Abstract
Sporadic Creutzfeldt–Jakob disease (sCJD) is the most frequent fatal human prion disease with a rapid progression and unknown mechanism. The synaptic vesicle (SV) cycle pathway has been a hot research field associated with many neurodegenerative diseases that affect synaptic function and thus may affect pathogenesis of the disorder. Here, we used the iTRAQ-based proteomic method and a KEGG pathway enrichment analysis to meticulously analyze all pathways involved in sCJD disease. In total, 1670 proteins were validated in pooled cerebrospinal fluid (CSF) from 20 patients with sCJD compared with that from 13 patients without CJD. The demographic analysis demonstrated that 557 proteins were upregulated and 595 proteins were downregulated with a 1.5-fold change, and 690 proteins involved in 39 pathways changed significantly (p ≤ 0.05) according to the enrichment analysis. The SV cycle pathway and proteins involved were further evaluated, and 14 proteins were confirmed to participate in the SV cycle pathway due to increased expression. Six key proteins, such as AP2A1, SYT1, SNAP25, STXBP1, CLTB, and Rab3a, showed the same trend by western blot as detected by iTRAQ. This is the first study to use high-throughput proteomics to accurately identify and quantify proteins in the SV cycle pathway of a neurodegenerative disease. These results will help define the mechanism and provide new insight into the pathogenetic factors involved in the SV cycle pathway in patients with sCJD. We hope that promising biomarkers can be identified in the CSF of patients with sCJD and other neurodegenerative disorders to help predict disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creutzfeldt–Jakob disease (CJD) is a lethal, rapidly progressing, and transmissible neurodegenerative disease of the central nervous system (CNS) [1] associated with the presence of a misfolded prion protein isoform. CJD is in the group of transmissible spongiform encephalopathies called human prion diseases [2]. The three CJD variations are called sporadic, hereditary, and acquired CJD. Among them, sporadic CJD (sCJD) is the most frequent condition with unclear mechanism [3]. Mean age of onset is 60 years but the range is relatively wide with no sex bias. The clinical course is usually <2 years, and most patients die within 1 year after onset [4, 5]. A definitive diagnosis of sCJD requires pathological confirmation in the CNS and detection of spongiform degeneration and/or prion protein (PrP)Sc in brain tissues [6]. However, biopsy is unlikely to be performed for the clinical diagnosis. Although cerebrospinal fluid (CSF) has a low protein concentration (nearly 0.4 mg/ml), the constant exchange between the CNS and peripheral blood can provide data on CNS status and thus has become the optimal sample source to evaluate neurodegenerative diseases [7].
The composition of synaptic vesicles (SVs) has been thoroughly studied, as SVs are essential for propagating nerve impulses through synapses. SVs undergo biogenesis, membrane fusion, and docking to release neurotransmitters and follow a continuous cycle [8]. Presynaptic terminals play an important role in cognitive reserve by balancing the effects of age-related pathologies on cognitive function [9], and the membranes and proteins are subsequently recycled by endocytosis. Emerging evidence indicates that presynaptic dysfunction may be an early event in neurodegeneration [10–12]. The exocytosis and endocytosis processes involved in the SV cycle are regulated and require the coordinated interactions of numerous proteins and protein–protein relevant cycles, such as assembly and disassembly of the soluble N-ethylmaleimide-sensitive fusion protein attachment receptor (SNARE) complex; MUNC18-1 (STXBP), which plays important roles in neurosecretion; Ras-related protein Rab-3A (Rab3A); and synaptotagmin 1 (SYT1), which has important roles mediating SV exocytosis [13]; and the clathrin/AP2 complex, which modulates endocytosis. Therefore, the fidelity of neurotransmitter function relies partly on SV use and recycling at each synapse, as well as the information flow distributed across many nerve terminals [14]. The SV cycle and its associated proteins have been implicated in neurodegenerative diseases, as they exhibit an immense effect on function or the disease mechanism [15, 16]. Thus, the SV cycle significantly affects neurotransmitter release, and a disrupted SV cycle may affects synaptic function and the pathogenesis of sCJD.
Isobaric tags for relative and absolute quantitation (iTRAQ) combined with multidimensional liquid chromatography (LC) and mass spectroscopic (MS) analysis is a high-throughput, highly stable method with no sample restrictions that is used widely in proteomics [17]. Identifying biological pathways is one of the most important results of this approach. Understanding all pathway mechanisms is common in these analyses, and a single disease can consist of ≥120 biological pathways [18, 19]. In this study, we quantified 1670 proteins and detected at least 2 unique peptides, 596 upregulated proteins, and 557 downregulated proteins with 1.5-fold changes in expression. A Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis confirmed 703 biological pathways after enrichment, and 39 pathways were significant (p < 0.05). We performed a sub-network-based analysis on the SV cycle pathway (ko04721), which was highly expressed in the analysis. The SV cycle serves as the main cell-to-cell communication method in the CNS and is a multistep, tightly regulated mechanism described by vesicle exocytosis, vesicle fusion with the plasma membrane, and release of the vesicular endosome to exert various functions. For example, release of neurotransmitters from neurons mediates synaptic transmission, which is essential for brain and neuron function [20]. Excess demands on neurotransmitter release and the SV cycle ultimately result in synaptic dysfunction [21]. Thus, the SV cycle and its differentially expressed proteins, SNAP25, RAB3A, MUNC18–1, SYT1, and clathrin/AP2, exert huge effects on synaptic function, play an important role in the sCJD mechanism, and may be potential biomarkers.
Experimental Procedures
Sample Preparation and Experimental Design
All CSF samples and the study protocol were approved by the Research Ethics Committee of the National Institute for Viral Disease Control and Prevention, China, CDC. All patients signed informed consent forms before samples were collected. Twenty CSF samples were collected from patients with sCJD (age, 40–79 years; mean, 60.15 years), and 13 were collected from patients without CJD (age, 44–76 years; mean, 58.23 years) during surgeries performed from October 2014 to March 2015. All samples were stored at −80 °C until use. The CSF samples were separated into 100-ml aliquots (three tubes/sample) before freezing to minimize the effect of freeze–thaw cycles and to facilitate performing multiple assays. The patients with sCJD included males and females diagnosed by the China CJD Surveillance Center according to the WHO CJD diagnostic criteria described previously [22] and were followed for later treatment. The patients without CJD included males and females; two had epilepsy, one had viral encephalitis due to an unknown pathogen, one had meningoencephalitis, one had limbic encephalitis, one had neuritis, and seven had unknown diagnoses. All samples were confirmed free of blood contaminants, as blood contamination can lead to false-positive protein 14-3-3 results [23]. All sCJD samples in our experiment tested positive for protein 14-3-3. The samples with and without sCJD were pooled and divided into three separate groups to eliminate interpatient variations irrelevant to sCJD. A 60-μl aliquot of each CSF sample was pooled to produce a single analytical sample of 400 μl for each subgroup. The clinical symptoms of each group are summarized in Table 1, and the experimental design is shown as a workflow diagram (Fig. 1).
