Abstract
T helper 17 (Th17) cells are vital components of the adaptive immune system involved in the pathogenesis of most autoimmune and inflammatory syndromes, and adiponectin(ADN) is correlated with inflammatory diseases such as multiple sclerosis (MS) and type II diabetes. However, the regulatory effects of adiponectin on pathogenic Th17 cell and Th17-mediated autoimmune central nervous system (CNS) inflammation are not fully understood. In this study, we demonstrated that ADN could inhibit Th1 and Th17 but not Th2 cells differentiation in vitro. In the in vivo study, we demonstrated that ADN deficiency promoted CNS inflammation and demyelination and exacerbated experimental autoimmune encephalomyelitis (EAE), an animal model of human MS. Furthermore, ADN deficiency increased the Th1 and Th17 cell cytokines of both the peripheral immune system and CNS in mice suffering from EAE. It is worth mentioning that ADN deficiency predominantly promoted the antigen-specific Th17 cells response in autoimmune encephalomyelitis. In addition, in vitro and in vivo, ADN upregulated sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor γ (PPARγ) and inhibited retinoid-related orphan receptor-γt (RORγt); the key transcription factor during Th17 cell differentiation. These results systematically uncovered the role and mechanism of adiponectin on pathogenic Th17 cells and suggested that adiponectin could inhibit Th17 cell-mediated autoimmune CNS inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naïve CD4+ T cells are activated by antigen presented by antigen-presenting cells (APCs) and subsequently differentiate into different T helper (Th) cell subsets, including Th1, Th2, Th9, Th17, Th22, and Tregs [1–3]. As vital components of the adaptive immune system, Th cells regulate immunity and inflammation [4, 5]. Specifically, Th17 cells and the inflammatory cytokines that they express have been implicated in the pathogenesis of most autoimmune syndromes, including rheumatoid arthritis (RA), psoriasis, multiple sclerosis (MS), and inflammatory bowel disease (IBD) [6–8]. Under the regulation of retinoid-related orphan receptor-γt (RORγt) and the stimulation of interleukin (IL)-1β, IL-6, and IL-23, Th17 cells differentiate from naïve CD4+ T cells and express a series of effector cytokines, including IL-17A, IL-17F, IL-21, IL-22, IL-26, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [9, 10]. Although these cytokines exhibit biological roles in chronic inflammation and autoimmune diseases, IL-17A plays a critical role in autoimmmune inflammation, such as the development of experimental autoimmune encephalomyelitis (EAE), an animal model of MS [10–12]. High expression of IL-17 in demyelinating lesions is found in patients with MS [13]. Furthermore, lack of IL-17 signaling or Th17 cell differentiation will relieve EAE [14, 15]. According to current study, IL-17/Th17 plays an essential role in autoimmune inflammation and immune activation.
Many studies have demonstrated that adipocytokines are key mediators linking adipose tissue, inflammation, and immunity [16]. Adiponectin, a 33-kDa protein, is one of the most abundant adipocytokines and is primarily released from adipocytes [17]. The circulating level of adiponectin negatively correlates with obesity, type II diabetes, insulin resistance, liver diseases, and inflammatory responses [18]. As a key metabolic regulator, adiponectin plays crucial roles in insulin sensitization, vascular protection, anti-atherosclerosis, anti-diabetes, and, most importantly, anti-inflammation and immune suppression [19–21]. Although adiponectin has anti-inflammatory properties and the circulating levels of adiponectin decrease in these inflammatory diseases, it is also found that adiponectin was increased in a number of autoimmune and inflammatory diseases [22]; therefore, the roles of adiponectin in autoimmune and inflammation may be controversial and remain largely unknown. Thus, it is necessary to clarify the role of adiponectin in classic autoimmune inflammatory diseases. For this reason, we utilized EAE, a mice model of autoimmune inflammatory central nervous system disease (MS), to reveal the potential cellular and molecular mechanism of adiponectin in regulating autoimmune diseases. The recent finding of significantly decreased adiponectin levels in the sera of patients with MS compared to those observed in healthy controls indicates that adiponectin may play a protective role in the pathogenesis of MS [23]. Many other studies have demonstrated that adiponectin and leptin play opposite roles in inflammation and immunity [24]. The level of circulating leptin, a pro-inflammatory adipocytokine, increases in mice during the onset of EAE [25], and leptin-deficient mice are resistant to the development of EAE [26]. As a whole, all of these studies suggest that adiponectin might play a protective role in inflammation and immunity. In addition, one study focused on Tregs and demonstrated that adiponectin could alleviate EAE [27]. However, it also reported that adiponectin could indirectly activate Th1 and Th17 via dendritic cell-secreted cytokines under physiological conditions [28], but this finding cannot explain the immune-suppressive role of adiponectin under the pathological conditions of autoimmune inflammatory diseases. Therefore, the direct role and mechanisms of adiponectin in regulating the function and differentiation of classical pathogenic, proinflammatory, and autoimmune Th17 cells need to be thoroughly investigated.
It is well-known that adiponectin could activate the signaling of AMP-activated protein kinase (AMPK) via adiponectin receptor1/2 [29]. Our previous study demonstrated a dynamic interactive relationship between AMPK and sirtuin 1 (SIRT1) in pancreatic cancer cells [30]. Studies show that sirtuins involve in immune inflammation, especially SIRT1, could inhibit monocyte differentiation and ameliorate chronic inflammation and autoimmune diseases [31, 32]. Also, some studies have illustrated that AMPK-SIRT1 could regulate peroxisome proliferator-activated receptor γ (PPARγ) and influence various biological mechanism [33]. In addition, both animal and human studies have demonstrated that the expression of adiponectin is under the control of PPARγ [34]. PPARγ agonists can upregulate the circulating adiponectin level in both humans and mice [35–37]. Importantly, PPARγ agonists protect mice from EAE induction, and PPARγ specific inhibits Th17 differentiation and suppresses central nervous system autoimmunity in EAE [38]. We recently found that adiponectin is required for the PPARγ-mediated improvement of endothelial function [39]. These findings indicate that adiponectin may play protective roles in inflammatory and immune diseases. However, it remains unclear whether adiponectin has a regulatory effect on SIRT1 and PPARγ in Th cells and plays protective roles in autoimmune diseases by suppressing pathogenic Th17 cells differentiation.
