Abstract
Parkinson’s disease (PD) having a complex and multi-factorial neuropathology includes mainly the degeneration of the dopaminergic nigrostriatal pathway, which is a cumulative effect of depleted endogenous antioxidant enzymes, increased oxidative DNA damage, mitochondrial dysfunction, excitotoxicity, and neuroinflammation. The present study was designed to investigate the neuroprotective effect of a potent antioxidant from Urtica dioica in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of parkinsonism. MPTP was administered intranigrally for the induction of PD in male Wistar rats. Behavioral alterations were assessed in between the study period. Animals were sacrificed immediately after behavioral session, and different biochemical, cellular, and neurochemical parameters were measured. Intranigrally repeated administration of MPTP showed significant impairment of motor co-ordination and marked increase of mito-oxidative damage and neuroinflammation in rats. Intranigral MPTP significantly decreases the dopamine and its metabolites with impairment of dopaminergic cell density in rat brain. However, post-treatment with the potent antioxidant fraction of Urtica dioica Linn. (UD) (20, 40, 80 mg/kg) improved the motor function, mito-oxidative defense alteration significantly and dose dependently in MPTP-treated rats. In addition, the potent antioxidant fraction of UD attenuated the pro-inflammatory cytokines (TNF-α and IL-β) and restored the level of dopamine and its metabolites in MPTP-induced PD in rats. Moreover, minocycline (30 mg/kg) with lower dose of UD (20 mg/kg) had significantly potentiated the protective effect of minocycline as compared to its effect with other individual drug-treated groups. In conclusion, Urtica dioica protected the dopaminergic neurons probably by reducing mito-oxidative damage, neuroinflammation, and cellular alteration along with enhanced neurotrophic potential. The above results revealed that the antioxidant rich fraction of UD contain flavonoids and phenolic compounds, which have a promising approach in therapeutics of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc) along with symptoms of muscle rigidity, tremor, bradykinesia (slowing of movement), postural instability, and cognitive problems. Current evidence supports the role for mitochondrial dysfunction, oxidative stress, neuroinflammation, and abnormal protein accumulation as early triggers of neuronal death in PD pathogenesis [1]. SNpc dopaminergic cells have high levels of basal oxidative stress compared to other dopaminergic neuronal populations [2]. A diminished activity of the mitochondrial enzymes in the SNpc of PD patients suggested that oxidative stress is linked to mitochondrial dysfunction and neuroinflammation which promotes α-synuclein oligomerization and aggregation [3]. It also states that pro-inflammatory cytokines are triggered by oxidative stress which amplifies the process of inflammation and apoptotic program [4]. The accumulation of α-synuclein is a central step in the pathogenesis of PD and a key constituent of intraneuronal proteinaceous inclusions known as Lewy bodies. The accumulation of α-synuclein has also been suggested to trigger mitochondrial oxidative stress and alterations in Ca2+ permeability [5].
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is the gold standard toxin-based animal model of PD which replicates almost all of the pathological hallmarks of human PD [6]. The pathological and systemic symptoms of PD involve severe motor deficits like bradykinesia, rigidity, resting tremors, and postural instability along with oxidative stress, mitochondrial dysfunction, excitotoxicity, and neuroinflammation [7]. Thus, the degeneration of dopaminergic neurons could be led either by necrosis or apoptosis. Given these characteristics, the MPTP-lesioned experimental model of PD is an excellent system for assessing the therapeutic potential of drugs for the treatment of PD.
Minocycline is a clinically available antibiotic and anti-inflammatory drug that also demonstrates neuroprotective properties in a variety of experimental models of neurological disorders. It has been found that minocycline produced its action in the central nervous system by suppressing the microglial activation and reducing the pro-inflammatory cytokine release in different in vivo as well as in vitro studies [8]. Many investigators reported its anti-inflammatory, antiapoptotic, and antioxidant properties [9, 10]. The main biological effects of minocycline, which are involved in the pathogenesis of several neurological disorders, include inhibition of microglial activation, attenuation of apoptosis, and suppression of reactive oxygen species (ROS) production [8].
In the theory of traditional medicine, Urtica dioica Linn. is widely used as antioxidant, anti-inflammatory, anticancer, and immunomodulatory as well as in various other diseases [11–13]. Recently, it has been found that Urtica dioica Linn. has neuroprotective features including antioxidant and anti-inflammatory properties [14–16]. The compounds which are reported from the plant are beta-sitosterol, trans-ferulic acid, dotriacontane, erucic acid, ursolic acid, scopoletin, rutin, quercetin, and p-hydroxylbenzalcohol which support its antioxidant property [17]. However, its property has not yet been evaluated against any animal models of PD. Keeping in mind the therapeutic potential of both the drugs and oxidative damage as main target, the present study has been further extended to explore the mechanism and its combination of Urtica dioica Linn. (UD) and minocycline against MPTP-induced behavioral, cellular, and neurochemical alterations.
Materials and Methods
Plant Material
The plant was collected from the local area of Ranikhet (Uttarakhand) in the month of August and authenticated by Dr. H.B. Singh from the Department of Raw Material Herbarium and Museum, National Institute of Science Communication and Information Resources, New Delhi Ref. NISCAIR/RHMD/CONSULT/2013/2313/93, October 3, 2013.
Extraction and Fractionation of Plant Material
The ground plant powder (500 gm) was extracted by hot extraction process using Soxhlet apparatus with (80 % v/v) hydroalcohol. The extract was concentrated under reduced pressure to obtain a green semi-solid residue. The 80 % hydroalcoholic extract was suspended in 500 ml of distilled water and sequentially partitioned by different solvents, viz., petroleum ether, ethyl acetate, and n-butanol, in increasing order of polarity. The various fractions obtained were concentrated under vacuum, and percentage yield was calculated.