The workflow of the designed study used for searching important pathway associated with Creutzfeldt–Jakob disease discovery. Sample preparation for Itraq-ESI-MS/MS and important steps in the analysis of proteomic data for the detection of extremely differently expressed pathway. For each experiment on CSF samples, iTRAQ tags were assigned to a biological replicated reference sCJD samples (pooled and divided into three groups: tagged with 118,119 and 121, respectively) and non-CJD samples (tagged with 113, 114, 116) .The same pattern was repeated in all 10 experiments. The data analysis flow chart depicted in this figure is described in the “Experimental Procedures” section
Sample Preparation
The 400-μl sample aliquots from both patient groups were placed in 3KD ultrafiltration tubes, centrifuged at 14,000×g for 15 min; the filtrate was discarded, and 8 M urea was added. These steps were repeated three times. The samples were homogenized in an extraction buffer containing 4 % SDS, 1 mM DTT, and 150 mM Tris–HCl (pH 8). After 3-min incubation in boiling water, the homogenate was sonicated on ice. The crude extract was incubated in boiling water again and clarified by centrifugation at 16,000×g at 25 °C for 10 min. Protein quantity was measured using the bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL, USA). A 200-μg portion of protein from each sample was incorporated into 30-μl STD buffer (4 % SDS, 100 mM DTT, and 150 mM Tris–HCl, pH 8.0). The detergent, DTT, and other low molecular-weight components were removed using UA buffer (8 M urea and 150 mM Tris–HCl, pH 8.0) by repeated ultrafiltration (30 kD Microcon; Millipore Corp., Billerica, MA, USA) Then, 100 μL of 0.05 M iodoacetamide in UA buffer was added to block reduction of cysteine residues, and the samples were incubated for 20 min in the dark. The filters were washed three times with 100-μl UA buffer and twice with 100-μl DS buffer (50 mM trimethylammonium bicarbonate, pH 8.5). The samples were subjected to an overnight trypsin treatment, using a trypsin-to-protein ratio of 1:100 depending on the quantitative results.
iTRAQ Labeling and Strong Fractionation by Cation Exchange Chromatography
Protein peptides (100 μg) from each group were labeled using the 8plex iTRAQ reagents multiplex kit (ABI, Foster City, CA, USA) (isobaric tags 113, 114, and 116 for the group without CJD and isobaric tags 118, 119, and 121 for the sCJD group). The 8plex iTRAQ reagents were allowed to reach room temperature, centrifuged, and reconstituted with 50-μl isopropyl alcohol to dissolve the iTRAQ labeling reagent. The tubes were vortexed and centrifuged. The iTRAQ labeling reagents were added to the corresponding peptide samples and reacted at room temperature for 1 h. A 100-μl aliquot of water was added to stop the labeling reaction. A 1-μl aliquot of the sample was removed from each group to test labeling and extraction efficiency, and the sample was subjected to a matrix-assisted laser desorption ionization procedure after Ziptip desalting. The six sample groups were pooled and vacuum-dried. Each pool of mixed peptides was lyophilized and dissolved in solution A (2 % acetonitrile (ACN) and 20 mM ammonium formate, pH 10). Then, the samples were loaded onto a reversed-phase column (Luna C18, 4.6 × 150 mm; Phenomenex, Torrance, CA, USA) and eluted using a step linear elution program: 0–10 % buffer B (500 mM KCl, 10 mM KH2PO4 in 25 % ACN, pH 2.7) for 10 min, 10–20 % buffer B for 25 min, 20–45 % buffer B for 5 min, and 50–100 % buffer B for 5 min at a flow rate of 0.8 ml/min. The samples were collected each min and centrifuged for 5–45 min. The fractions (about 40) collected were finally combined into ten pools and desalted on C18 cartridges (Empore™ standard density SPE C18 cartridges, bed I.D. 7 mm, 3 ml volume; Sigma, St. Louis, MO, USA).
LC–Electrospray Ionization–MS/MS Analysis
The reconstituted peptides were analyzed with the Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled with a nano high-performance liquid chromatography (UltiMate 3000 LC Dionex; Thermo Fisher Scientific) system. The peptides were loaded onto a C18-reversed-phase column (3 μm-C18 resin, 75 μm × 15 cm) and separated on an analytical column (5 μm C18 resin, 150 μm × 2 cm; Dr. Maisch GmbH, Ammerbuch, Germany) using mobile phase A: 0.5 % formic acid (FA)/H2O and B 0.5 % FA/ACN at a flow rate of 300 nl/min, using a 150-min gradient. Spectra were acquired in data-dependent mode. The ten most intense ions were selected for MS scanning (300–1800 m/z, 60,000 resolution at m/z 400, accumulation of 1 × 106 ions for a maximum of 500 ms, 1 microscan). The isolation window was 1.3 m/z, and the MS/MS spectra were accumulated for 150 ms using an Orbitrap. MS/MS spectra were measured at resolution of 15,000 at m/z 400. Dynamic precursor exclusion was allowed for 120 s after each MS/MS spectrum measurement and was set to 17,500 at m/z 200. Normalized collision energy was 30 eV and the underfill ratio, which specifies the minimum percentage of the target value likely to be reached at the maximum fill time, was defined as 0.1 %. The instrument was run with peptide recognition mode enabled.
Sequence Database Search and Data Analysis
The raw mass data were processed for the peptide data analysis using Proteome Discoverer 1.4 (ver. 1.4.0.288; Thermo Fisher Scientific) with a false discovery rate (FDR = N(decoy)*2/((N(decoy) + N(target))) <1 % and expected cutoff or ion score <0.05 (with 95 % confidence) for searching the Uniprot Human Complete Proteome database. Protein probabilities were assigned using the Protein Prophet algorithm [24], and proteins with at least two unique peptides were identified. The upregulated or downregulated proteins in both replicates with relative quantification p values <0.05 and 1.5-fold changes were selected as being differentially expressed in the data. The following options were used to identify the proteins: peptide mass tolerance = ±10 ppm, MS/MS tolerance = 0.6 Da, enzyme = trypsin, missed cleavage = 2, fixed modification: iTRAQ8plex (K) and iTRAQ8plex (N-term), variable modification: oxidation (M), database pattern = decoy.