We previously found that adiponectin could regulate endothelial cell and dendritic cell differentiation in vitro [40, 41]. In this study, we investigated in vitro the effects of adiponectin on T helper cells differentiation and, interestingly, found that exogenous adiponectin inhibited Th1 and Th17 cell differentiation from naïve CD4 positive splenocyte cells. Furthermore, we demonstrated that adiponectin deficiency increased Th1 and Th17 cytokine expression in the peripheral immune system and CNS of mice with induced EAE. Moreover, adiponectin deficiency prominently promoted an antigen-specific Th17 cell response in autoimmune encephalomyelitis. In addition, we found that adiponectin could downregulate RORγt, a key transcription factor during Th17 cell differentiation in vivo and in vitro. These results suggested that adiponectin may play protective roles in inflammatory and autoimmune diseases via the inhibition of pathogenic Th17 cell differentiation.
Materials and Methods
Mice and EAE Induction
Female C57BL/6 background adiponectin knockout mice [42] and C57BL/6 wild-type (WT) mice (Academy of Military Medical Science, Beijing, China) 6 to 8 weeks old were used to induce EAE. The mice were maintained and fed in a specific pathogen-free condition in the Experimental Animal Center of Tianjin Medical University. The care and treatment for mice were approved by Animal Ethics Committee of Tianjin Medical University and were in accordance with guidelines for animal care. Each mouse was immunized subcutaneously (s.c.) with 100 μg of myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (CL. Bio-Scientific CO., LTD, Xi’an, China) that was emulsified in complete Freund’s adjuvant (Difco, Detroit, MI) containing 200 μg of heat-killed Mycobacterium tuberculosis H37RA (Difco, Detroit, MI) on day 0. On day 0 and day 2 after the immunization, each mouse was given 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) in 200 μl of PBS intraperitoneally (i.p.). The clinical scores were monitored daily based on the clinical symptoms on a scale from 0 to 6 for 39 consecutive days (score of 0–6: 0 = no clinical signs, 0.5 = partially paralyzed tail, 1 = paralyzed tail, 2 = paralyzed tail and weakness in hind limbs, 3 = one hind limb paralyzed, 4 = both hind limbs paralyzed, 5 = both hind limbs paralyzed and partial or complete paralysis of forelimbs, and 6 = moribund or dead).
Histology and Immunohistochemistry
The spinal cords were fixed in 10 % (weight/volume) formalin solution at 4 °C overnight, paraffin-embedded and cut into sections of 5 μm in thickness. Representative sections were stained with hematoxylin and eosin (H&E) to reveal inflammatory infiltrates. The immunohistochemistry of rabbit anti-mouse F4/80 (AbD Serotec), rabbit anti-mouse CD3 (eBiosciences), and rabbit anti-mouse CD4 (BioLegend) was examined to quantify the infiltrating components. DAB peroxidase substrate solution was used to detect HRP-conjugated anti-rabbit antibody.
Real-Time PCR
The total RNA was extracted from tissues using TRIzol reagent (Invitrogen, CA, USA). Random hexamer primers and ImProm-II reverse transcriptase were used to generate cDNA. Gene expressions were assessed using a SYBR Green real-time PCR system (Invitrogen, CA, USA) with an Applied Biosystem Prism 7000 sequence detection system. For each sample, duplicate test reactions were analyzed to determine the expression of interested genes, and the results were normalized to the GAPDH expression. The melting curves were analyzed to ensure the specificity of the qPCR reaction. The primer sequences of mouse genes were as follows:
-
IL-1β forward: GGACATAATTGACTTCACCATGGAA, reverse: CAGTCCAGCCCATACTTTAGGAA;
-
IL-4 forward: TCATCGGCATTTTGAACGAG, reverse: TTTGGCACATCCATCTCCG;
-
IL-6 forward: AGCCAGAGTCCTTCAGAGAG, reverse: GATGGTCTTGGTCCTTAGCC;
-
IL-17A forward: CTCCAGAAGGCCCTCAGACTAC, reverse: AGCTTTCCCTCCGCATTGACACAG;
-
IL-17F forward: GAGGATAACACTGTGAGAGTTGAC, reverse: GAGTTCATGGTGCTGTCTTCC;
-
IL-22 forward: CATGCAGGAGGTGGTACCTT, reverse: CAGACGCAAGCATTTCTCAG;
-
IFN-γ forward: GCATTCATGAGTATTGCCAAGTTT, reverse: GATTCCGGCAACAGCTGGT;
-
TNF-α forward: ACCCTCACACTCAGATCATC, reverse: GAGTAGACAAGGTACAACCC;
-
T-bet forward: GCCAGGGAACCGCTTATATG, reverse: GACGATCATCTGGGTCACATTGT;
-
RORα forward: AGAACAACACCGTGTACTTTG, reverse: CTGTAGGACGTGCTGAAG;
-
RORγt forward: AGTGTAATGTGGCCTACTCCT, reverse: GCTGCTGTTGCAGTTGTTTCT;
-
CD68 forward: TTGGGAACTACACACGTGGGC, reverse: CGGATTTGAATTTGGGCTTG
Recombinant Mouse Adiponectin Purification
p-PROEX HTb plasmid containing the mouse adiponectin gene was used to express and purify mouse adiponectin. The plasmid was transfected into E. coli and purified using a Ni-NTA agarose column. The concentration of recombinant mouse adiponectin was determined using the bicinchoninic acid (BCA) assay kit (Pierce Biotechnology Inc., Rockford, USA). SDS-PAGE revealed that the protein purity was greater than 95 %.
