Abstract
Mitogen-activated protein kinases (MAPKs) are expressed in postmitotic neurons and act as important regulators in intracellular signaling. In addition to their nuclear distribution and roles in regulating gene expression, MAPKs, especially the extracellular signal-regulated kinase (ERK) subclass, reside in peripheral dendritic spines and synapses, including the postsynaptic density (PSD) microdomain. This peripheral pool of MAPKs/ERKs is either constitutively active or sensitive to changing synaptic input. Active MAPKs directly interact with and phosphorylate local substrates to alter their trafficking and subcellular/subsynaptic distributions, through which MAPKs regulate function of substrates and contribute to long-lasting synaptic plasticity. A number of physiologically relevant substrates of MAPKs have been identified at synaptic sites. Central among them are key synaptic scaffold proteins (PSD-95 and PSD-93), cadherin-associated proteins (δ-catenin), Kv4.2 K+ channels, and metabotropic glutamate receptors. Through a reversible phosphorylation event, MAPKs rapidly and efficiently modulate the function of these substrates and thus determine the strength of synaptic transmission. This review summarizes the recent progress in cell biology of synaptic MAPKs and analyzes roles of this specific pool of MAPKs in regulating local substrates and synaptic plasticity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mitogen-activated protein kinase (MAPK) family is a large group of serine/threonine protein kinases [1]. These kinases are activated via a characteristic cascade involving consecutive and sequential events at four levels: Ras/Rac GTPases, MAPK kinase kinases (Raf or MEKK), MAPK kinases (MEK), and MAPKs. Ras and Rac are anchored to the inner surface of the plasma membrane. They are activated (from GDP- to GTP-bound) by Ras-guanine nucleotide exchange factors (RasGEFs), including Sos and Ras-guanine nucleotide releasing factors (RasGRFs, a neuron-specific set of RasGEFs), and are inactivated (from GTP- to GDP-bound) by their intrinsic GTPase activity and GTPase activating proteins (GAPs). Once activated, Ras and Rac act as a switch to transmit extracellular or intracellular signals to the rest of MAPK cascades. The prototypic MAPK subclass is extracellular signal-regulated kinases (ERK). Among different isoforms (ERK1/2/3/4/5/7), ERK1/2 have drawn the most attention and have been most extensively studied. Two additional subclasses of MAPKs are the c-Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPK) and p38 MAPKs [2]. All MAPKs share the basic biochemical properties such as binding to a similar domain and phosphorylating a common proline-directed motif (S/TP) [3]. Nevertheless, MAPK subclasses are heterogeneous in upstream activators, downstream substrates, the amino acid sequence around binding and phosphorylation sites, and thus physiological roles.
MAPKs are primarily distributed in the cytoplasm. Upon phosphorylation by MEKs (i.e., activation), MAPKs translocate to the nucleus to phosphorylate a discrete set of transcription factors which in turn regulate gene expression related to cellular growth, differentiation, and survival in mammalian proliferative cells [1]. MAPKs are also expressed in postmitotic neurons of adult mammalian brains. Activation of these brain MAPKs in a cytosolic pool triggers the transcription-dependent regulation of synaptic transmission and plasticity [4–6]. In addition to the cytoplasmic and nuclear distribution, ERK and JNK are notably present in neuronal peripheral structures, such as dendritic spines and synapses [7–10]. ERK, especially ERK2, coexists with all MAPK cascade components within the postsynaptic density (PSD) microdomain [11, 12]. Thus, MAPKs are not limited to the long-lasting regulation of gene expression in the nucleus. They also act locally to regulate synaptic activities.