Bioinformatics and Pathway Analyses
We performed a gene ontology (http://www.geneontology.org, GO) bioinformatics analysis on the differentially expressed proteins with a 1.5-fold change to catalog the molecular functions, cellular components, and biological processes. The interactions among these proteins regarding the biological pathways were determined using Pathway Studio software and the ResNet database to better understand these differentially expressed proteins in relation to the published literature. The Pathway Maps tool was used to enrich the pathways, and p values were calculated based on a hypergeometric distribution, with the default database used as the background. Significant pathway enrichment was defined as a corrected FDR of p ≤ 0.05, and proteins with ≥1.5-fold change were considered differentially abundant proteins. The pathway enrichment analysis of the proteins was conducted with information from the Kyoto Encyclopedia of Genes and Genome (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html) using the following formula [25]:
where N is the number of all proteins within the KEGG pathway annotation information, n is the number of differentially regulated proteins with KEGG pathway annotation information, M is the number of proteins for a given KEGG pathway annotation, and m is the number of differentially regulated proteins following a given KEGG pathway annotation. KEGG pathways with p values ≤0.05 were considered enriched KEGG pathways for patients with sCJD vs. those without.
Identification of Differentially Expressed Proteins by Western Blotting
The samples were added to an equal volume of 2× loading buffer and boiled for 10 min at 100 °C, separated by SDS-polyacrylamide gel electrophoresis, and transferred to a hydrophobic polyvinylidene difluoride membrane (Hybond-P PVDF membrane; GE Healthcare, Amersham Biosciences, Pittsburgh, PA, USA) according to standard protocols. The membranes were blocked for 1 h with 5 % nonfat dry milk in TBST at room temperature and incubated overnight at 4 °C on a shaker with the following primary antibodies: anti-SNAP25 (ab109105, Abcam, Cambridge, MA, USA); anti-STXBP (ab109023; Abcam); anti-Rab3A, (ab3335; Abcam) anti-SYT1 (14,511–1-AP); anti-clathrin (10,852–1-AP); and anti-AP2A1 (11,401–1-AP) diluted 1:5000 in TBST. As clathrin has three component chains and AP2 consists of three subunits that are expressed similarly, we randomly chose one isoform of each protein for identification. The membranes were washed three times with 30-ml TBST for 10 min each and incubated 1 h in horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (Abcam) diluted 1:1000 in PBST at room temperature. Then, the membranes were washed three times with 30-ml PBST for 10 min each. Enhanced chemiluminescence (Clarity™ Western ECL Substrate, cat. no. 170–5060; Bio-Rad Laboratories, Hercules, CA, USA) and the western blotting detection system (Amersham Biosciences) were used to detect the target proteins in the dark, according to the manufacturer’s instructions.
Densitometric and statistical analyses were performed with Quantity One 4.6.2 (Bio-Rad) and SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL, USA), respectively. Differential expression of a protein was considered significant when the p value was <0.05 according to Student’s t test.
Results
Identification of peptides and proteins in CSF of the groups with and without sCJD. The pooled CSF samples from both groups lead to the identification of 10,078 peptides with FDR <0.1 % at the peptide level (Supplemental Table S1), including 1917 proteins originally identified within a 1 % global FDR and with high confidence of the correct peptide sequence assignment (Supplemental Table S2). After correction within the three replicate groups, 1670 proteins were finally identified (Supplemental Table S3).
Functional classifications of the identified proteins. A total of 1152 proteins (1.5-fold change differentially expressed proteins) were functionally annotated using Protein Center software. The three main annotation types were cellular components, molecular functions, and biological process (Fig. 2a–c). The GO slim approach, which is defined specifically for a high-throughput protein database, reduced the multiple GO annotations to a set of 20 easily manageable high level terms that were used to search the database and were transformed into percentages for evaluation. All 1152 differentially expressed proteins were classified into 34 functional groups (Fig. 2d), of which cellular components possessed 8 GO terms, the most representative was “cell part”; molecular function possessed 11 GO terms, the most representative was “catalytic activity and binding”; and biological process possessed 15 GO terms, the most representative was “metabolic process and cellular processes.” The ontology analysis of the proteins identified the associations and diversity of molecular functions, such as catalytic activity (32.30 %) and receptor activity (28.50 %). Most of the proteins identified were involved in metabolism (22.10 %) or cellular processes (20.00 %). The data in Fig. 2d support the numbers of differentially expressed proteins in the three main annotation types. Finally, a large number of identified proteins were localized to the cell (31.20 %) and extracellular space (17.20 %), as the samples were CSF with differences in normal subcellular fractionation.
Classification of enriched pathway for KEGG pathway characteristics (p ≤ 0.05). a Upregulated and downregulated proteins are divided into three groups: differentially expressed proteins with 1.2-fold change, differentially expressed proteins with 1.5-fold change, and differentially expressed proteins with 2.0-fold change. b All 1.5-fold change proteins are categorized and with enrichment analysis into 39 pathways
Molecularly enriched pathways associated with differentially expressed proteins in the groups with and without sCJD. To further identify the biological pathways of the differentially expressed proteins, the total number of proteins expressed and their fold changes are shown in Fig. 3a. The demographic analysis demonstrated that 96 proteins were upregulated and 301 proteins were downregulated with a 2.0-fold change; 557 proteins were upregulated and 595 proteins were downregulated with a 1.5-fold change; and 835 proteins were upregulated and 835 proteins were downregulated with a 1.2-fold change (Fig. 3a). We confirmed that 557 upregulated proteins and 595 downregulated proteins were obtained using the criteria of no less than a 1.5-fold change; the former participated in 1509 biological pathways and the latter participated in 284 biological pathways (Supplemental Table S4: 1.5 fold-change of differentially expressed proteins in the KEGG pathway analysis). An enrichment analysis was carried out for the upregulated and downregulated proteins with at least a 1.5-fold change to determine the affected pathways. The analysis was performed by matching the differentially expressed proteins to the proteins annotated with the KEGG pathway database, and the frequencies of the differentially expressed proteins in the sCJD group for each KEGG pathway were compared to statistically determine their involvement in that KEGG pathway. As a result, 39 KEGG pathways were critically affected, with p values <0.05 (Fig. 3b) (Table 2).