In Vitro Free Radical Scavenging Activity
DPPH Radical Scavenging Activity
The antioxidant activity of UD whole plant extract and its fractions (petroleum ether fraction (PEF), ethyl acetate fraction (EAF), n-butanol fraction (NBF), and aqueous fraction (AF)) were assessed by determining its ability to scavenge DPPH radical. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) is a stable free radical [18]. The 0.1-mM solution of DPPH in methanol was prepared. Then, 1 ml of this solution was added to 2 ml of test drug solution at different concentrations (50–250 μg/ml). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then, the absorbance was measured at 517 nm. Ascorbic acid was used as standard.
The percentage of scavenging activity was determined using the following formula:
where A control is the absorbance of DPPH and A sample is the absorbance of DPPH with the test sample.
Nitric Oxide Scavenging Activity
Nitric oxide radical scavenging activity was performed according to the method of Garrat [19]. Nitric oxide radical is generated from a reaction mixture containing sodium nitroprusside (20 mM) in phosphate-buffered saline (pH 7.4) when incubated at 25 °C for 30 min. The nitric oxide radical thus generated interacts with oxygen to produce nitrite ion, which is assayed by mixing with an equal amount of Griess reagent (1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphthylethylenediamine dihydrochloride in water), and its absorbance was measured at 570 nm. Decrease in absorbance in the presence of different concentrations of the test sample (50–250 μg/ml) indicated the nitric oxide scavenging activity. Ascorbic acid was used as standard. The percentage of nitric oxide scavenging activity was determined using the following formula.
where A control is the absorbance of reaction mixture without the test sample; A sample is the absorbance of reaction mixture with various concentrations of sample.
Animals
Young male Wistar rats (180–250 g) procured from Central Animal House, ISF College of Pharmacy, Moga, India, were used in the study. Animals were acclimatized to laboratory conditions at room temperature prior to experimentation. Following surgery, animals were kept under standard conditions of a 12-h light/dark cycle with food (Ashirwad Industries, Mohali, India) and water ad libitum in groups of two in plastic cages with soft bedding. All the experiments were carried out between 09:00 and 15:00 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) bearing the registration no. IAEC/CPCSEA/M9/2013/P167 and carried out in accordance with the guidelines of the committee for the purpose of Control and Supervision of Experimentats on Animals (CPCSEA), Government of India, on animal experimentation.
Intranigral Administration of MPTP
Brain surgery was performed by using a stereotactic apparatus as per the previously described protocol [20]. Animals were anaesthetized with ketamine (100 mg/kg, i.p) followed by diazepam (5 mg/kg, i.p.) administration and then positioned in the frame of a stereotaxic apparatus. A midline sagittal incision was made in the scalp to expose bregma. MPTP (1 μmol in 2 μl of saline) was infused bilaterally through a cannula implanted 2.0 mm above the SNpc through a Hamilton syringe with 30-gauge stainless needle at a rate of 0.7 μl/min at a site with the following coordinates adapted from the rat brain atlas: anterio-posterior 5.0 mm, middle-lateral 2.0 mm, and ventral depth 7.8 mm from the bregma, midline, and skull surface, respectively [21]. The syringe was allowed to stay in position for the next 2 min to prevent back diffusion of the drug. Sham operations were subjected to the same procedure, but were infused with 2 μl of sterile saline 0.9 % instead of MPTP. Immediately after surgery, all rats were administered gentamicin (0.1 ml, 10,000 IU) i.p. to prevent sepsis formation. Special care of the animals was taken during the post-operative period.
Drugs and Treatment Schedule
The following drugs were used: MPTP (Sigma Chemicals Co., St. Louis, Mo, USA) was dissolved in normal saline and administered by intranigral injection on day 1, 7, and 14 at a 1 μmol/2 μl dose. Urtica dioica was dissolved in sterile water for injection and administered intragastrically (i.g.) at a dose body weight of rats for 14 days. Minocycline (30 mg/kg; Ranbaxy Ltd., Gurgaon, India) and selegiline (10 mg/kg; Intas Pharmaceuticals) were suspended in 0.5 % w/v sodium carboxymethyl cellulose (CMC) and administered in a dose of 0.5 ml/100 g body weight at a dose body weight of rats per oral for 14 days after 14 days of MPTP administration.
Experimental Procedure
Brain surgery was performed using a stereotactic apparatus. Cannulas were implanted bilaterally 2 mm above the SNpc. The animals were divided into seven groups (n = 10). Group 1 served as vehicle control for MPTP and received 2 μl saline bilaterally on days 1, 7, and 14. Group 2 received MPTP (1 μM/2 μl) on days 1, 7, and 14. Groups 3, 4, and 5 received EAF of UD (20, 40, and 80 mg/kg, p.o.) for 14 days (15–28 days). Groups 6 and 7 received EAF of UD (20 mg/kg + minocycline (30 mg/kg) and selegiline (10 mg/kg)) for 14 days. The motor activities were assessed using a rotarod, a grip strength meter, and a narrow beam walk test during the study.
Experimental Protocol and Procedure
Animals were randomly divided into seven groups of 10 animals in each group. MPTP was repeatedly administered on days 1, 7, and 14 intranigrally in all groups except in the vehicle-treated group. Different behavioral performances and biochemical analyses have been carried out as per scheme in Fig. 1.
Measurement of Body Weight
Animal body weight was recorded on the first and last days of experimentation. Percent change in body weight was calculated as
Behavioral Assessment
Rotarod Activity
The rotarod was commercially available from Medicraft INCO, Ambala, Haryana, India, consisting of two, three, or four compartments of 75 mm width each with a rotating rod of 25 mm diameter having speeds of 5, 10, 15, 20, and 25 rpm with time interval counter in each compartments. The rats were exposed to a prior training session to acclimatize them to rotarod performance. Rats were placed individually on a rotating rod with a diameter of 7 cm (speed 25 rpm). The cutoff time was 180 s [22].