Given the ultrastructural location of MAPKs in synapses, local substrates of MAPKs were deemed to exist. In fact, several synaptic proteins have been identified as sufficient substrates of MAPKs and have been individually characterized in details. These substrates include scaffolding proteins (PSD-95 and PSD-93), cadherin-associated proteins (δ-catenin and plakophilin 4, PKP4), Kv4.2 potassium channels, and group I metabotropic glutamate receptors (mGluR1 and mGluR5 subtypes). MAPKs (mainly ERK and JNK) through phosphorylating specific serine and threonine residues in these proteins control their trafficking and synaptic delivery and thus determine the strength and efficacy of excitatory synapses. This review summarized the progress of studies on the synaptic species of MAPKs and discussed each individual substrate of MAPKs in the synaptic zone in their interactions with MAPKs.
Localization of MAPKs in Synapses
MAPKs are certainly expressed in postmitotic neurons of adult human and animal brains. While a large amount of MAPKs usually resides in the cytoplasm and nucleus, a significant fraction of MAPKs also situates within peripheral dendritic spines and synapses. This was consistently shown in different brain regions investigated. In a subcellular fractionation study, enriched ERK1/2 proteins were found in the synaptosomal fraction, while nuclear ERK1/2 were about one third of those in homogenates, in the rat frontal cortex and pons/medulla [7]. In a biochemical procedure that can selectively isolate synapse- and extrasynapse-enriched membranes, ERK1/2 were found at both synaptic and extrasynaptic sites in the rat striatum with more ERK1/2 proteins in the extrasynaptic location [10]. The similar synaptic distribution of ERK1/2 was seen in the prefrontal cortex [10]. In addition to ERK, JNK (JNK1, 2, and 3 isoforms) was visible within synapses in rat striatal and cortical neurons [13].
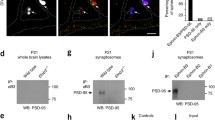
An isoform gradient in terms of ERK1 versus ERK2 abundance in the synaptic location is noteworthy. A higher level of ERK2 existed at synaptic sites as opposed to a smaller amount of ERK1 at these sites in the rat striatum and prefrontal cortex, while the two isoforms showed no obvious isoform gradient at extrasynaptic sites [10]. Similarly, a high ERK2 to ERK1 ratio exhibited in the synaptic plasma membrane of rat hippocampal neurons [11]. Moreover, ERK2 seemed to be the only isoform that was detected in the PSD microdomain [11]. Within the PSD, ERK2 coexisted with all the MAPK cascade components as detected in purified PSD fractions and in immunohistochemical analysis at the electron microscopic level (Fig. 1) [12].
Coexistence of the Ras-ERK cascade components in the PSD fraction. ERK2 is enriched in the PSD microdomain of neurons and is activated via a prototypic cascade. Ras which is anchored to the inner surface of the plasma membrane is activated by the Shc-Grb2-Sos1 pathway or RasGRF. Active Ras then sequentially activates Raf which activates MEK to activate ERK2. Activated ERK2 interacts with and phosphorylates a set of local substrates to modulate synaptic transmission and plasticity
Synaptic MAPKs are constitutively active. An example is the detection of a high level of dually phosphorylated ERK (pERK), an active state of ERK, in synaptic fractions in the rat striatum and prefrontal cortex under normal conditions [10, 13]. Similarly, basal pERK immunostaining was present in dendritic spines and synapses in pyramidal neurons of the rat visual cortex as well as in presynaptic axonal boutons forming connections with dendritic spines of pyramidal neurons [8]. Like pERK, active JNK was localized in striatal and cortical synapses [13]. Of note, the pERK2 to pERK1 ratio was higher than the ERK2 to ERK1 ratio. In fact, pERK2 signals were predominant while pERK1 signals were minimal at synaptic sites under basal conditions [10].
Synaptic MAPKs were highly responsive to changing synaptic input. Visual stimulation induced a rapid increase in the number of pERK-labelled synapses in the rat visual cortex [8]. Contextual fear conditioning activated ERK in a pool associated with synapses in addition to a nuclear pool in mouse hippocampal neurons [9]. Dopamine stimulation with an indirect dopamine receptor agonist amphetamine increased ERK2 but not ERK1 phosphorylation in the synaptic but not extrasynaptic compartment in the rat striatum and prefrontal cortex [10]. Thus, synaptic MAPKs represent a class of kinases that are readily regulated in an activity-dependent manner.