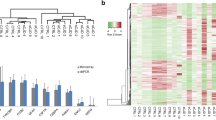
Hierarchical clustering analysis of the enriched pathway and proteins involved in the synaptic vesicle cycle pathway. Hierarchical clustering using Cluster 3.0 software for the proteins identified in the enrichment analysis within the 39 enriched pathways yielded six major sample clusters. A total of 690 proteins that satisfied the criteria and presented a mean 1.5-fold expression change or greater were classified as significantly downregulated or upregulated in sCJD disease were included in the analysis. The samples without CJD were labeled 113 (1), 114 (1), and 116 (1), and the sCJD samples were labeled 118 (2), 119 (2), and 121 (2). A heat map analysis was performed for the differentially expressed proteins among the three biological replicates between the two groups. The log10 value of the MS signal intensity is shown beside the heat map with the gradient (Fig. 4a). We also prepared a small heat map of the SV cycle pathway using hierarchical clustering. The accessions to the left of the pathway heat map represent the 14 proteins involved in the SV cycle pathway. The samples without sCJD are labeled 113 (1), 114 (1), and 116 (1), and the sCJD samples are labeled 118 (2), 119 (2), and 121 (2). The heat map shows no differences between the two groups. Mean fold changes ranged from 1.7 to 4.3. (Fig. 4b). Fourteen proteins were involved in the SV cycle pathway and were differentially expressed in the two groups: AP2 (O95782, 1.9-fold change; Q96CW1, 2.1-fold change; and O94973, 3.0-fold change); RAB3A (P20336,4.9-fold change); ATP (P21283, 2.8-fold change; Q16864, 1.7-fold change; and Q9UI12, 2.1-fold change); clathrin (Q00610, 2.1-fold change; P09496, 1.8-fold change; and P09497, 1.9-fold change); SNAP25 (P60880, 1.8-fold change), SYT1 (J3KQA0, 2.3-fold change); complexin-2 (Q6PUV4, 3.9-fold change); and Munc18-1 (P61764, 2.0-fold change).
Heat map showing levels of differentially expressed proteins for which relative quantitative values were obtained for all 20 sCJD and 13 non-CJD cases (n = 690). a Hierarchical clustering of sCJD cases based on the relative expression of these proteins generated two clusters comprising 20 cases of sCJD and 13 cases of non-CJD, respectively. b Heat map of proteins in the synaptic vesicle cycle pathway, proteins in other groups tagged with 114, 116, 118, 119, and 121 were severely antithesis with the group tagged with 113
Schematic diagram illustrating the synaptic vesicle cycle pathway and the proteins involved. The SV cycle pathway was one of the most significantly upregulated protein-enriched pathways (Fig. 5). The proteins involved and essential to this pathway included AP2, RAB3A, ATP, clathrin, SNAP25, SYT1, complexin-2, and MUNC18-1 and were all differentially expressed. All of these proteins were expressed at significantly higher levels in the sCJD group than in the group without CJD. These results suggest that patients with sCJD have hypernomic synaptic transmitter transport performance. Among these proteins, SYT1, RAB3A, SNAP25, and MUNC18-1 participate in exocytosis, whereas clathrin and AP2 are important for the endosomes involved in endocytosis. The pathway is shown in Fig. 5, and the proteins participating in this process in excess are marked in red.
Schematic summary of the synaptic vesicle cycle pathway and key proteins involved differentially expressed proteins. Budding: transmitters first filling the synaptic vesicle by active transport. Docking: vesicles filled with transmitters docked at the active zone. Priming: full-filling vesicles make themselves competent for Ca2+-triggered fusion-pore opening. Fusion: SYT1 mediate transmitters pouring out from the vesicles into synaptic cleft. Endocytosis: vesicles recycle via clathrin/AP2-mediated endocytosis and with recycling via endosomes. SNAP25, SYT1, MUNC18-1, Rab3A, clathrin, and AP2 marked with red square are the key proteins involved in the synaptic vesicle cycle pathway. Exocytosis steps are indicated by red arrows and endocytosis steps and recycling by green arrows
Identification of proteins essential for the synaptic vesicle cycle pathway. A western blot analysis further confirmed the results obtained in CSF. The samples from the patients in the two groups were pooled and subsequently divided into three subgroups to verify the changes in abundance of the six key proteins (SYT1, RAB3A, SNAP25, MUNC18-1, clathrin, and AP2) involved in the SV cycle pathway. As a result, SYT1, RAB3A, SNAP25, MUNC18-1, clathrin, and AP2 expression levels were clearly higher in the sCJD group than in the group without sCJD (Fig. 6a).
Western blots and quantitative analysis of six upregulated proteins in the pooled CSF samples of sCJD and non-CJD. a Same aliquot of CSF from 20 sCJD cases or 13 non-CJD cases were taken and pooled separately. Twenty microliters of each pooled CSF sample was loaded in 10 % SDS-PAGE confirmed the sCJD disease upregulation of SNAP25, SYT1, MUNC18-1, Rab3A, clathrin, and AP2; n = 3 (Student’s t test; mean ± SD; *p ≤ 0.05). Representative blots of the tested protein with predicted molecular weight is presented. All tested proteins were shown with the corresponding total amount of protein used for normalization. b Quantitative analysis of each of the tested protein’s relative fold change with predicted molecular black numerical value weight versus that of their mean protein concentrations, respectively. The average values were calculated for three times and presented as mean ± SD; Student’s t test; *p < 0.05; ** p < 0.01
The quantitative analysis of relative fold-changes on each blot after normalization to individual protein concentrations in the CSF samples (350 ± 8 mg/l for the sCJD and 440 ± 10 mg/l for control group) and normalization to the control group showed significantly increased levels of Rab3A, 1 AP2, SYT1, SNAP25, STXBP, and clathrin in the sCJD group compared with those in the control group. These results agree with those of the iTRAQ experiment (Fig. 6b).