T Helper Cell Differentiation
First, a single cell suspension was isolated from the spleens of wild-type mice by mashing and passing through a 40 μm cell strainer (BD Biosciences CA, USA) with RPMI1640 medium. Anti-mouse CD4 magnetic microbeads (Miltenyi Biotec, Auburn, CA) were used to purify CD4+ T cells. The cells were seeded into an anti-mouse CD3 (5 μg/ml) and anti-mouse CD28 (1 μg/ml) pre-coated 24-well plate at the concentration of 1 × 106 cells per well and cultured with cytokines (R&D systems, USA) used to induce T cell differentiation for 96 h. To induce Th1 cells, 10 ng/ml IL-12 and 10 μg/ml anti-mouse-IL-4 were used. To induce Th2 cells, 10 ng/ml IL-4 and 10 μg/ml anti-mouse IFN-γ were used. To induce Th17 cells, 20 ng/ml IL-6, 10 ng/ml IL-23, 1 ng/ml TGF-β, 10 μg/ml anti-mouse IL-4, and 10 μg/ml anti-mouse IFN-γ were used. Recombinant mouse adiponectin was absent or present in parallel wells at the concentration of 10 μg/ml to show its effect on T helper cell differentiation.
Ex Vivo Re-stimulation with MOG35–55
A single spleen cell suspension was seeded to a 96-well round plate at a concentration of 2 × 105 cells per well. The cells were treated with 33 μg/ml MOG35–55 and cultured for 96 h. The supernatant was collected, and the cytokine productions were quantified using the Bio-Plex cytokine assay. For the cell proliferation assay, 0.5 μCi 3H thymidine was incorporated to each well for the last 16 h of culture. Then, cells were harvested with UniFilter-96 GF/C plates (PerkinElmer, CA, USA), and the radioactivity was measured as counts per minute (cpm) by a TopCount NxT ß-counter (PerkinElmer, CA, USA) and normalized against the protein concentration.
Multiple Cytokine ELISA Assay
The Bio-Plex cytokine assay system (Bio-Rad) was used to analyze the cytokines released from cells under certain culture conditions. After culturing, the supernatant of the cells was collected and diluted using the diluents that were included in the kit and then quantitated according to the manufacturer’s instructions.
Flow Cytometry
Cells that separated from EAE mice were first re-stimulated with the cell stimulation cocktail (plus protein transport inhibitors) (eBioscience, CA, USA) for 5 h and were then collected and washed with 1× PBS containing 2 % FBS and 0.1 % NaN3. To stain the cell surface markers, APC-conjugated rat anti-mouse CD4 antibody was used. Then, the cells were fixed in an IC fixation buffer followed by washing with a permeabilization buffer (eBioscience, CA, USA). Then, PE-conjugated rat anti-mouse IL-17A (BioLegend, CA, USA) and PE-conjugated rat anti-mouse IFN-γ (eBioscience, CA, USA) were used to stain the intracellular cytokines. Accuri C6 (BD, CA, USA) was used to analyze the cells that were labeled with different antibodies according to the manufacturer’s instructions. FlowJo software (Tree star, Ashland, OR) was used to analyze the fluorescence-activated cell sorter (FACS) data.
Antibodies and Western Blotting
Western blot analysis was conducted to detect the expression of SIRT1, PPAR-γ, and ROR-γt protein in the Jurkat cells and primary CD4+ T cells. Jurkat cells were treated with adiponectin (10 μg/ml) for 24 h. Primary CD4+ T cells were separated from the spleen of adiponectin KO mice WT mice with EAE. Antibody for PPAR-γ was purchased from Cell Signaling. SIRT1 and RORγt were purchased from Abcam. β-actin was purchased from Sungene. RIPA lysis buffer with protease inhibitors was used for the whole-cell lysate. Protein concentrations were examined using the BCA method (Biomed, Beijing, China). The total protein was separated with 10 % SDS-PAGE, transferred onto PVDF membranes, and subsequently detected using various primary antibodies. The Chemiluminescent HRP Substrate (Millipore, MA, USA) was used to detect the antibody-antigen complexes.
Statistical Analysis
The results shown are representative data of at least three independently repeated experiments. All of the data are presented as the mean ± SEM. Statistical significance was determined by a one-way ANOVA or Student’s t test. In all of the statistical comparisons, error bars are ±s.e.m., and p value <0.05 was used to indicate a significant difference.