Synaptic Scaffolds
Given the presence of MAPKs in synaptic structures, specific substrates of MAPKs are reasoned to coexist locally. In fact, MAPK-phosphorylated proteins as detected using an antibody against the consensus MAPK phosphorylation motif were highly enriched in the PSD of rat brains, while Akt-phosphorylated proteins were not [14]. A JNK inhibitor (SP600125) broadly reduced the phosphorylation signals in cultured hippocampal neurons, indicating a role of JNK in this event. The MAPK-mediated phosphorylation of PSD proteins changed rapidly in response to altered synaptic activity. Many MAPK-phosphorylated proteins were concentrated in the core of the PSD, similar to the distribution pattern of local scaffold proteins. Indeed, a following mass spectrometry analysis identified several well-known PSD scaffolds, including PSD-95 (also known as synapse-associated protein 90, SAP-90), Chapsyn-110, SAP-associated protein 4 (SAPAP4), Shank3, and Homer1 [14], as potential targets of MAPKs. PSD-95 is the best studied member of the membrane-associated guanylate kinase (MAGUK) family and is a key multimeric scaffold for clustering receptors, ion channels, and signaling proteins in the PSD microdomain. Its phosphorylation by MAPKs has been individually investigated and confirmed in a separate study [15]. As described in this early study, p38γ bound to the third PDZ (PSD-95/Discs-Large/ZO-1) domain of PSD-95, which enabled the kinase to phosphorylate PSD-95 at T287 and S290 in vitro and S290 in cells in response to cellular stresses. PSD-95 phosphorylation was insensitive to the inhibition of p38α and p38β, but was abolished by an interaction-dead peptide that disrupted the interaction between p38γ and PSD-95, establishing the isoform-selective role of p38γ. ERK2 also phosphorylated PSD-95 at T287 and S290 residues, which, unlike the p38γ-mediated phosphorylation, was independent of PDZ-binding. The mitogen-induced PSD-95 phosphorylation appears to be mediated by ERK. In neurons, p38γ formed complexes with PSD-95 in synaptic fractions. These results support PSD-95 as a sufficient biochemical substrate of p38γ and ERK, although whether and how MAPKs regulate PSD-95 function is less clear.
PSD-93 is another PDZ-containing scaffold. Like PSD-95, PSD-93 interacts with a large number of synaptic proteins to support and organize synaptic networks and thus participates in the control of synaptic strength and efficacy [16]. PSD-93 is believed to be regulated by a phosphorylation-dependent mechanism. In support of this, serine and threonine phosphorylation was evident in PSD-93 proteins based on several phosphoproteomic studies [17–19], although responsible protein kinases catalyzing PSD-93 phosphorylation have not been identified in those studies. Noticeably, PSD-93 contains multiple proline-directed MAPK phosphorylation motifs (S/TP), suggesting the possibility that PSD-93 might be phosphorylated by a proline-directed kinase. In an effort to identify PSD-93-associated kinases, Guo et al. [20] indeed have found that recombinant ERK2 but not JNK directly bound to PSD-93 in vitro. In rat striatal neurons, native ERK from synaptosomal fractions was associated with PSD-93. Active ERK2 phosphorylated PSD-93 at a serine site (S323). This phosphorylation is believed to be constitutively active. These results indicate that synaptic PSD-93 is likely a substrate of local ERK. Thus, synaptic ERK can regulate synaptic transmission via PSD-93 in addition to PSD-95.
