and Anxiolytic Effect of Souvenaid, a Novel Nutraceutical, Is Mediated by Alox15 Activity in the Prefrontal Cortex. Mol Neurobiol 54, 6032–6045 (2017). https://doi.org/10.1007/s12035-016-0138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0138-2