Results
Adiponectin Inhibited Th1 and Th17 Cell Differentiation In Vitro
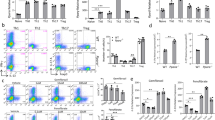
In this study, we first investigated the influence of exogenous adiponectin on Th1, Th2, and Th17 cell differentiation. Naïve CD4+ splenic cells were differentiated to Th1 by IL-12 and anti-IL-4, Th2 by IL-4 and anti-IFN-γ, and Th17 by TGF-β, IL-6, IL-23, anti-IFN-γ, and anti-IL-4. And recombinant adiponectin was presented during the differentiation. The signature cytokine proteins that were produced from different T helper cells were examined by a cytokine immunoassay after the rest and restimulation of T cells with PMA and ionomycin. Compared to the Th0 cells, which had no cytokine stimulation, the Th1 cells had highest amounts of IFN-γ, Th2 cells expressed an abundance of IL-4, and Th17 cells produced the greatest abundance of IL-17, demonstrating that these cells were successfully differentiated (Fig. 1a–c). However, as presented with the adiponectin, the IFN-γ expression in Th1 cells, the IL-6 and IL-17 production in Th17 cells were significantly reduced (Fig. 1a, c). No inhibitions were observed of IL-4 secretion or intracellular expression in differenced Th2 cells (Fig. 1b). Then, the expression of signature cytokine transcripts of different T helper cell lineages was examined by quantitative PCR. Consistently, the results showed that exogenous adiponectin inhibited the expression of cytokine IFN-γ and its transcription factor T-bet during Th1 cell differentiation (Fig. 1d). Adiponectin also inhibited the expression of Th17 cytokines IL-6, IL-17A, IL-7F, IL-21, IL-22, and IL-23R and transcription factors RORα and RORγt in Th17 cell differentiation (Fig. 1f, g). Because of the dramatic inhibition of IL-6 transcript expression in Th17 cells, we examined IL-6 transcription factor STAT3 and found that adiponectin also suppressed STAT3 expression during Th17 cell differentiation (Fig. 1f). However, adiponectin did not inhibit the expression of IL-4 and its transcription factor GATA-3 in Th2 cell differentiation (Fig.1e). These results indicated that adiponectin had suppressive effects on Th1 and Th17 cell differentiation but not on Th2 cell differentiation in vitro.
Adiponectin (ADN) inhibited the differentiation of Th1 and Th17 cells in vitro. Naïve CD4+ splenic cells were treated with adiponectin during differentiation to the different effector Th cells. Supernatant and mRNA were detected after differentiation. ELISA results of IFN-γ (a), IL-4 (b), IL-6 and IL-17 (c). d Real-time PCR of Th1 cell-related cytokine and transcription factor. e Real-time PCR of Th2 cell-related cytokine and transcription factor. (f, g) Real-time PCR of Th17 cell-related cytokines and transcription factors. mRNA was extracted from differentiated Th1 cells, Th2 cells, and Th17 cells. The results were normalized against GAPDH. The data are expressed as the mean fold change ± SEM(*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test)
Adiponectin Deficiency Promoted CNS Inflammation and Demyelination and Exacerbates Autoimmune Encephalomyelitis
Based on the results of the suppressive effect of adiponectin on Th1 and Th17 cells, we found that adiponectin deficiency could exacerbate the development of EAE, an animal model of multiple sclerosis, mainly mediated by pathogenic T cells, including Th1 and Th17 cells. In Fig. 2a, EAE was inducted in both adiponectin-deficient and wild-type mice using MOG35–55 peptides. Daily recorded clinical scores of EAE showed that adiponectin-deficient mice had more severe disease development than wild-type control mice did. However, there were no differences in the disease onset time between the adiponectin-deficient and wild-type mice. These results suggested that adiponectin deficiency may influence the EAE progression but not delay the disease initiation.
Adiponectin knock-out (KO) mice were more susceptible to EAE than wild-type littermates (WT). a Average EAE clinical scores of KO mice and WT mice after being immunized with MOG35–55 peptide. Female WT mice (n = 15) and adiponectin KO mice (n = 15) that were 6–8 weeks old were treated with MOG35–55 peptide that was emulsified in CFA. The clinical scores of all the mice were assessed daily according to the same criteria for 39 continuous days. b Hematoxylin and eosin (H&E) staining of representative spinal cord sections from adiponectin KO mice WT mice. c Fast blue staining of a representative spinal cord section from adiponectin KO mice and WT mice. d Inflammatory cell numbers in two regions of the same area from H&E staining slides from adiponectin KO mice and WT mice. e Real-time PCR of inflammatory cytokines in spinal cords of adiponectin KO mice and WT mice. The data are expressed as the mean ± SEM(*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test)
Inflammation and demyelination in the central nervous system are the two main pathological characters of EAE and are also important parameters that are used to compare the severity of EAE. To assess the inflammation and demyelination, spinal cords were isolated and assessed using H&E staining and luxol fast blue staining, respectively. There was significantly increased inflammation (Fig. 2b) and demyelination (Fig. 2c) in adiponectin-deficient EAE mice compared to wild-type EAE mice. Furthermore, we chose two regions of the same area from each H&E staining slide and counted the inflammatory cell numbers in these regions. Compared to the spinal cord samples from wild-type EAE mice, there were more inflammatory cells infiltrations in the spinal cords of adiponectin-deficient EAE mice (Fig. 2d). In addition, the mRNA level of the inflammatory cytokines TNF-α and IL-1β and the macrophage cell marker CD68 was also evaluated by quantitative PCR analysis (Fig. 2e). The results showed that the spinal cords from adiponectin-deficient mice had a relatively higher inflammatory response than wild-type mice after suffering from EAE. These data indicated that there was more severe inflammation and demyelination in adiponectin-deficient mice. Taken together, adiponectin-deficient mice were more susceptible to EAE, suggesting the protective roles of adiponectin in EAE.