Gait Abnormalities
The apparatus consists of 50-cm wooden strips supported by two pedestals at each end, with height of 100 cm above the ground. The rats have to traverse a narrow beam which is suspended between a start platform and their home cage. It is important to make sure that the entire apparatus is placed at a height of at least 100 cm above the ground, so as to make sure that the animal fears the height and really attempts to reach the goal box. All rats must be trained to walk over a beam for 5 days before testing. A ceiling of 120 s is employed at the end of which the rat is removed and placed in the cage by hand and receives, if it is the case, a score of 120 s. The test procedure is identical for all rats tested and performed in the same environment preferably in the morning. Time taken to traverse the rat from the start platform to their home cage was measured along with their number of slipping errors [23].
Grip Strength
The grip strength test is a widely used non-invasive method designed to evaluate mouse limb strength. It has been used to investigate the effects of neuromuscular disorders and drugs. It is based on the natural tendency of the mouse to grasp a bar or grid when it is suspended by the tail. During this test, the mouse grips with either both forelimbs and hind limbs to a single bar or a mesh. Metering is performed with precision force gauges in such a manner as to retain the peak force applied on a digital display. The values may be either recorded manually or automatically via an optional RS-232 interface available on the computerized model(s) [24].
Biochemical Assessments
The animals were sacrificed under anesthesia, and brains were removed and rinsed with ice-cold isotonic saline after the last behavioral test. Brains were then kept on an ice tray, and the striatum and cortex areas were separated and weighed. Ten percent (w/v) tissue homogenates were prepared in 0.1-M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000×g for 15 min, and aliquots of the supernatants were separated and used for biochemical estimation.
Measurement of Lipid per Oxidation
The extent of lipid per oxidation in the striatum and cortex was determined quantitatively by performing the method as described by Wills [25]. The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid at 532 nm using a Shimadzu spectrophotometer (Japan). The values were calculated using the molar extinction coefficient of a chromophore (1.56 × 105 M−1 cm−1) and expressed as percentage of control.
Estimation of Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide, was determined by a colorimetric assay with Griess reagent (0.1 % N-(1-napththyl)ethylenediamine dihydrochloride, 1 % sulphanilamide, and 5 % phosphoric acid). Equal volumes of the supernatant and the Griess reagent were mixed, and the mixture was incubated for 10 min at room temperature in the dark. The absorbance was measured at 540 nm using the Shimadzu spectrophotometer (Japan). The concentration of nitrite in the supernatant was determined from the sodium nitrite standard curve and expressed as percentage of control [26].
Estimation of Reduced Glutathione Levels
Reduced glutathione was estimated according to the method described by Ellman [27]. One milliliter of supernatant was precipitated with 1 ml of 4 % sulfosalicylic acid and cold digested for 1 h at 4 °C. The samples were then centrifuged at 1200×g for 15 min at 4 °C. To 1 ml of the supernatant obtained, 2.7 ml of phosphate buffer (0.1 mmol/l, pH 8) and 0.2 ml of 5,5ʹ-dithiobis(2-nitrobenzoic acid) (DTNB) was added. The yellow color developed was measured at 412 nm using the Shimadzu spectrophotometer (Japan). Results were calculated using molar extinction coefficient of the chromophore (1.36 × 104 mol/l−1 cm−1) and expressed as percentage of control.
Catalase Estimation
Briefly, the assay mixture consisted of 12.5 mM H2O2 in phosphate buffer (50 mM of pH 7.0) and 0.05 ml of supernatant from the tissue homogenate (10 %), and the change in absorbance was recorded at 240 nm. The results were expressed as millimolars of H2O2 decomposed per milligram of protein per minute [28].
Protein Estimation
The protein content was estimated by Gornall’s biuret method using bovine serum albumin as a standard [29].
Mitochondrial Complex Estimation
Isolation of Rat Brain Mitochondria
Rat brain mitochondria were isolated by the method of Berman and Hastings [30]. The brain regions were homogenized in isolation buffer with EGTA (215 mM mannitol, 75 mM sucrose, 0.1 % BSA, 20 mM HEPES, 1 mM EGTA, pH 7.2). Homogenate was centrifuged at 13000×g for 5 min at 4 °C. Pellet was re-suspended in isolation buffer with EGTA and spun again at 13000×g for 5 min. The resulting supernatant was transferred to new tubes and topped off with isolation buffer with EGTA and again spun at 13,000×g for 10 min. The pellet containing pure mitochondria was re-suspended in isolation buffer without EGTA.
Complex-I (NADH Dehydrogenase Activity)
Complex-I was measured spectrophotometrically by the method of King and Howard [31]. The method involves catalytic oxidation of NADH to NAD+ with subsequent reduction of cytochrome C. The reaction mixture contained 0.2 M glycylglycine buffer pH 8.5, 6 mM NADH in 2 mM glycylglycine buffer, and 10.5 mM cytochrome C. The reaction was initiated by addition of a requisite amount of solubilized mitochondrial sample, and absorbance change was followed at 550 nm for 2 min.
Complex-II (Succinate Dehydrogenase Activity)
Complex-II was measured spectrophotometrically according to King [32]. The method involves oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer pH 7.8, 1 % BSA, 0.6 M succinic acid, and 0.03 M potassium ferricyanide. The reaction was initiated by the addition of mitochondrial sample, and absorbance change was followed at 420 nm for 2 min.
MTT Ability
The MTT assay is an indirect method to measure the activity of complex-III. MTT, a pale yellow substrate, produces a purple product when incubated with living cells, and the number of viable cells/well is directly proportional to the product, followed by solubilization with DMSO, which can be measured according to Mosmann [33]. In general, each culture well was incubated in 10 μl of MTT culture medium and the plate was incubated in a humidified atmosphere of 5 % CO2+ 95 % air at 37 °C for 3 h. The culture medium was then aspirated off, and the cells were lysed with 50 % DMSO. The absorbance of the resulting medium was measured by an ELISA reader at 580 nm wavelength.