Cadherin-Associated Proteins
δ-catenin (i.e., δ-2-catenin) is a member of a cadherin-associated protein family. It is expressed specifically in neurons where it is important for cadherin-mediated cell-to-cell adhesion related to synaptic plasticity, learning and memory, and normal cognitive function [21, 22]. A mass spectrometry study has identified δ-catenin as one of possible substrates of MAPKs in the rat brain PSD [14]. In the same study, δ-catenin was then individually investigated for its regulation by MAPKs. In details, in in vitro assays, ERK2 and JNK1 both strongly phosphorylated the immunoprecipitated δ-catenin proteins. In real neurons from rat brains, endogenous δ-catenin was phosphorylated at a proline-directed site (S447) as detected by a phospho- and site-specific antibody against S447-phosphorylated δ-catenin. The S447-phosphorylated δ-catenin was enriched in the PSD fraction under normal conditions and was reduced by the JNK inhibitor (SP600125) but not the MEK/ERK inhibitor (PD98059). Thus, JNK is the main mediator of basal S447 phosphorylation. Of note, δ-catenin S447 phosphorylation was subjected to the activity-dependent regulation. Enhancing activity of cultured neurons with the GABAA receptor antagonist bicuculline dephosphorylated S447, while silencing activity with the sodium channel blocker tetrodotoxin increased S447 phosphorylation. This JNK-mediated increase in S447 phosphorylation targeted δ-catenin for proteasomal degradation, leading to reduction of total δ-catenin levels. Furthermore, mutation of S447 to alanine increased dendritic branching in hippocampal cultures, indicating the role of S447 phosphorylation in the dendrite branching function. Perhaps, reducing S447 phosphorylation limits JNK-mediated degradation of δ-catenin and thereby enhances the dendritic branching.
Plakophilin 4 (PKP4) is another cadherin-associated protein containing multiple proline-directed phosphorylation sites. It was phosphorylated by ERK2 and JNK1 in vitro [14]. Thus, PKP4 could be a putative MAPK substrate in the PSD location. Cadherin-mediated adhesion and/or signaling are critical for morphological and functional plasticity of synapses [23]. It is thus conceivable that synaptic MAPKs through phosphorylating and regulating cadherin-associated proteins participate in the modulation of synaptic structures and activities.
Kv4.2 K+ Channels
The Kv4.2 K+ channel encodes a transient A-type K+ current in the dendrites of hippocampal CA1 pyramidal and other neurons. These currents exert a profound influence over synaptic network activity and plasticity [24]. Kv4.2 is densely localized to distal dendrites [25], placing the channel as a potential target of local ERK. Indeed, pharmacological studies show that (1) the MEK inhibitors (PD98059 and U0126) enhanced Kv4.2 currents recorded in hippocampal and spinal dorsal horn neurons, indicating a basal inhibition of Kv4.2 channels by ERK, and (2) ERK linked protein kinase A (PKA) and protein kinase C (PKC) to Kv4.2, resulting in the inhibition of Kv4.2 currents [26, 27]. Complementary to these pharmacological results, biochemical studies reveal that Kv4.2 was phosphorylated by ERK [28, 29]. The phosphorylation occurred in the cytoplasmic C-terminus (CT) of the pore-forming α-subunit at three residues (T602, T607, and S616). Site-directed mutagenesis studies discovered that ERK phosphorylation at these residues significantly altered biophysical properties and channel function. In details, ERK elicited the electrophysiological changes in neurons: a rightward shift of the activation curve and an overall reduction in the Kv4.2-mediated outward K+ current, which is mimicked by the site-directed mutation at T607, changing the threonine to aspartate (T607D) to mimic phosphorylation. Thus, direct phosphorylation of Kv4.2 at T607 mediates the ERK inhibition of the channel. This regulation is believed to result from changes in gating kinetics of Kv4.2 currents since ERK phosphorylation had no effect on protein expression or surface membrane localization. Interestingly, the ERK phosphorylation sites in Kv4.2 do not always have a similar effect. The S616D mutation induced an effect opposite to that seen in the T607D mutation. This dual regulation underscores the complex of the Kv4.2 regulation by ERK. Alternatively, these ERK-sensitive phosphorylation sites may also be sensitive to other MAPKs. Multiple MAPKs can work in concert to precisely control the response of the channel to a given stimulus.