Adiponectin Deficiency Increased Th1 and Th17 Cells and Their Signature Cytokine Expression in the Peripheral Immune System
To further determine whether the cytokine production related to T helper cells in the peripheral immune system is upregulated by adiponectin deficiency, total RNA was extracted from the lymph nodes of two groups of mice with induced EAE. Quantitative PCR was used to analyze and compare the relative mRNA expression level for Th1, Th2, Th17, and inflammatory cytokines in both adiponectin-deficient and wild-type mice (Fig. 3a–e). The mRNA levels of inflammatory cytokines IL-1β and TNF-α (Fig. 3a), Th1 cytokine IFN-γ(Fig. 3b), and several Th17 cell-related cytokines, such as IL-6, IL-17A, IL-17F, and IL-22, were significantly increased in the lymph nodes of adiponectin-deficient EAE mice (Fig. 3d), in contrast to the Th2 cytokine IL-4(Fig. 3c). We also measured the mRNA expression level of transcription factors of different Th cells and found that, similar to the cytokine transcripts results, adiponectin deficiency increased the expression of Th1 factor T-bet and the Th17-related factors RORα, RORγt, and STAT3 (Fig. 3b, e) but did not influence the Th2 factor GATA-3(Fig. 3c). These data supported the roles of adiponectin against inflammation and activity in Th1 and Th17 cells but not Th2 cells. Furthermore, to intuitively detect the inhibitory effect of adiponectin on Th1 and Th17, PE-conjugated rat anti-mouse IL-17A and IFN-γ were used to stain the intracellular cytokines of the cells that were separated from the spleen and lymph nodes of two groups of EAE mice. In agreement with the mRNA level, adiponectin deficiency increased the key cytokine expression in Th1 and Th17 cells (Fig. 3f). Notably, the increase in IL-17A was more obvious than IFN-γ in CD4+ T cells from adiponectin knockout mice with EAE.
Increased Th1 and Th17 cells in the peripheral immune system of adiponectin KO mice with EAE. a Real-time PCR of inflammatory cytokine production in adiponectin KO and WT mice. b Real-time PCR of Th1 cell-related cytokine and transcription factor. c Real-time PCR of Th2 cell-related cytokine and transcription factor. d Real-time PCR of Th17 cell-related cytokines. e Real-time PCR of Th17 cell-related transcription factors. f Flow cytometry of IFN-γ and IL-17A in CD4+ T cells from the spleens and lymph nodes of both adiponectin KO mice and WT mice with EAE. mRNA was extracted from the lymph nodes of both adiponectin KO mice (n = 6) and WT mice (n = 6) 14 days after immunization with MOG35–55. (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test)
Adiponectin Deficiency Promoted T Cell Infiltration and Enhanced Th1 and Th17 Cytokine Expression in CNS
Because EAE is a pathogenic T cell-mediated CNS inflammatory disease, T cell infiltration in CNS is another parameter of CNS inflammation. To further confirm the compositions of the inflammatory T cell infiltrates, immunohistochemistry was used to analyze the T cell infiltration in spinal cords. In adiponectin-deficient EAE mice, there were more CD3+ and CD4+ cell infiltrations in the CNS (Fig. 4a). These infiltrations suggested that adiponectin deficiency may influence the CNS inflammation by at least partially elevating inflammatory T cell infiltration.
Increased Th1 and Th17 cell cytokines production in the spinal cords of adiponectin KO mice with EAE. a Immunohistochemistry analysis of the expression of inflammatory markers in the spinal cords of adiponectin KO mice and WT mice at the disease peak of EAE (day 17 after immunization). Sections of spinal cords from adiponectin KO EAE mice (n = 6) and WT EAE mice (n = 6) were immunostained for rabbit anti-mouse CD3 antibody and rabbit anti-mouse CD4 antibody. b Real-time PCR of Th1 cell-related cytokine and transcription factor. c Real-time PCR of Th2 cell-related cytokine and transcription factor. d Real-time PCR of Th17 cell-related transcription factors. e Real-time PCR of Th17 cell-related cytokines. The mRNA expression levels of each gene in spinal cords from adiponectin KO EAE mice (n = 6) and WT EAE mice (n = 6) were compared using real-time PCR analysis. (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test)
Th1 and Th17 cells are two key mediators of inflammation in the CNS of EAE. Based on the above results, to determine which type of effecter T cell cytokine expression was influenced in the spinal cord of EAE, spinal cords were collected for quantitative PCR analysis to compare the mRNA levels of Th1, Th2, and Th17 cell-related cytokines between adiponectin-deficient and wild-type mice. This analysis demonstrated that the expression of several cytokines, including the Th1 cell cytokine IFN-γ and Th17 cytokines IL-6, IL-17A, IL-17F, IL-22, and IL-23 receptor were elevated in the spinal cords of adiponectin-deficient EAE mice compared to their respective wild-type controls (Fig. 4b, e). Furthermore, adiponectin-deficient EAE mice also displayed a markedly increased expression of transcription factors, including T-bet, which mediated the differentiation of Th1 cells, and RORγt and STAT3, which were involved in the differentiation of Th17 cells (Fig. 4b, d). The decreased expression of Th2 cell cytokine IL-4 was observed in the spinal cords of adiponectin-deficient EAE mice, but no significant change in transcription factor GATA-3, which controlled the differentiation of Th2 cells, was observed (Fig. 4c). These data demonstrated that adiponectin deficiency increased the transcripts of inflammatory Th1 and Th17 cell cytokines but not Th2 cell cytokines and may decrease the Th2 transcripts to certain levels, suggesting that adiponectin protected CNS inflammation partially through the inhibition of Th1 and Th17 cytokine expression and/or Th1 and Th17 cell infiltration in the CNS.