Cytochrome Oxidase Assay
Cytochrome oxidase activity was assayed in brain mitochondria according to the method of Sottocassa [34]. The assay mixture contained 0.3 mM reduced cytochrome C in 75 mM phosphate buffer. The reaction was started by the addition of solubilized mitochondrial sample, and absorbance change was recorded at 550 nm for 2 min.
Estimation of TNF-α and IL-1β
The quantification of TNF-α and IL-1β was done by the help and instructions provided by RayBiotech, Inc., using Quantikine rat TNF-α and IL-1β immunoassay kits.
Histopathology of Brain Tissue
Tissue Section Preparation
Remaining animals were deeply anaesthetized and perfused transcardially via the ascending aorta with cold phosphate-buffered saline (0.1 M, pH 7.4) followed by fixative solution containing 4 % (w/v) paraformaldehyde in 0.1 M PBS solution (pH 7.4). The whole brain was dissected out and fixed overnight at 4 °C in the same buffer containing 4 % (w/v).
The brain was then washed with 0.1 M PBS (pH 7.4) for 1 h, dehydrated in alcohol, and then embedded in paraffin wax. Serial coronal sections (5 mm thickness) of the whole brain were then obtained.
Hematoxylin and Eosin Staining
The paraffin sections of the whole brain (thickness 5 mm) were dewaxed and rehydrated with alcohol for hematoxylin and eosin (H&E) staining. The neurons in striatum and cortex were examined under electron microscopy, and photomicrographs were prepared.
Measurement of Dopamine Levels by HPLC
Striatal dopamine was estimated with Waters standard system consisting of a high-pressure isocratic pump, a 20-μl sample injector valve, a C18 reversed-phase column, and an electrochemical detector. Data were recorded and analyzed with the help of Empower Software. Mobile phase consisted of 2 % citric acid, 2 % KHPO4, 1 mM EDTA, 1.2 % MeOH, and 70 mg/ml of sodium octyl sulfate. pH of the mobile phase was adjusted to 3 with the help of HCl (6 N). Electrochemical conditions for the experiment were at +0.800 V, and sensitivity ranges from 5–50 nA. Separation was carried out at a flow rate of 1 ml/min. Samples (20 μl) were injected manually. On the day of experiment, frozen striatum samples were thawed and they were homogenized in homogenizing solution containing 0.1 M perchloric acid. After that, the samples were centrifuged at 12,000×g for 5 min. The supernatant was further filtered through 0.25-μm nylon filters before injecting in the HPLC injection pump. Data were recorded and analyzed with the help of Empower Software [35].
Statistical Analysis
Values are expressed as mean ± S.E.M. The behavioral assessment data were analyzed by a repeated-measures two-way analysis of variance (ANOVA) with drug-treated groups as between-subject factors and sessions as within-subject factors. The biochemical estimations were separately analyzed by one-way ANOVA. Post hoc comparisons between groups were made using Bonferroni test. P < 0.05 was considered significant.
Results
In Vitro Antioxidant Studies
The several concentrations of the hydroalcoholic extract of UD and its fractions were tested for their antioxidant activity in different in vitro models, viz., DPPH and NO assay. It has been observed that the ethyl acetate fraction has shown potent antioxidant potential as compared to other fractions. The ethyl acetate fraction showed DPPH and NO radical scavenging activity with IC50 of 78.99 ± 0.171 and 101.39 ± 0.306, respectively. The IC50 value of the hydroalcholic extract and its fractions in DPPH and NO free radical scavenging assay are shown in Table 1.
Effect of Potent Antioxidant EAF of UD on Body Weight in MPTP-Administered Rats
Intranigral administration of MPTP significantly decreased body weight on the 28th day as compared to the sham control group. Further, post-treatment with potent antioxidant EAF of UD (20, 40, 80 mg/kg, p.o.) significantly and dose dependently improved the body weight in MPTP-treated rats (Fig. 2). In addition, selegiline (10 mg/kg) also restored their body weight in the MPTP-treated groups. However, combination of minocycline (30 mg/kg, p.o.) with the lower dose of EAF of UD (20 mg/kg, p.o.) did not influence body weight significantly in comparison to EAF of UD-treated groups.
Effect of potent antioxidant EAF of UD on body weight in MPTP-administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (Two-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica
Effect of Potent Antioxidant EAF of UD on Rotarod Performance and Grip Strength Alteration in MPTP-Administered Rats
Intranigral administration of MPTP significantly (P < 0.001) decreased the rotarod activity and the grip strength in a Chatillon grip strength meter apparatus during the 14th, 21st, and 28th days as compared to the sham control group. Further, post-treatment with potent antioxidant EAF of UD (20, 40, 80 mg/kg, p.o.) and selegiline (10 mg/kg) which started on day 15 significantly and dose dependently improved the motor coordination assessed in rotarod activity as well as using the grip strength meter on days 21 and 28 in MPTP-treated rats. Moreover, the treatment with minocycline (30 mg/kg) and lower dose of EAF UD (20 mg/kg) also increased the grip strength and fall of time in MPTP-treated rats but not significantly. [A two-way ANOVA revealed on rotarod activity on group (F (6, 2) = 650.1, P < 0.001), session (F (6, 2) = 495.6, P < 0.001), and interaction drug treatment × session group (F (12, 6) = 68.65, P < 0.001) and a two-way ANOVA revealed on grip strength on group (F (6, 2) = 16.47, P < 0.001), session (F (6, 2) = 8.966, P < 0.001), and interaction drug treatment × session group (F (12, 6) = 1.577, P < 0.001)] (Fig. 3a).