Group I mGluRs
Group I mGluRs (mGluR1/5) are G protein-coupled receptors (GPCR). These receptors activate phospholipase Cβ1 to enhance phosphoinositide hydrolysis, which yields diacylglycerol and inositol-1,4,5-triphosphate (IP3) to trigger Ca2+ and PKC signaling pathways, respectively [30]. Group I mGluRs are expressed in broad brain regions and are vigorously involved in the regulation of neuronal activities and excitatory synaptic transmission [30]. As opposed to other groups of mGluRs, group I mGluRs are mostly peri- and postsynaptic [31, 32] and act as key regulators in the PSD microdomain. Moreover, the postsynaptic localization provides mGluR1/5 with the spatial opportunity to interact with synaptic protein kinases, including MAPKs.
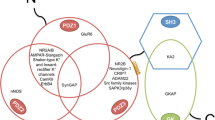
Indeed, mGluR1/5 have recently been found to be targets of ERK. mGluR1/5, as typical GPCRs, have an intracellular CT. The long-form splice variants (mGluR1a, mGluR5a, and mGluR5b) are characterized by particularly long CT tails. Through the CT domain, mGluR1/5 are readily accessible to various submembranous interacting partners [33–35]. Protein kinases, e.g., PKC and Ca2+/calmodulin-dependent protein kinase II, are among mGluR1/5-associated regulators. These kinases interact with and phosphorylate mGluR1/5 CT and thereby regulate subcellular distribution of mGluR1/5 and their functions [36–39]. The proline-directed kinase joins the mGluR1/5-associated kinase family given the existence of multiple S/TP phosphorylation motifs in the distal CT region of these receptors. Indeed, both cyclin-dependent kinase 5 and ERK were found to phosphorylate a common serine site within the homer-binding domain (−PPSPF-) conserved in mGluR1a (S1154) and mGluR5 (S1126) [40, 41]. This phosphorylation seemed to enhance the mGluR5 interaction with the homer EVH1 domain [40] in an activity-dependent manner [41]. In cultured cortical neurons, the serine phosphorylation of immunoprecipitated mGluR5 was reduced by U0126, indicating the existence of basal and ERK-dependent phosphorylation of mGluR5 [41]. In a remarkable model recently discovered by Park et al. [42], the dopamine D1 receptor agonist or brain-derived neurotrophic factor (BDNF) activated ERK1/2, leading to inducible and reversible phosphorylation of mGluR5 at S1126 in striatal neurons as detected by a phospho- and site-specific antibody (Fig. 2). This dopamine- and BDNF-induced mGluR5 phosphorylation was sensitive to the MEK/ERK inhibitor U0126 [42].
ERK links dopamine and BDNF signals to mGluR5. Stimulation of dopamine D1 receptors (D1R) or BDNF stimulation of TrkB activates ERK in cultured striatal neurons. Active ERK then phosphorylates a serine residue (S1126) on C-terminal tails of mGluR5. This forms an ERK-sensitive heterologous signaling pathway to activity-dependently modulate mGluR5 function
mGluR5 S1126 phosphorylation seems to be an essential element in enduring mGluR5 and behavioral plasticity in response to chronic psychostimulant exposure. Administration of the psychostimulant cocaine increased mGluR5 S1126 phosphorylation in the striatum of adult mice [42]. This cocaine-sensitive phosphorylation of mGluR5 S1126 is important for behavioral plasticity, i.e., behavioral sensitization to cocaine, based on the finding that motor sensitization to cocaine was markedly reduced in mice that express mutant mGluR5 which could not be phosphorylated at S1126 [42]. Mechanistic studies show that the ERK-mediated mGluR5 S1126 phosphorylation facilitated Pin1-mediated prolyl-isomerization of mGluR5. This led to potentiation of mGluR5 activity in triggering NMDA receptor-mediated currents, which underlies synaptic plasticity and cocaine-evoked motor sensitization [42].