Adiponectin Deficiency Promoted Antigen Specific Th17 Cell Response in EAE Mice
We next investigated whether adiponectin deficiency might influence the MOG35–55-specific proinflammatory Th1 and Th17 response in EAE. The cells of the spleen of MOG35–55-induced EAE mice were assessed for T cell proliferation and cytokine production under MOG35–55 peptide stimulation. To this end, the splenic cells were restimulated with MOG35–55 in vitro for 96 h. We first used 3H-thymidine incorporation to determine the antigen specific T cells proliferation and found that the cells from adiponectin-deficient EAE mice had a higher proliferation rate than the wild-type littermates did (Fig. 5a). This result indicated that adiponectin deficiency may enhance antigen specific T cell proliferation. However, which type(s) of T cells were more proliferated was not clear. Thus, we further detected which types of Th cell cytokines were influenced by adiponectin deficiency. The cell culture supernatant of MOG35–55-stimulated splenocytes was used for multiple cytokines assays. The adiponectin-deficient cells had higher levels of Th17 cell cytokines IL-17 and IL-6 production and inflammatory cytokine TNF-α and IL-1β production. However, the other cytokines were not increased significantly in adiponectin-deficient cells compared to the wild-type cells under MOG35–55 stimulation (Fig. 5c). After normalizing the cytokine production to cell proliferation, the level of IL-17 was still higher in adiponectin-deficient mice, suggesting that the overproduction of IL-17 was not simply a result of the proliferated cell numbers but also because of the deficiency of adiponectin (Fig. 5b). These data demonstrated that adiponectin deficiency significantly promotes the Th17 cell response and CNS inflammation by enhancing the inflammatory Th17 cell response.
Adiponectin deficiency promoted antigen-specific Th17 cell response in EAE mice. Spleen cells from adiponectin KO mice and WT mice were collected 14 days after immunization and were re-stimulated with MOG35–55 again in vitro. a Cell proliferation was assessed by incorporating 3H thymidine for the last 16 h of culture. b Relative level of IL-17 production after normalization against proliferation. c Cytokine production was evaluated using the Bio-Plex cytokine assay
Adiponectin Suppressed the Differentiation of Th17 Cells by Upregulating SIRT1 and PPARγ and Inhibiting RORγt
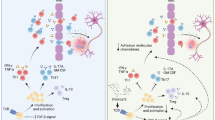
Our previous study demonstrated a dynamic interactive relationship between AMPK, activated by adiponectin and sirtuin 1 (SIRT1) in pancreatic cancer (PC) cells [30]. Therefore, we wondered whether adiponectin suppressed Th17 cells via SIRT1 and/or its downstream pathway. Jurkat cells were treated with or without exogenous adiponectin (10 μg/ml) for 24 h and were then collected to detect the key signaling regarding Th17 cell differentiation. In Fig. 6a, the results showed that adiponectin could upregulate SIRT1 and PPARγ and inhibit RORγt in vitro. RORγt is a key transcription factor in Th17 cell differentiation, and PPARγ can selectively inhibit RORγt [38]. However, the exact interaction among SIRT1, PPARγ, and RORγt in T cells is not clear. Therefore, we used shRNA to knockdown SIRT1 and detect the change in PPARγ and RORγt. As expected, SIRT1 knockdown downregulated PPARγ and upregulated RORγt (Fig. 6b). From these results, we inferred that adiponectin could inhibit Th17 cell differentiation via the SIRT1-PPARγ-RORγt pathway. Furthermore, CD4+ T cells that were separated from the spleen of adiponectin knockout and wild-type mice were used to detect SIRT1, PPARγ, and RORγt. Adiponectin deficiency could significantly decrease the level of SIRT1 and PPARγ; in contrast, RORγt was upregulated in adiponectin knockout mice with EAE (Fig. 6c). In summary, these results suggested that adiponectin could suppress the differentiation of Th17 cells by upregulating SIRT1 and PPARγ and inhibiting RORγt in vitro and in vivo.
The signaling mechanisms of adiponectin regulated Th17 cell differentiation. a Western blot of SIRT1/PPARγ/RORγt in Jurkat cells that were treated with adiponectin or not for 24 h. b Western blot of SIRT1/PPARγ/RORγt in Jurkat cells after knocking down the gene of SIRT1. c Western blot of SIRT1/PPARγ/RORγt in CD4+ T cells that were separated from the spleen of adiponectin KO mice WT mice with EAE. d Mode pattern for the effect of adiponectin on the SIRT1/PPARγ/RORγt pathway
Discussion
Many reports have demonstrated that Th cells, as vital components of the adaptive immune system, regulate immunity and inflammation [4, 5]. Notably, Th17 cells are a special proinflammatory lineage of effector/memory Th cells and have been implicated in the pathogenesis of nearly all major autoimmune syndromes [7]. Based on these, our major study about autoimmune inflammation focused on the effects of adiponectin on Th cells, especially Th17 cells.
A previous report demonstrated that, under physiological conditions, adiponectin could activate Th17 and Th1 via dendritic cell [28]. However, most studies showed that adiponectin acted as a negative modulator of the autoimmune diseases [22, 43]. Such adiponectin could inhibit skin inflammation of psoriasiform via suppressing γδ-T cells [44]. In addition, another study showed that adiponectin deficiency could exacerbate EAE due to a defect in Treg cells. Meanwhile, a lack of adiponectin led to the activation of Th1 cells. Interestingly, compared to wild-type mice, APCs of adiponectin deficient mice did not differ in supporting the proliferation of CD4+ T cells [27]. Thus, present studies suggest that adiponectin may have different, even diverse effects under different conditions, such as pathology and physiology and indicate that the roles of adiponectin in autoimmune and inflammation are controversial. Hence, the effect of adiponectin on autoimmune requires systematic study.