a Effect of potent antioxidant EAF of UD on rotarod performance and grip strength alteration in MPTP-administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (Two-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica. b Effect of potent antioxidant EAF of UD on time taken to cross the straight runway and foot error counts on narrow beam walk performance in MPTP-administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (Two-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica
Effect of Potent Antioxidant EAF of UD on Time Taken to Cross (Latency) and Foot Errors on Narrow Beam Walk Apparatus in MPTP-Administered Rats
Intranigral administration of MPTP significantly increased the time to cross the straight runway and foot errors (gait abnormalities) on a narrow beam walk apparatus during the 14th, 21st, and 28th days as compared to the sham control group. However, post-treatment with potent antioxidant EAF of UD (20, 40, 80 mg/kg) and selegiline (10 mg/kg) which started on day 15 significantly and dose dependently attenuated the increased latency and foot errors on days 21 and 28 in MPTP-treated rats. Moreover, the treatment with minocycline (30 mg/kg) and lower dose of EAF of UD (20 mg/kg) also decreased the latency time and foot errors in MPTP-treated rats but not significantly. [A two-way ANOVA revealed on cross latency on group (F (6, 2) = 281.6, P < 0.001), session (F (6, 2) = 286.4, P < 0.001), and interaction drug treatment × session group (F (12, 6) = 34.94, P < 0.001) and a two-way ANOVA revealed on foot slips on group (F (6, 2) = 157.4, P < 0.001), session (F (6, 2) = 159.2, P < 0.001), and interaction drug treatment × session group (F (12, 6) = 22.23, P < 0.001)] (Fig. 3b).
Effect of Potent Antioxidant EAF of UD on Oxidative Parameters in MPTP-Administered Rats
Intranigral infusion of MPTP significantly increased lipid peroxidation and nitrite concentration and diminished the level of endogenous antioxidant enzymes in the striatum and cortex region of the brain as compared to the sham control group. Wherever, chronic administration of EAF of UD (40 and 80 mg/kg, p.o.) and selegiline (10 mg/kg) administration significantly decreased the increased concentration of lipid peroxidation and nitrite concentration and further restored the decreased activity of GSH and catalase in MPTP-treated rats. In addition, the co-administration of minocycline (30 mg/kg) with the lower dose of UD (20 mg/kg) synergistically potentiated the neuroprotective effect as compared to their individual effects: striatum, lipid peroxidation (LPO) F (6, 14) = 55.40, P < 0.001; nitrite F (6, 14) = 39.10, P < 0.001; reduced glutathione F (6, 14) = 29.06, P < 0.001; catalase F (6, 14) = 16.43, P < 0.001 and cortex, LPO F (6, 14) = 50.10, P < 0.001; nitrite F (6, 14) = 45.41, P < 0.001; reduced glutathione F (6, 14) = 40.92, P < 0.001; catalase F (6, 14) = 16.89, P < 0.001 (Fig. 4a, b).
a Effect of potent antioxidant EAF of UD against MPTP-induced oxidative stress parameters (LPO and nitrite) in striatum and cortex of rat brain. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (One-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica. b Effect of potent antioxidant EAF of UD on endogenous antioxidants (reduced glutathione and catalase) activity against MPTP-induced rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (One-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica
Effect of Potent Antioxidant EAF of UD on Mitochondrial Complex Enzymes in MPTP-Administered Rats
Systemic intranigral administration of MPTP significantly declined all the mitochondrial enzyme complexes (I, II, III, and IV) as compared to the sham control group. Chronic administration of UD significantly attenuated mitochondrial enzyme dysfunction activity as compared to the MPTP-treated group. Treatment with higher dose of UD (40 and 80 mg/kg) and selegiline (10 mg/kg) significantly restored the decreased activity of mitochondrial enzymes as compared to MPTP and lower dose of UD-treated groups. Further, co-administration of minocycline (30 mg/kg) and UD (20 mg/kg) did not give significant results in comparison to other individuals. [Complex I F (6, 14) = 20.75, P < 0.001; complex II F (6, 14) = 27.94, P < 0.001; complex III F (6, 14) = 12.54, P < 0.001; complex IV F (6, 14) = 19.30, P < 0.001] (Fig. 5a, b).
a Effect of potent antioxidant EAF of UD on mitochondrial enzymes (NAD(P)H dehydrogenase and succinate dehydrogenase in MPTP-administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (One-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica. b Effect of potent antioxidant EAF of UD on cytochrome c oxidase and cell viabilities in MPTP-administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (One-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica
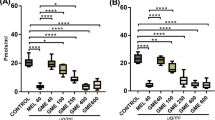
Effect of Potent Antioxidant EAF of UD on TNF-α and IL-1β levels in Administered Rats
Intranigral administration of MPTP significantly increased TNF-α and IL-1β levels in the striatum and cortex as compared to sham-treated rats. However, post-treatment with potent antioxidant EAF of UD (40, 80 mg/kg; p.o.) and selegiline (10 mg/kg) significantly attenuated TNF-α and IL-1β levels in the striatum and cortex as compared to MPTP control rats. Co-administration of minocycline (30 mg/kg) with lower dose of EAF of UD (20 mg/kg) also significantly attenuated the increase in the neuroinflammatory markers and potentiates the anti-inflammatory effect in comparison to the individual drug-treated group. [TNF-α F (6, 14) = 9.982, P < 0.0002; IL-1β F (6, 14) = 8.380, P < 0.0005] (Fig. 6).