Discussion
The SV cycle, including presynaptic nerve terminal release of neurotransmitters by exocytosis and endocytosis by the membrane is essential for continuous neuronal communication, and the participating proteins are continuously recycled [16, 26]. Exocytotic release of neurotransmitters from SVs contributes to nervous system function [27], and the SVs are retrieved from the plasma membrane by endocytosis to complete the next exocytosis cycle; both of these processes are key rate-limiting steps in the SV cycle [28]. Significant dysfunction in this process contributes to lethal consequences due to impaired neuronal communication [29]. Figure 5 shows the SV cycle process and the proteins involved. Accumulating evidence suggests that the SV cycle is associated with many neurodegenerative diseases, including Parkinson’s disease and sCJD [16, 30]. A disturbance in synaptic transmission, such as an imbalance between excitatory and inhibitory synapses, affects the pathogenesis of seizures and epilepsy [31]. A properly functioning SV cycle meets the demands for synaptic transmission of complex information originating within the brain [32] and avoids depleting local vesicle stores during synaptic transmission due to profound failure [33]. Greater demands on neurotransmitter release and the SV pool could ultimately result in synaptic dysfunction [21]. SYT1, RAB3A, SNAP25, Munc18-1, clathrin, and AP2 are the key proteins modulating exocytosis and endocytosis within the SV cycle (Fig. 5).
RAB3A and SYTI mediate SV exocytosis with opposite actions but cooperatively play critical regulatory roles in vesicle exocytosis at central synapses. SYT1 modulates neurotransmitter release; calcium-dependent exocytosis is mediated by synaptotagmins, which act as calcium sensors [34], and exert an important role in spontaneous exocytosis of SVs. Some studies have reported that SYT1 plays an antagonistic role for MUNC18-1 in SNARE zippering and opening pores [35] and provide the final impetus for complete assembly of the SNARE complex to fuse to the membrane [36, 37]. One study confirmed that an on-and-off switch regulates the association between Rab3 and the membrane to facilitate repeated release [38]. SYT1 was first identified in CSF from patients with Alzheimer’s disease (AD), who show a concomitant decrease in brain SYT1 expression. Overexpression of SYT1 positively regulates short-term synaptic plasticity at developing neuromuscular junctions [39]. RAB3A is one of the most important proteins in the Ras superfamily of small (21–25 kDa) GTP-binding proteins and participates in regulating internal trafficking, exocytosis, neurotransmission, and vesicle endocytosis. RAB3A regulates the last stages of calcium-dependent exocytosis downstream of vesicle docking [40] and is believed to select and limit the number of SVs released at a central synapse. RAB3A is the most abundant Rab3 isoform and competes with the SYTI interaction with syntaxin 1B during synaptic vesicle exocytosis [13, 41]. MUNC18-1 (also known as STXBP1) is an evolutionally conserved neuronal Sec1/MUNC-18 protein primarily expressed in the brain that plays a critical role in SV release in several species. MUNC18-1 is a key regulator of neurosecretory function [42]. It also acts as a chaperone to initiate SNARE assembly, which is essential for membrane fusion [43]. The SNARE-binding protein complexin bidirectionally regulates spontaneous release of SVs [44] during fusion, acts as a fusion hub to prevent premature exocytosis in the absence of calcium [45], and regulates the timing and properties of SYT1 activity [46]. SNAP-25 is a part of the SNARE complex that helps transport vesicles containing neurotransmitters to the inner plasma membrane during synaptic exocytosis [47] with an important role of regulating intracellular calcium dynamics and possibly network excitability [48]. All of these proteins are involved in exocytosis during the SV cycle. Clathrin and AP2 are both important for endocytosis, and their expression levels changed significantly in our study. Clathrin is located at the synaptic plasma membrane to retrieve the SV membrane and regenerate SVs at some synapses under particular physiological conditions. Most studies have shown that clathrin is pivotal for endocytosis at hippocampal synapses, even though the ultrafast endocytosis process is clathrin independent. [49] The AP2 adaptor complex is the major clathrin-associated adaptor that recognizes cargo at the plasma membrane. AP2 is thought to be a molecular bridge for most cells and biological processes by interacting with the cytoplasmic tails of cell surface transmembrane proteins and participating in the molecular machinery of the clathrin coat, thus helping concentrate specific cargo molecules into endocytic pits [50]. AP2 has also been described as a “pivotal” protein central to clathrin-mediated endocytosis, as the interactions between AP2 and proteins are generally considered the primary aspect of the endocytic machinery [51].
Proteins within the SV cycle pathway are also associated with other neurodegenerative diseases. An abnormal accumulation of α-synuclein/rab3a binding is found in the cerebellum, pons, and cerebral cortex of patients with multiple system atrophy (MSA), suggesting that SV trafficking is a vulnerable target in MSA [52], and increased binding of RAB3A to α-synuclein in cases of Lewy bodies (LBs) also suggests that the exocytosis of neurotransmitters may be destroyed in LB diseases [53]. Previous studies have reported that some sCJD-related proteins are involved in signal transduction and neuronal activity and present as an accumulation of a membrane-associated form of RAB3A, suggesting that RAB3A-mediated exocytosis is a dysfunction of sCJD pathology and could contribute to the cognitive impairment of patients suffering from sCJD [54]. A SYT1 mutation in humans and mice causes presynaptic disorders, disrupts neurotransmission, and negatively affects functional neurodevelopment, resulting in a range of movement disorders and mental deficiencies [55]. SYT1 expression tends to decrease in the hypothalamus and cortex of aged mice [56] but was upregulated in our analysis.
Increased MUNC18-1 expression results in a larger readily releasable vesicle pool, leading to increased synaptic endurance and faster recovery from fatigue [57]. MUNC18-1 mutations have been associated with epileptic encephalopathy, movement disorders, and intellectual disabilities. MUNC18-1 is associated with cognitive function in old age and may contribute to protecting against dementia related to age-associated pathologies as well. A postmortem proteomic analysis of the prefrontal cortex in patients with schizophrenia showed that MUNC18-1 is upregulated in gray matter membrane microdomains [58]. Increased MUNC18-1 expression induces consecutive behaviors that correlate with hyperlocomotion, decreased social interaction, and cognitive deficits in mutant mice, which are similar to patients with schizophrenia [59]. SNARE proteins may participate in the pathogenesis of schizophrenia by underlying dysfunctional neuroexocytosis [60], and both reduced and excessive SNAP-25 expression have been reported in the CSF of patients with attention deficit disorder, schizophrenia, and AD. SNAP-25 is also an important marker of functional synapses [61]. Overexpression of SNAP-25 in cultured hippocampal neurons is related to impaired synaptic transmission [62]. Sharma et al. identified SNAP25 as a neuroprotective target regulated by other chaperones.