Therefore, we conducted thorough study to explore the potential regulatory effect of adiponectin on Th cells. In our study, adiponectin could inhibit the differentiation of Th1 and Th17 cells at both the protein and mRNA levels in vitro (Fig. 1). Then, we utilized EAE which is mainly triggered by pathogenic Th cells to explore the effects of adiponectin on Th cell subtypes. In our experience, during the induction of EAE, mice that were given PTX intraperitoneally (i.p.) would have lower clinic scores than intravenous (i.v.) injection. Thus, we gave mice PTX i.p. to better observe the difference of EAE clinic scores between WT and KO mice. As expected, EAE significantly aggravated in KO group compared with WT group (Fig. 2). Previous studies hypothesized that the differentiation of CD4+ T cells into Th1 cells, which mainly secrete IFN-γ, is crucial in the initial steps of EAE induction. In addition, the transition from Th1 cells to Th2 cells is important in protecting mice from EAE [45, 46]. With the discovery of Th17 cells and IL-17, many studies indicated that Th17 cells are the primary mediators of inflammation and demyelination in EAE [5, 47]. Although Th17 cells are considered the main pathogenic factors in EAE, these studies could not exclude the roles of Th1 cells when examining the mechanism of EAE pathogenesis [48]. Consistently, our studies also demonstrated that, when induced with EAE, in both of the peripheral immune system and the central nervous system of adiponectin-deficient mice, there was a higher expression of Th1 and Th17 cytokines. In addition, the transcription factor expression of Th1 and Th17 cells increased in adiponectin-deficient mice. However, the expression of cytokines and transcription factors in Th2 cells did not differ between the two groups of mice (Figs. 3 and 4).
As flow cytometry (Fig. 3f) demonstrated, an increase of IL-17A was more obvious than IFN-γ in CD4+ T cells from adiponectin knockout mice with EAE. In addition, in spinal cords, the site of inflammation, the activity was consistent with the situation in the peripheral immune system. Quantitative PCR showed that IL-17 cytokine and the Th17 cell-related transcription factor RORγt significantly increased compared with other factors in the spinal cords of adiponectin-deficient EAE mice than in wild-type EAE mice (Fig. 4). These results suggested that the protective role of adiponectin might primarily because of the inhibition of Th17 cells. Therefore, we isolated and cultured the spleen cells from both two groups of mice after they suffered from EAE. After restimulation with MOG35–55, the specific antigen of EAE, the level of IL-17 in the supernatant of splenocytes increased greatly in adiponectin-deficient mice, while the change in wild-type mice was relatively small. Even after normalizing the cytokine production to cell proliferation, the level of IL-17 was still much higher in adiponectin-deficient mice than in wild-type mice (Fig. 5). These results indicated that adiponectin might protect mice from autoimmune diseases by prominently suppressing Th17 cells.
Studies showed that adiponectin receptors were expressed on T cells [49], suggesting that adiponectin may directly interact with naïve T cells and affect Th17 cell differentiation. It is well known that RORγt is a key transcription factor during the differentiation of Th17 cells from naïve CD4+ T cells [50] and our study showed that the expression of RORγt was significantly increased in peripheral immune organ and CNS of adiponectin-deficient mice suffer from EAE compared with wild-type mice (Figs. 3 and 4). These suggested that adiponectin might affect Th17 cell differentiation via inhibiting the RORγt signaling pathway. Therefore, we focused on RORγt pathway and further explored the potential molecular mechanism of adiponectin-mediated Th17 cell suppression. Some studies demonstrated that PPARγ agonists could protect mice from suffering EAE, and importantly, PPARγ inhibits Th17 differentiation specifically and suppresses the central nervous system autoimmunity in EAE. Moreover, PPARγ is involved in the inhibition of the RORγt expression in T cells [38]. In addition, the expression of adiponectin could be controlled by PPARγ in both animal and human studies [34], and PPARγ agonists could upregulate the circulating adiponectin level [35–37]. Thereby, current data implied that adiponectin might influence the PPARγ/RORγt pathway and restrain the Th17 differentiation.
It is known that the signaling of AMPK can be activated by adiponectin [29]. Our previous study [30] demonstrated that in pancreatic cancer (PC) cells, there was a dynamic interaction relationship between AMPK and sirtuin 1 (SIRT1). Studies have demonstrated that SIRT1 activation could relieve EAE [51]. Thus, we further probed into the interaction between the SIRT1 and PPARγ/RORγt pathways. As expected, in this study, we found that SIRT1 knockdown could downregulate PPARγ and upregulate RORγt in T cells. Furthermore, adiponectin could upregulate SIRT1 and PPARγ and inhibit RORγt in T cells both in vitro and in vivo (Fig. 6). Interpreting the above results, adiponectin could inhibit Th17 cells via the SIRT1/PPARγ/RORγt pathway.
In conclusion, these results indicated that adiponectin inhibits pathogenic Th17 differentiation by suppressing Th17 cytokine IL-17, its associated cytokine IL-6 and the transcription factors of Th17 cells. These results suggested that adiponectin may play protective roles in inflammatory and autoimmune diseases via the inhibition of pathogenic Th17 cell differentiation.