Effect of potent antioxidant EAF of UD on TNF-α and IL-1β levels in administered rats. Data was expressed as mean ± S.E.M. P ≤ 0.01 as compared to sham control (a); P < 0.05 as compared to the MPTP-treated group (b); P < 0.05 as compared to EAF of UD (20) (c); P < 0.05 as compared to EAF of UD (40) (d). (One-way ANOVA followed by Bonferroni test). MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, EAF of UD ethyl acetate fraction of Urtica dioica
Effect of Potent Antioxidant EAF of UD on Cellular or Histopathological Changes in MPTP-Administered Rats
Brain histology of sham animals showed typical histopathological structures of neurons in the cerebral cortex and striatum. Administration of MPTP significantly enhanced the level of neuroinflammatory cells along with their degeneration (apoptosis) resulting in decreased cell density in both cerebral cortex and striatum as compared with the sham group. However, treatment with EAF of UD (80 mg/kg) attenuated these histological abnormalities. Co-administration of minocycline (30 mg/kg) with lower dose of EAF of UD (20 mg/kg) further restored histological alterations of neuronal cells as compared to MPTP control and lower and medium dose of EAF of UD (Fig. 7).
Representative photomicrographs of striatum and cerebral cortex of rat brain sections. Sections were stained with hematoxylin and eosin. a Sham control: neurons are intact. b MPTP control: mild-moderate infiltration of inflammatory cells with large number of apoptotic cells. c Treated with EAF of UD (80 mg/kg): slight inflammation of neurons with less apoptotic cells. d Treated with minocycline (30 mg/kg) + EAF of UD (20 mg/kg): neurons are preserved with slight inflammation. (HE stain 6250)
Effect of Potent Antioxidant EAF of UD on Neurochemical Analysis in MPTP-Administered Rats
Aging resulted in a significant decrease in dopamine level and its metabolites in the forebrain. Chronic administration of MPTP resulted in further decrease in the levels of dopamine in the extracellular spaces of aged animals as well as in matured animals; however, a slight decrease in the dopamine level was observed in young animals (Table 2). Dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were decreased significantly (P < 0.001) in the MPTP-administered group, as compared to the sham group. In contrast, potent antioxidant EAF of UD (80 mg/kg) and selegiline (10 mg/kg) and co-administration of minocycline (30 mg/kg) with lower dose of EAF of UD (20 mg/kg) restored DA and its metabolite content as compared to the MPTP-administered group. [DA F (5, 12) = 158.6, P < 0.001; DOPAC F (5, 12) = 15.32, P < 0.001; HVA F (5, 12) = 17.89, P < 0.001].
Discussion
Many neurodegenerative investigations revealed that oxidative stress followed by mitochondrial dysfunction and neuroinflammation could be the major cause behind the dopaminergic neurodegeneration in PD [36, 37]. In the present study, we demonstrated the neuroprotective potential of the antioxidant fraction from UD in MPTP-induced behavioral, biochemical, and cellular abnormalities in rats. Our findings revealed that repeated administration of MPTP into SNpc in rats produced stable motor deficit and significant increase in mito-oxidative damage in the striatal brain region, where post-treatment of potent antioxidant EAF of UD significantly and dose dependently attenuated MPTP-induced behavioral, cellular, and neurochemical alterations.
Repeated intranigral administration of MPTP for 14 days significantly impaired body weight and motor coordination assessed by different behavioral parameters in comparison to the sham control group. The striatal mito-oxidative damage and bradykinesia could be partially responsible for decreasing food intake and motor coordination. Post-treatment with ethyl acetate antioxidant fraction of UD significantly reversed the alteration in body weight and motor coordination. Systemic intranigral administration of MPTP, which is a mitochondrial toxin, leads to depletion of cellular ATP and secondary mitochondrial dysfunction complication. Mitochondrial dysfunction is one of the hallmarks of pathogenesis caused by ROS inducing cellular alteration in the neurodegenerative disorders [38].
It has been well reported that MPTP-induced motor abnormalities were short lived and reversed to normal within a week due to compensatory excess release of dopamine. Hence, repeated administration of MPTP in three doses at an interval of 1 week was done to reproduce the irreversible damage of dopaminergic neurons like human PD [39]. The chronic administration of potent antioxidant EAF of UD (20, 40, 80 mg/kg) significantly and dose dependently improved the body weight, rotarod activity, and grip strength and attenuated the increase in latency to cross a straight runway and foot errors in MPTP-treated rats. Moreover, minocycline (30 mg/kg) with lower dose of potent antioxidant EAF of UD (20 mg/kg) improved the body weight and motor abilities and potentiated the performance using a narrow beam walk apparatus (attenuated gait abnormalities) as compared to the per se groups.
MPTP-injected rats showed significant increase in MDA levels and nitrite concentration and decreased the endogenous antioxidants (reduced glutathione and catalase activity), which shows association of motor impairment with oxidative damage. The results are consistent with earlier reports suggesting the role of oxidative stress in neuronal cell death associated with PD [40], where our present findings revealed that UD (20, 40, 80 mg/kg) significantly and dose dependently attenuated the increase in MDA level and nitrite concentration and restored the reduced glutathione and catalase activity suggesting its antioxidant potential (Fig. 8).
Mitochondria of SNpc in PD subjects showed a significant accumulation of α-synuclein, which was associated with impairment in complex-I activity and increased oxidative stress [41]. In the present study, we found that intranigral administration of MPTP significantly decreased the activity of various electron transport complexes, viz., NADH dehydrogenase, succinic dehydrogenase, cytochrome oxidase, and mitochondria redox activity, in the striatal and other major regions of rat brain. It has been reported that changes in mitochondrial membrane permeability and impairment in mitochondrial enzymes induced mitochondrial membrane potential loss and cytochrome c release, leading to caspase-3 activation (Fig. 8) [42]. Recently, Fattahi et al. reported that Urtica dioica has shown to produce the antioxidant and antiapoptotic effect in MCF-7 human breast cancer cell line [43]. In our present study, we found that reduced mitochondrial complex enzymes could be restored by post-treatment with potent antioxidant EAF of UD in a dose-dependent manner and selegiline as standard maker in MPTP-treated rats. Moreover, our combinational treatment of lower dose of EAF of UD (20 mg/kg) with minocycline (30 mg/kg) produced a synergistic effect on restoration of reduced mitochondrial enzyme activities.