Some studies have suggested that clathrin-coated pits and vesicles are involved in the generation of PrPsc; thus, inhibiting clathrin-mediated endocytosis of PrPc might play a therapeutic role inhibiting replication of PrP [63]. Clathrin and AP2 complex with amyloid precursor protein (APP) in patients with AD, which is essential for correct APP trafficking and avoidance of endolysosomal defects and neuronal dysfunction [64]. Whether clathrin/AP-2 plays the same role in sCJD pathogenesis requires further research.
In this study, the expression of all the proteins involved in the SV cycle pathway increased, whereas other studies have shown that the expressions of SNAP-25 and RAB3A decrease in the cerebral cortex and cerebellum in patients with sCJD [30, 65], and increased synaptic protein expression is observed in Purkinje cell axons [30]. We wondered whether the same proteins are expressed differently in different tissues or in different diseases. Epileptic seizure causes changes in neuronal distribution of MUNC18-1, as the protein decreases in the nuclear fraction but increases in the cytoplasmic fraction [66]. Expression of SNAP-25 increases in the CSF of patients with schizophrenia and AD; however, expression of the SNAP25 complex decreases in the frontal cortex of patients with Huntington’s disease [67]. Some studies have detected neurodegenerative disease-related changes in protein accumulation, aggregation, and function, which may contribute to imbalanced synaptic function [68]. The mechanism of differentially expressed proteins in different diseases or in the same disease but different tissues requires in-depth study.
In our study, we used the high-throughput iTRAQ-LC-MS/MS proteomic method to identify differentially expressed proteins in patients with sCJD and discussed the SV cycle pathway and the proteins involved. These data may help in the understanding of the mechanism of the SV cycle and the key proteins involved in neurodegenerative diseases, particularly in sCJD, and provide clues for screening potential biomarkers to make an early diagnosis, assess disease progression, and monitor the effects of drugs during sCJD treatment trials. The SV cycle and protein expression analysis provides an initial approach to study inborn errors of neurotransmitters. In particular, this study may provide novel insight into the pathophysiology of neuronal communication in these disorders. Our results reflect the importance of the SV cycle pathway and the proteins involved in sCJD. The SV cycle pathway plays a crucial role in the expression of sCJD and is closely associated with its pathogenesis; thus, further research is needed.
Abbreviations
- sCJD:
-
Sporadic Creutzfeldt–Jakob disease
- CSF:
-
Cerebrospinal fluid
- SV:
-
Synaptic vesicle
- CNS:
-
Central nervous system
- SNARE:
-
Soluble N-ethylmaleimide-sensitive fusion protein attachment receptor
- SNAP-25:
-
Synaptosomal-associated protein of 25 kDa
- SYTI:
-
Synaptotagmin1
- iTRAQ:
-
Isobaric tags for relative and absolute quantitation
- LC:
-
Liquid chromatography
- AD:
-
Alzheimer’s disease
- LB:
-
Lewy body
References
Chen C, Xiao D, Zhou W, Shi Q, Zhang H, Zhang J, Tian C, Zhang J, Dong X (2014) Global protein differential expression profiling of cerebrospinal fluid samples pooled from Chinese sporadic CJD and non-CJD patients. Mol Neurobiol 49(1):290–302. doi:10.1007/s12035-013-8519-2
Steinacker P, Rist W, Swiatek-de-Lange M, Lehnert S, Jesse S, Pabst A, Tumani H, von Arnim CA, Mitrova E, Kretzschmar HA, Lenter M, Wiltfang J, Otto M (2010) Ubiquitin as potential cerebrospinal fluid marker of Creutzfeldt–Jakob disease. Proteomics 10(1):81–89. doi:10.1002/pmic.200900246
Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11(7):618–628. doi:10.1016/S1474-4422(12)70063-7
Brown P, Cathala F, Castaigne P, Gajdusek DC (1986) Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol 20(5):597–602. doi:10.1002/ana.410200507
Zhang XM, Xiao K, Zhou W, Chen C, Lv Y, Chen LN, Shi Q, Dong XP (2014) Analysis of the compliance and the related influence factors in the follow-up process of surveillance for Creutzfeldt-Jakob disease in China. Prion 8(5):359–368. doi:10.4161/19336896.2014.983747
Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K, et al. (1995) Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 5(4):459–466
Dislich B, Wohlrab F, Bachhuber T, Muller SA, Kuhn PH, Hogl S, Meyer-Luehmann M, Lichtenthaler SF (2015) Label-free quantitative proteomics of mouse cerebrospinal fluid detects beta-site APP cleaving enzyme (BACE1) protease substrates in vivo. Mol Cell Proteomics 14(10):2550–2563. doi:10.1074/mcp.M114.041533
Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490(7419):201–207. doi:10.1038/nature11320
Ramos-Miguel A, Hercher C, Beasley CL, Barr AM, Bayer TA, Falkai P, Leurgans SE, Schneider JA, Bennett DA, Honer WG (2015) Loss of Munc18-1 long splice variant in GABAergic terminals is associated with cognitive decline and increased risk of dementia in a community sample. Mol Neurodegener 10:65. doi:10.1186/s13024-015-0061-4
Gray BC, Siskova Z, Perry VH, O’Connor V (2009) Selective presynaptic degeneration in the synaptopathy associated with ME7-induced hippocampal pathology. Neurobiol Dis 35(1):63–74. doi:10.1016/j.nbd.2009.04.001
Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J (2009) Presenilins are essential for regulating neurotransmitter release. Nature 460(7255):632–636. doi:10.1038/nature08177
Burgoyne RD, Morgan A (2011) Chaperoning the SNAREs: a role in preventing neurodegeneration? Nat Cell Biol 13(1):8–9. doi:10.1038/ncb0111-8
Geppert M, Sudhof TC (1998) RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci 21:75–95. doi:10.1146/annurev.neuro.21.1.75