Other Synaptic Substrates
In addition to the aforementioned substrates, other synaptic proteins have been found to have a close relationship with local MAPKs. Among these proteins is the AMPA receptor which is deemed to be regulated by the Ras family of small GTPases (Ras, Rap1, and Rap2) and their downstream MAPKs [43]. Different MAPK pathways seem to exert different impacts on AMPA receptor trafficking and thus differentially regulate distinct forms of synaptic plasticity. The Ras-dependent ERK enhanced phosphorylation of AMPA receptors with long CT (GluA2L at S841) and drove synaptic delivery of GluA2L AMPA receptors during long-term potentiation, whereas the Rap1-dependent p38 MAPK enhanced phosphorylation of AMPA receptors with only short CT (GluA2/3) and mediated removal of GluA2/3 AMPA receptors during long-term depression [43–46]. ERK and p38 were unlikely to directly phosphorylate AMPA receptors. Other kinases downstream to ERK and p38 are thought to link respective ERK and p38 to AMPA receptors [43]. In addition, the Rap2-activated JNK mobilized protein phosphatase 2B to dephosphorylate AMPA receptors with long CT (GluA1 and GluA2L) and triggered synaptic removal of these receptors. Of note, Ras and Rap were able to control AMPA receptor trafficking and synaptic plasticity in individual synapses without affecting different types of synapses spatially separated by <1 μm [47]. Thus, Ras and Rap can precisely control synaptic plasticity in a synapse-specific manner.
In addition to the postsynaptic zone, the presynaptic end is another working site for MAPKs. Visual stimulation rapidly increased pERK immunoreactivity in axon terminals of visual cortical neurons, suggesting a role of ERK played at the presynaptic level [8]. The presynaptic substrates of MAPKs have been less studied. One possible substrate of this kind is synapsin I, a phosphoprotein that regulates the synaptic vesicle binding to actin cytoskeleton and modulates transmitter release. MAPKs stoichiometrically phosphorylated synapsin I at S62, S67, and S549 [48]. This phosphorylation significantly reduced the synapsin I binding to the actin cytoskeleton system. Thus, MAPKs by affecting synapsin I regulate the release machinery in the axonal boutons and control transmitter release and presynaptic plasticity [49].
Conclusions
It is certain that MAPKs act at both nuclear and synaptic sites. Biochemical and functional studies focusing on the synaptic pool of MAPKs have discovered a number of physiologically relevant substrates of MAPKs in the local synaptic location. These substrates are directly or indirectly phosphorylated by MAPKs and transmit the MAPK signals to the regulation of synaptic transmission and plasticity. However, it is noticed that the number of synaptic substrates identified so far is far less than the number of ERK-dependent targets in the nucleus [50, 51]. The total of synaptic substrates known at present is also much less than the number suggested by mass-spectrometric analysis [14]. Thus, more synaptic substrates are expected to be identified in future studies. In addition to the posttranslational modification, MAPKs may participate in local protein synthesis. Translation of mRNA residing in dendrites and synapses occurs and plays a critical role in synaptic plasticity [52]. Activation of local MAPKs may accommodate the need of new protein synthesis at potentiated synapses for structural changes in relation to long-term synaptic plasticity. In support of this notion, an inducible dendritic protein synthesis was abolished by U0126 [53]. The studies on this topic are very limited. Future studies will examine the presence of the MAPK-associated translational machinery within the dendritic and synaptic locus and the precise role of MAPKs in local new protein synthesis and long-lasting synaptic plasticity. Whether synaptic MAPKs translocate to the nucleus to exert a remote role is another interesting question. An attractive scenario is that synaptic MAPKs, once activated in response changing synaptic input, rapidly traffic to the nucleus to regulate gene expression, which transcription-dependently modulates synaptic activities. This spatiotemporal model defines the activation of MAPKs at the synaptic site as an initial step that cross-talks with the nucleus and determines long-term changes in the synapses containing activated MAPKs. At present, the available evidence for and against this model is limited.