Abbreviations
- ADN:
-
Adiponectin
- Th:
-
T helper
- CNS:
-
Central nervous system
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- IFN-γ:
-
Interferon-γ
- MS:
-
Multiple sclerosis
- EAE:
-
Experimental autoimmune encephalomyelitis
- MOG:
-
Myelin oligodendrocyte glycoprotein
- H&E:
-
Hematoxylin-eosin
- ELISA:
-
Enzyme-linked immunosorbent assay
- STAT:
-
Signal transducer and activator of transcription
- T-bet:
-
T box transcription factor
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- SIRT1:
-
Sirtuin 1
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- RORγt:
-
Retinoid-related orphan receptor-γt
References
Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA (2004) Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117:515–526
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489
Jutel M, Akdis CA (2011) T-cell subset regulation in atopy. Curr Allergy Asthma Rep 11:139–145
Coffman RL, Carty J (1986) A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol 136:949–954
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201:233–240
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133
Yang J, Sundrud MS, Skepner J, Yamagata T (2014) Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci 35:493–500
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919
Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F (2005) IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 174:3695–3702
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 cells. Annu Rev Immunol 27:485–517
Segal BM (2010) Th17 cells in autoimmune demyelinating disease. Semin Immunopathol 32:71–77
Algood HM, Allen SS, Washington MK, Peek RM Jr, Miller GG, Cover TL (2009) Regulation of gastric B cell recruitment is dependent on IL-17 receptor a signaling in a model of chronic bacterial infection. J Immunol 183:5837–5846
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, et al. (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8:500–508
Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, et al. (2009) The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature 460:405–409
Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W (2010) IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 184:4307–4316
Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783
Wolf AM, Wolf D, Avila MA, Moschen AR, Berasain C, Enrich B, Rumpold H, Tilg H (2006) Upregulation of the anti-inflammatory adipokine adiponectin in acute liver failure in mice. J Hepatol 44:537–543
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, et al. (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866
Hatano Y, Matsumoto M, Ishikawa S, Kajii E (2009) Plasma adiponectin level and myocardial infarction: the JMS cohort study. J Epidemiol 19:49–55
Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I (2006) Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 131:853–861
Parker J, Menn-Josephy H, Laskow B, Takemura Y, Aprahamian T (2011) Modulation of lupus phenotype by adiponectin deficiency in autoimmune mouse models. J Clin Immunol 31:167–173
Musabak U, Demirkaya S, Genc G, Ilikci RS, Odabasi Z (2011) Serum adiponectin, TNF-alpha, IL-12p70, and IL-13 levels in multiple sclerosis and the effects of different therapy regimens. Neuroimmunomodulation 18:57–66
Kaur S, Zilmer K, Leping V, Zilmer M (2011) The levels of adiponectin and leptin and their relation to other markers of cardiovascular risk in patients with psoriasis. J Eur Acad Dermatol Venereol 25:1328–1333
Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, et al. (2005) Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A 102:5150–5155
Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, Bloom SR, Lechler RI, et al. (2001) Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol 166:5909–5916
Piccio L, Cantoni C, Henderson JG, Hawiger D, Ramsbottom M, Mikesell R, Ryu J, Hsieh CS, et al. (2013) Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol 43:2089–2100
Jung MY, Kim HS, Hong HJ, Youn BS, Kim TS (2012) Adiponectin induces dendritic cell activation via PLCgamma/JNK/NF-kappaB pathways, leading to Th1 and Th17 polarization. J Immunol 188:2592–2601
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, et al. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503:493–499
Huang B, Cheng X, Wang D, Peng M, Xue Z, Da Y, Zhang N, Yao Z, et al. (2014) Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1alpha signaling. Oncotarget 5:4732–4745
Vachharajani VT, Liu T, Wang X, Hoth JJ, Yoza BK, McCall CE (2016) Sirtuins link inflammation and metabolism. J Immunol Res 8167273:20
Park SY, Lee SW, Kim HY, Lee SY, Lee WS, Hong KW, Kim CD (2016) SIRT1 inhibits differentiation of monocytes to macrophages: amelioration of synovial inflammation in rheumatoid arthritis. J Mol Med 9:9
Choi YH, Bae JK, Chae HS, Kim YM, Sreymom Y, Han L, Jang HY, Chin YW (2015) Alpha-Mangostin regulates hepatic steatosis and obesity through SirT1-AMPK and PPARgamma pathways in high-fat diet-induced obese mice. J Agric Food Chem 63:8399–8406
Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52:1655–1663
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, et al. (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099
Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM (2002) The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 51:2968–2974
Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ (2006) Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 291:27
Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, et al. (2009) The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med 206:2079–2089
Wong WT, Tian XY, Xu A, Yu J, Lau CW, Hoo RL, Wang Y, Lee VW, et al. (2011) Adiponectin is required for PPARgamma-mediated improvement of endothelial function in diabetic mice. Cell Metab 14:104–115
Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK (2011) Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol 11:604–609
Yang H, Zhang R, Mu H, Li M, Yao Q, Chen C (2006) Adiponectin promotes endothelial cell differentiation from human peripheral CD14+ monocytes in vitro. J Cell Mol Med 10:459–469
Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L (2002) Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277:34658–34661
Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K, Rifkin IR (2009) The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol 182:340–346
Shibata S, Tada Y, Hau C S, Mitsui A, Kamata M, Asano Y, Sugaya M, Kadono T et al (2015) Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gamma delta-T cells. Nat Commun 6
Soleimani M, Jameie SB, Mehdizadeh M, Keradi M, Masoumipoor M, Mehrabi S (2014) Vitamin D3 influence the Th1/Th2 ratio in C57BL/6 induced model of experimental autoimmune encephalomyelitis. Iran J Basic Med Sci 17:785–792
Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, et al. (1995) B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707–718
Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S, Yosef N, Vaidya VS, et al. (2015) MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest 125:1069–1080
Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM (2008) IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med 205:1535–1541
Palmer C, Hampartzoumian T, Lloyd A, Zekry A (2008) A novel role for adiponectin in regulating the immune responses in chronic hepatitis C virus infection. Hepatology 48:374–384
Dong C (2014) Targeting Th17 cells in immune diseases. Cell Res 24:901–903
Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A (2010) Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol 30:328–339
Acknowledgments
This work was supported by the Ministry of Science and Technology of China through Grant No. 2012CB932503; the National Natural Science Foundation of China through Grants No. 91029705, 81172864, 81272317, 81302568, 81301026, and 31402097.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The care and treatment for mice were approved by Animal Ethics Committee of Tianjin Medical University and were in accordance with guidelines for animal care.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Kai Zhang and Yawei Guo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, K., Guo, Y., Ge, Z. et al. Adiponectin Suppresses T Helper 17 Cell Differentiation and Limits Autoimmune CNS Inflammation via the SIRT1/PPARγ/RORγt Pathway. Mol Neurobiol 54, 4908–4920 (2017). https://doi.org/10.1007/s12035-016-0036-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0036-7