Schweizer FE, Ryan TA (2006) The synaptic vesicle: cycle of exocytosis and endocytosis. Curr Opin Neurobiol 16(3):298–304. doi:10.1016/j.conb.2006.05.006
Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, Sudhof TC (2004) The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron 42(2):237–251
Esposito G, Ana Clara F, Verstreken P (2012) Synaptic vesicle trafficking and Parkinson’s disease. Dev Neurobiol 72(1):134–144. doi:10.1002/dneu.20916
Liu J, Pang C, Wei H, Song M, Meng Y, Ma J, Fan S, Yu S (2015) iTRAQ-facilitated proteomic profiling of anthers from a photosensitive male sterile mutant and wild-type cotton (Gossypium hirsutum L.). J Proteome 126:68–81. doi:10.1016/j.jprot.2015.05.031
Chen LN, Shi Q, Zhang BY, Zhang XM, Wang J, Xiao K, Lv Y, Sun J, Yang XD, Chen C, Zhou W, Han J, Dong XP (2015) Proteomic analyses for the global S-nitrosylated proteins in the brain tissues of different human prion diseases. Mol Neurobiol. doi:10.1007/s12035-015-9440-7
Shi Q, Chen LN, Zhang BY, Xiao K, Zhou W, Chen C, Zhang XM, Tian C, Gao C, Wang J, Han J, Dong XP (2015) Proteomics analyses for the global proteins in the brain tissues of different human prion diseases.MOL. Cell Proteomics 14(4):854–869. doi:10.1074/mcp.M114.038018
Wu LG, Hamid E, Shin W, Chiang HC (2014) Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol 76:301–331. doi:10.1146/annurev-physiol-021113-170305
Chapman MA (2014) Interactions between cell adhesion and the synaptic vesicle cycle in Parkinson’s disease. Med Hypotheses 83(2):203–207. doi:10.1016/j.mehy.2014.04.029
Lanska DJ (2001) Diagnosis of Creutzfeldt-Jakob disease: effect of clinical criteria on incidence estimates. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 56(10):1422–1423
Schmitz M, Ebert E, Stoeck K, Karch A, Collins S, Calero M, Sklaviadis T, Laplanche J, Golanska E, Baldeiras I, Satoh K, Sanchez-Valle R, Ladogana A, Skinningsrud A, Hammarin A, Mitrova E, Llorens F, Kim YS, Green A, Zerr I (2015) Validation of 14-3-3 protein as a marker in sporadic Creutzfeldt-Jakob disease diagnostic. Mol Neurobiol. doi:10.1007/s12035-015-9167-5
Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75(17):4646–4658
Zhang M, Luo SC (2016) Gene expression profiling of epithelial ovarian cancer reveals key genes and pathways associated with chemotherapy resistance. Genet Mol Res 15(1). doi:10.4238/gmr.15017496
Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P (2008) Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol 182(5):1007–1016. doi:10.1083/jcb.200804162
Kononenko NL, Haucke V (2015) Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron 85(3):484–496. doi:10.1016/j.neuron.2014.12.016
Fernandez-Alfonso T, Ryan TA (2006) The efficiency of the synaptic vesicle cycle at central nervous system synapses. Trends Cell Biol 16(8):413–420. doi:10.1016/j.tcb.2006.06.007
Rizzoli SO (2014) Synaptic vesicle recycling: steps and principles. EMBO J 33(8):788–822. doi:10.1002/embj.201386357
Ferrer I (2002) Synaptic pathology and cell death in the cerebellum in Creutzfeldt-Jakob disease. Cerebellum 1(3):213–222. doi:10.1080/14734220260418448
Casillas-Espinosa PM, Powell KL, O’Brien TJ (2012) Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia 53(Suppl 9):41–58. doi:10.1111/epi.12034
Gottmann K (2008) Transsynaptic modulation of the synaptic vesicle cycle by cell-adhesion molecules. J Neurosci Res 86(2):223–232. doi:10.1002/jnr.21484
Koenig JH, Ikeda K (1989) Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci 9(11):3844–3860 PMID:2573698
Sudhof TC (2013) A molecular machine for neurotransmitter release: synaptotagmin and beyond. Nat Med 19(10):1227–1231. doi:10.1038/nm.3338
Lou X, Shin J, Yang Y, Kim J, Shin YK (2015) Synaptotagmin-1 is an antagonist for Munc18-1 in SNARE zippering. J Biol Chem 290(16):10535–10543. doi:10.1074/jbc.M114.631341
Vrljic M, Strop P, Ernst JA, Sutton RB, Chu S, Brunger AT (2010) Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nat Struct Mol Biol 17(3):325–331. doi:10.1038/nsmb.1764
Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR (2010) Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat Struct Mol Biol 17(3):318–324. doi:10.1038/nsmb.1763
Cheng Y, Wang J, Wang Y, Ding M (2015) Synaptotagmin 1 directs repetitive release by coupling vesicle exocytosis to the Rab3 cycle. ELIFE 4. doi:10.7554/eLife.05118
Morimoto T, Wang XH, Poo MM (1998) Overexpression of synaptotagmin modulates short-term synaptic plasticity at developing neuromuscular junctions. Neuroscience 82(4):969–978
Coleman WL, Bykhovskaia M (2010) Cooperative regulation of neurotransmitter release by Rab3a and synapsin II. Mol Cell Neurosci 44(2):190–200. doi:10.1016/j.mcn.2010.03.007
Xie C, Li J, Guo T, Yan Y, Tang C, Wang Y, Chen P, Wang X, Liang S (2014) Rab3A is a new interacting partner of synaptotagmin I and may modulate synaptic membrane fusion through a competitive mechanism. Biochem Bioph Res Co 444(4):491–495. doi:10.1016/j.bbrc.2014.01.090
Han GA, Malintan NT, Collins BM, Meunier FA, Sugita S (2010) Munc18-1 as a key regulator of neurosecretion. J Neurochem 115(1):1–10. doi:10.1111/j.1471-4159.2010.06900.x
Ma L, Rebane AA, Yang G, Xi Z, Kang Y, Gao Y, Zhang Y(2015) Munc18-1-regulated stage-wise SNARE assembly underlying synaptic exocytosis. ELIFE 4. doi: 10.7554/eLife.09580
Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE (2009) Alternative zippering as an on-off switch for SNARE-mediated fusion. Science 323(5913):512–516. doi:10.1126/science.1166500
Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323(5913):516–521. doi:10.1126/science.1166505
Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT (2012) Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J Neurosci 32(50):18234–18245. doi:10.1523/JNEUROSCI.3212-12.2012