MPTP-lesioned rats showed a marked increase in neuroinflammatory markers like TNF-α and IL-1β. The chronic treatment with potent antioxidant EAF of UD (20, 40, 80 mg/kg) significantly and dose dependently attenuated the increased neuroinflammatory markers and showed its anti-inflammatory property (Fig. 8). Moreover, pre-treatment of minocycline with lower dose of UD potentiated the anti-inflammatory property in the rat brain which indicates the anti-inflammatory property. Further, selegiline treatment also produced a neuroprotective effect against the neuroinflammatory markers in MPTP-treated rats. Previous reports suggested that MPTP produced histopathological alterations in the striatum and cortex after in MPTP-induced rats [44]. In support to this, the present study showed significant alteration in the striatum and cortex region of the brain in MPTP-treated rats, which was further reversed by potent antioxidant EAF of UD (80 mg/kg) treatment.
Administration of MPTP is known to produce a decrease in the density of tyrosine hydroxylase (TH+) neurons indicating degeneration of the dopaminergic neurons in the nigrostriatal pathway and to cause a decrease in the dopamine levels, an important neurotransmitter involved in the motor function [45, 46]. In the present study, we also found that repeated MPTP infusion produced a significant decrease in striatal dopamine and its metabolite concentration which is strongly correlated with the observed motor deficit. However, we found that EAF of UD significantly and dose dependently restored the striatal dopamine and its metabolite concentration in MPTP-infused rats. Moreover, the treatment with minocycline (30 mg/kg) and UD (20 mg/kg) potentiate the restoration of striatal dopamine and its metabolite concentration in rat brain. Chronic administration of selegiline and MAO-B inhibitor also restored the dopamine and its metabolite concentration in MPTP-treated rats. Recently, it has been hypothesized that UD exert neuroprotective effects by acting as a selective MAO-B inhibitor in high throughput screening [16]. It has been already reported that PD is associated with elevated levels of MAO-B in the brain which generally metabolizes DA [47, 48] and creates reactive oxygen species [49].
Several natural compounds such as flavonoids, phenolics, and polyphenols exhibit neuroprotective effect against various neurodegenerative disorders by preventing oxidative stress-induced mitochondrial transition pore complex opening by decreasing the production of Bax and Bad protein, favoring an increase in the Bcl2–BclXL/Bax–Bak ratio [50]. UD has also been reported to contain flavonoids [13] and phenolics [51] including flavonol glycosides (kaemferol-3-O-glucoside and −3-O-rutinoside; quercetin-3-O-glucoside and −3-O-rutinoside; isorhamentin-3-O-glucoside; −3-O-rutinoside; and −3-oneohesperidoside) [52], some of which are major contributors in the antioxidant activity of UD. UD has been recently indicated to show free radical scavenging activity [53, 54]. In vitro DPPH and NO assay showed the ethyl acetate fraction of UD having a potent antioxidant potential. The HPTLC methods were also developed for the standardization of ethyl acetate fraction of UD. The HPTLC method was successfully validated with respect to International Conference on Harmonization (ICH) guidelines. The HPTLC analysis in our study showed the presence of a bioactive marker, i.e., ferulic acid. Ferulic acid is reported to be a phenolic compound. On the basis of in vitro antioxidant studies, the plant has a potent antioxidant potential due to the presence of flavanoids and phenolic compounds. Hence, these compounds might be responsible for its neuroprotective activity.
In conclusion, the findings of the present study raised the possibility that mito-oxidative damage-mediated pro-inflammatory markers may contribute to the neurodegeneration in the MPTP model of PD in rats (Fig. 8). On the other hand, the ethyl acetate antioxidant-rich fraction of UD showed neuroprotective actions due to its multiple pleiotropic effects, viz., strong anti-inflammatory, radical scavenging, and neuromodulating properties. Co-administration of minocycline (30 mg/kg) with lower dose of EAF of UD potentiates the neuroprotective property. Hence, the observed beneficial effects following UD administration in the present study may be due to its antioxidant and anti-inflammatory activity. Thorough verification might better clarify the mechanism of actions of UD and support the rationale for clinical use and in the treatment of movement disorders.