The phosphorylation-dependent modulation needs to be further investigated in many aspects. For instance, changes in protein ultrastructures derived from phosphorylation need to be defined and characterized in details. These changes constitute the structural basis for an altered state of the interaction of phosphorylated proteins with their interacting partners. In addition, each MAPK subclass possesses the ability to phosphorylate multiple synaptic substrates. A single synaptic protein is often phosphorylated by multiple subclasses of MAPKs or even by various protein kinases (PKA, PKC, etc.). Intimate crosstalk is believed to take place among these kinases in phosphorylating synaptic targets and needs to be investigated in depth. Similarly, phosphorylation can be studied together with other types of posttranslational modifications given that phosphorylation often coexists with other posttranslational modifications in regulating a same protein target. Finally, phospho- and site-specific antibodies are a useful tool to assess the regulation of a specific phosphorylation residue by a kinase in neurons of adult brains in vivo. Transgenic mice with a site-directed mutation (serine or threonine to alanine or aspartic acid mutation) are a valuable model to define a site-specific role of phosphorylation in regulating a phosphoprotein. Increased availability of these tools will greatly promote the studies on phosphorylation biology of synaptic MAPKs.
References
Volmat V, Pouyssegur J (2001) Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell 93:71–79
Gallo KA, Johnson GL (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3:663–672
Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC (1996) A structure basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol 16:6486–6493
Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317
Thomas GM, Huganir RL (2004) MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci 5:173–183
Wang JQ, Fibuch EE, Mao LM (2007) Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100:1–11
Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ (1995) Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15:1285–1297
Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Glustetto M (2007) Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE 2:e604
Sindreu CB, Scheiner ZS, Storm DR (2007) Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron 53:79–89
Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ (2013) Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res 1494:101–108
Suzuki T, Okumura-Noji K, Nishida E (1995) ERK2-type mitogen-activated protein kinase (MAPK) and its substrates in postsynaptic density fractions from the rat brain. Neurosci Res 22:277–285
Suzuki T, Mitake S, Murata S (1999) Presence of up-stream and downstream components of a mitogen-activated protein kinase pathway in the PSD of the rat forebrain. Brain Res 840:36–44
Xue B, Mao LM, Jin DZ, Wang JQ (2015) Regulation of synaptic MAPK/ERK phosphorylation in the rat striatum and medial prefrontal cortex by dopamine and muscarinic acetylcholine receptors. J Neurosci Res 93(10):1592–9
Edbauer D, Cheng D, Batterton MN, Wang CF, Duong DM, Yaffe MB, Peng J, Shang M (2009) Identification and characterization of neuronal mitogen-activated protein kinase substrates using a specific phosphomotif antibody. Mol Cell Proteomics 8:681–695
Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, Kuhlendahl F, Leal-Ortiz S, Goedert M, Garner C, Cuenda A (2004) Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem J 380:19–30
Xu W (2011) PSD-95 like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol 21:306–312
DeGiorgis JA, Jaffe H, Moreira JE, Carlotti CG Jr, Leite JP, Dosemeci A (2005) Phosphoproteomic analysis of synaptosomes from human cerebral cortex. J Proteome Res 4:306–315
Jaffe H, Vinade L, Dosemeci A (2004) Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun 321:210–218
Nada S, Shima T, Yanai H, Husi H, Grant SGN, Okada M, Akiyama T (2003) Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem 48:47610–47621
Guo ML, Xue B, Jin DZ, Mao LM, Wang JQ (2012) Interactions and phosphorylation of postsynaptic density 93 (PSD-93) by extracellular signal-regulated kinase (ERK). Brain Res 1460:18–25
Israely I, Costa RM, Xie CW, Silva AJ, Kosik KS, Liu X (2004) Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr Biol 14:1657–1663