Wilson MC, Mehta PP, Hess EJ (1996) SNAP-25, enSNAREd in neurotransmission and regulation of behaviour. Biochem Soc Trans 24(3):670–676
Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, Proux-Gillardeaux V, Galli T, Rossetto O, Frassoni C, Matteoli M (2004) SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41(4):599–610
Watanabe S, Trimbuch T, Camacho-Perez M, Rost BR, Brokowski B, Sohl-Kielczynski B, Felies A, Davis MW, Rosenmund C, Jorgensen EM (2014) Clathrin regenerates synaptic vesicles from endosomes. Nature 515(7526):228–233. doi:10.1038/nature13846
Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447. doi:10.1146/annurev.biochem.72.121801.161800
Rodemer C, Haucke V (2008) Clathrin/AP-2-dependent endocytosis: a novel playground for the pharmacological toolbox? Handb Exp Pharmacol 186:105–122. doi:10.1007/978-3-540-72843-6_5
Dalfó E, Ferrer I (2005) ?-synuclein binding to rab3a in multiple system atrophy. Neurosci Lett 380(1–2):170–175. doi:10.1016/j.neulet.2005.01.034
Dalfo E, Barrachina M, Rosa JL, Ambrosio S, Ferrer I (2004) Abnormal alpha-synuclein interactions with rab3a and rabphilin in diffuse Lewy body disease. Neurobiol Dis 16(1):92–97. doi:10.1016/j.nbd.2004.01.001
Gawinecka J, Cardone F, Asif AR, De Pascalis A, Wemheuer WM, Schulz-Schaeffer WJ, Pocchiari M, Zerr I (2012) Sporadic Creutzfeldt-Jakob disease subtype-specific alterations of the brain proteome: impact on Rab3a recycling. Proteomics 12(23–24):3610–3620. doi:10.1002/pmic.201200201
Baker K, Gordon SL, Grozeva D, van Kogelenberg M, Roberts NY, Pike M, Blair E, Hurles ME, Chong WK, Baldeweg T, Kurian MA, Boyd SG, Cousin MA, Raymond FL (2015) Identification of a human synaptotagmin-1 mutation that perturbs synaptic vesicle cycling. J Clin Invest 125(4):1670–1678. doi:10.1172/JCI79765
Jiang CH, Tsien JZ, Schultz PG, Hu Y (2001) The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci U S A 98(4):1930–1934. doi:10.1073/pnas.98.4.1930
Toonen RF, Wierda K, Sons MS, de Wit H, Cornelisse LN, Brussaard A, Plomp JJ, Verhage M (2006) Munc18-1 expression levels control synapse recovery by regulating readily releasable pool size. Proc Natl Acad Sci U S A 103(48):18332–18337. doi:10.1073/pnas.0608507103
Behan AT, Byrne C, Dunn MJ, Cagney G, Cotter DR (2009) Proteomic analysis of membrane microdomain-associated proteins in the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder reveals alterations in LAMP, STXBP1 and BASP1 protein expression. Mol Psychiatry 14(6):601–613. doi:10.1038/mp.2008.7
Uriguen L, Gil-Pisa I, Munarriz-Cuezva E, Berrocoso E, Pascau J, Soto-Montenegro ML, Gutierrez-Adan A, Pintado B, Madrigal JL, Castro E, Sanchez-Blazquez P, Ortega JE, Guerrero MJ, Ferrer-Alcon M, Garcia-Sevilla JA, Mico JA, Desco M, Leza JC, Pazos A, Garzon J, Meana JJ (2013) Behavioral, neurochemical and morphological changes induced by the overexpression of munc18-1a in brain of mice: relevance to schizophrenia. Transl Psychiatry 3:e221. doi:10.1038/tp.2012.149
Johnson RD, Oliver PL, Davies KE (2008) SNARE proteins and schizophrenia: linking synaptic and neurodevelopmental hypotheses. Acta Biochim Pol 55(4):619–628
Thompson PM, Kelley M, Yao J, Tsai G, van Kammen DP (2003) Elevated cerebrospinal fluid SNAP-25 in schizophrenia. Biol Psychiatry 53(12):1132–1137
Owe-Larsson B, Berglund M, Kristensson K, Garoff H, Larhammar D, Brodin L, Low P (1999) Perturbation of the synaptic release machinery in hippocampal neurons by overexpression of SNAP-25 with the Semliki Forest virus vector.EUR. J Neurosci 11(6):1981–1987
Shyng SL, Heuser JE, Harris DA (1994) A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol 125(6):1239–1250
Poulsen E, Larsen A, Zollo A, Jørgensen A, Sanggaard K, Enghild J, Matrone C (2015) New insights to clathrin and adaptor protein 2 for the design and development of therapeutic strategies. Int J Mol Sci 16(12):29446–29453. doi:10.3390/ijms161226181
Ferrer I, Rivera R, Blanco R, Marti E (1999) Expression of proteins linked to exocytosis and neurotransmission in patients with Creutzfeldt-Jakob disease. Neurobiol DIS 6(2):92–100
Zhang YP, Wan P, Wang HQ, Zhao H, Xu YX, Yang R, Zhu CQ (2011) Effect of neuronal excitotoxicity on Munc18-1 distribution in nuclei of rat hippocampal neuron and primary cultured neuron. Neurosci Bull 27(3):163–172. doi:10.1007/s12264-011-1007-7
Smith R, Klein P, Koc-Schmitz Y, Waldvogel HJ, Faull RL, Brundin P, Plomann M, Li JY (2007) Loss of SNAP-25 and rabphilin 3a in sensory-motor cortex in Huntington’s disease. J Neurochem 103(1):115–123. doi:10.1111/j.1471-4159.2007.04703.x
Marttinen M, Kurkinen KM, Soininen H, Haapasalo A, Hiltunen M (2015) Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol Neurodegener 10:16. doi:10.1186/s13024-015-0013-z
Acknowledgments
We thank Xiaoping Dong, Qi shi, and Wei zhou for their assistance with the sample preparation. This study was supported by the Ministry of Agriculture of China, 948 projects (2014-S9), and the Foundation of Chinese Ministry of Science and Technology (project no. 2015BAI07B02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All CSF samples and the study protocol were approved by the Research Ethics Committee of the National Institute for Viral Disease Control and Prevention, China, CDC. All patients signed informed consent forms before samples were collected.
Additional information
Chunyu Wang and Deming Zhao are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, C., Zhao, D., Shah, S.Z.A. et al. Proteome Analysis of Potential Synaptic Vesicle Cycle Biomarkers in the Cerebrospinal Fluid of Patients with Sporadic Creutzfeldt–Jakob Disease. Mol Neurobiol 54, 5177–5191 (2017). https://doi.org/10.1007/s12035-016-0029-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0029-6