References
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4(11):600–609
Giordano S, Darley-Usmar V, Zhang J (2014) Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol 2:82–90
Banerjee R, Starkov AA, Beal MF, Thomas B (2009) Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta 1792(7):651–663
Hartmann A (2004) Postmortem studies in Parkinson’s disease. Dialogues Clin Neurosci 6(3):281–293
Celsi F, Pizzo P, Brini M et al (2009) Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta 1787(5):335–344
Ma K-L, Gao J-H, Huang Z-Q et al (2013) Motor function in MPTP-treated tree shrews (Tupaia belangeri chinensis). Neurochem Res 38(9):1935–1940
Warner TT, Schapira AH (2003) Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol 53(Suppl 3):S16–S23, discussion S23-15
Plane JM, Shen Y, Pleasure DE, Deng W (2010) Prospects for minocycline neuroprotection. Arch Neurol 67(12):1442–1448
Peng J, Xie L, Stevenson FF et al (2006) Nigrostriatal dopaminergic neurodegeneration in the weaver mouse is mediated via neuroinflammation and alleviated by minocycline administration. J Neurosci 26(45):11644–11651
Chen M, Ona VO, Li M et al (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6(7):797–801
Mavi A, Terzi Z, Özgen U et al (2004) Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), malva neglecta (malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), urtica dioica (urticaceae). Biol Pharm Bull 27(5):702–705
Koch E (2001) Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Med 67(6):489–500
Akbay P, Basaran AA, Undeger U, Basaran N (2003) In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother Res 17(1):34–37
Golalipur M, Ghafari S, Latimfimoghadam M, Kaboli S (2011) Alteration of dentate gyrus astrocytes in diabetic rats: protective role of Urtica dioica. Int J Morphol 29(4):1307–1312
Patel SS, Udayabanu M (2013) Effect of Urtica dioica on memory dysfunction and hypoalgesia in an experimental model of diabetic neuropathy. Neurosci Lett 552:114–119
Mazzio E, Deiab S, Park K, Soliman KF (2013) High throughput screening to identify natural human monoamine oxidase B inhibitors. Phytother Res 27(6):818–828
Ji T, Liu C, Wang A et al (2007) Studies on the chemical constituents of Urtica dioica L. grown in Tibet Autonomous Region. Zhong yao cai = Zhongyaocai = J Chin Med Mater 30(6):662–664
Suh S-S, Hwang J, Park M et al (2014) Phenol content, antioxidant and tyrosinase inhibitory activity of mangrove plants in Micronesia. Asian Pac J Trop Med 7(7):531–535
Garratt DC (1964) The quantitative analysis of drugs. Springer, New York
Veerendra Kumar MH, Gupta YK (2002) Intracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in rats. Pharmacol Biochem Behav 73(3):565–571
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates: hard cover edition. Academic press, Cambridge
Kulkarni S (2008) Practical pharmacology and clinical pharmacy. 1st. 38–39
Kumar P, Kalonia H, Kumar A (2012) Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol 674(2–3):265–274
Thakur KS, Prakash A, Bisht R, Bansal PK (2015) Beneficial effect of candesartan and lisinopril against haloperidol-induced tardive dyskinesia in rat. J Renin Angiotensin-Aldosterone Syst 16(4):917–929
Wills E (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Luck H (1965) Catalase. Methods of enzymatic analysis. 885–888
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73(3):1127–1137
King TE, Howard RL (1967) [52] Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10:275–294
King TE (1967) [58] Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol 10:322–331
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol 32(2):415–438
Bishnoi M, Chopra K, Kulkarni SK (2008) Protective effect of L-type calcium channel blockers against haloperidol-induced orofacial dyskinesia: a behavioural, biochemical and neurochemical study. Neurochem Res 33(9):1869–1880
Perier C, Vila M (2012) Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med 2(2):a009332
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62(5):803–819
Witte ME, Geurts JJ, De Vries HE et al (2010) Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10(5):411–418
Bisht R, Kaur B, Gupta H, Prakash A (2014) Ceftriaxone mediated rescue of nigral oxidative damage and motor deficits in MPTP model of Parkinson’s disease in rats. Neurotoxicology 44C:71–79
Seet R, Lee C-YJ, Lim EC et al (2010) Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med 48(4):560–566
Zhu M, Li W, Lu C (2012) Role of alpha-synuclein protein levels in mitochondrial morphology and cell survival in cell lines. PLoS One 7(4):e36377
Ricci JE, Gottlieb RA, Green DR (2003) Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 160(1):65–75
Fattahi S, Ardekani AM, Zabihi E et al (2013) Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev 14(9):5317–5323
Ferro MM, Bellissimo MI, Anselmo-Franci JA et al (2005) Comparison of bilaterally 6-OHDA-and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods 148(1):78–87
Reksidler AB, Lima MM, Dombrowski P et al (2008) Repeated intranigral MPTP administration: a new protocol of prolonged locomotor impairment mimicking Parkinson’s disease. J Neurosci Methods 167(2):268–277
Kuroiwa H, Yokoyama H, Kimoto H et al (2010) Biochemical alterations of the striatum in an MPTP-treated mouse model of Parkinson’s disease. Metab Brain Dis 25(2):177–183
Mallajosyula JK, Chinta SJ, Rajagopalan S et al (2009) Metabolic control analysis in a cellular model of elevated MAO-B: relevance to Parkinson’s disease. Neurotox Res 16(3):186–193
Riederer P, Laux G (2011) MAO-inhibitors in Parkinson’s disease. Exp Neurobiol 20(1):1–17
Nagatsu T, Sawada M (2006) Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. J Neural Transm Suppl 71:53–65
Mandel S, Youdim MB (2004) Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med 37(3):304–317
Ioana N, Viorica I, Diana-Carolina I, Valeria R (2013) Preliminary research regarding the therapeutic uses of Urtica dioica l note ii. The dynamics of accumulation of total phenolic compounds and ascorbic acid. Farmacia 61(2):276–283
Chaurasia N, Wichtl M (1987) Flavonol glycosides from Urtica dioica. Planta Med 53(5):432–434
Ghaima KK, Hashim NM, Ali SA (2013) Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale)
Kataki MS, Murugamani V, Rajkumari A et al (2012) Antioxidant, hepatoprotective, and anthelmintic activities of methanol extract of Urtica dioica L. Leaves. Pharm Crops 3(1):38–46
Acknowledgments
This study was partially funded by the Punjab State Council of Science and Technology (PSCST), Chandigarh (India), reference no. PSCST/795, to Rohit Bisht. We express our gratitude to the management and Mr. Parveen Garg, Honarable Chairman, ISF College of Pharmacy, Moga (India), for providing the needed facilities and financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) bearing the registration no. IAEC/CPCSEA/M9/2013/P167 and carried out in accordance with the guidelines of the committee for the purpose of Control and Supervision of Experimentats on Animals (CPCSEA), Government of India, on animal experimentation.
Rights and permissions
About this article
Cite this article
Bisht, R., Joshi, B.C., Kalia, A.N. et al. Antioxidant-Rich Fraction of Urtica dioica Mediated Rescue of Striatal Mito-Oxidative Damage in MPTP-Induced Behavioral, Cellular, and Neurochemical Alterations in Rats. Mol Neurobiol 54, 5632–5645 (2017). https://doi.org/10.1007/s12035-016-0084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0084-z