Martinez MC, Ochiishi T, Majewski M, Kosik KS (2003) Dual regulation of neuronal morphogenesis by a δ-catenin-cortactin complex and Rho. J Cell Biol 162:99–111
Kosik KS, Donahue CP, Israely I, Liu X, Ochiishi T (2005) δ-Catenin at the synaptic-adherens junction. Trends Cell Biol 15:172–178
Hoffman DA, Magee JC, Colbert CM, Johnston D (1997) K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387:869–875
Sheng M, Tsaur ML, Jan YN, Jan LY (1992) Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron 9:271–284
Hu HJ, Glauner KS, Gereau RW 4th (2003) ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90:1671–1679
Yuan LL, Adams JP, Swank M, Sweatt JD, Johnson D (2002) Protein kinase modulation of dendritic K+ channels in hippocampal involves a mitogen-activated protein kinase pathway. J Neurosci 22:4860–4868
Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD (2000) The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem 75:2277–2287
Schrader LA, Bimbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD (2006) ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol 290:C852–861
Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322
Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P (1996) Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 8:1488–1500
Kuwajima M, Hall RA, Aiba A, Smith Y (2004) Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol 474:589–602
Enz R (2007) The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. Bioessays 29:60–73
Enz R (2012) Metabotropic glutamate receptors and interacting proteins: evolving drug targets. Curr Drug Targets 13:145–156
Fagni L (2012) Diversity of metabotropic glutamate receptor-interacting proteins and pathophysiological functions. Adv Exp Med Biol 970:63–79
Dhami GK, Ferguson SS (2006) Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther 111:260–271
Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ (2013) Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J Neurosci 33:3402–3412
Kim CH, Lee J, Lee JY, Roche KW (2008) Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res 86:1–10
Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ (2011) Posttranslational modification biology of glutamate receptors and drug addiction. Front Neuroanat 5:19
Orlando LR, Ayala R, Kett LR, Curley AA, Duffner J, Bragg DC, Tsai LH, Dunah AW, Young AB (2009) Phosphorylation of the homer-binding domain of group I metabotropic glutamate receptors by cyclin-dependent kinase 5. J Neurochem 110:557–569
Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, Park JM, Dong X, Xiao B, Worley PF (2012) Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci 15:836–844
Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF (2013) A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell 154:637–650
Stornetta RL, Zhu JJ (2011) Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist 17:54–78
McCormack SG, Stornetta RL, Zhu JJ (2006) Synaptic AMPA receptors exchange maintains bidirectional plasticity. Neuron 50:75–88
Qin Y, Zhu Y, Baumqart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ (2005) State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev 19:2000–2015
Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R (2002) Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110:443–455
Zhu JJ (2009) Activity level-dependent synapse-specific AMPA receptor trafficking regulates transmission kinetics. J Neurosci 29:6320–6335
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ (1996) Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A 93:3679–3683
Vara H, Onofri F, Benfenati F, Sassoe-Pognetto M, Giustetto M (2009) ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc Natl Acad Sci U S A 106:9872–9877
Treisman R (1996) Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol 8:205–215
Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S (2009) Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol 16:1026–1035
Sutton MA, Schuman EM (2006) Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127:49–58
Gong R, Tang SJ (2006) Mitogen-activated protein kinase signaling is essential for activity-dependent dendritic protein synthesis. Neuroreport 17:1575–1578
Acknowledgments
This work was supported by the NIH grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mao, LM., Wang, J.Q. Synaptically Localized Mitogen-Activated Protein Kinases: Local Substrates and Regulation. Mol Neurobiol 53, 6309–6315 (2016). https://doi.org/10.1007/s12035-015-9535-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9535-1