Abstract
c-Jun N-terminal kinases (JNKs), which belong to a mitogen-activated protein kinase (MAPK) family, are involved in the regulation of several physiological functions in mammals and act as mediators of apoptosis, obesity, and memory storage in the brain, including the processes of neuronal de- and regeneration. JNK subfamily is encoded by three separate but related genes: jnk1, jnk2, and jnk3, giving rise to at least ten distinct splice variants of the JNK proteins. JNK3 is thought to be a major contributor to neurodegeneration in mammalian brain. The role of JNK1 in the pathological processes affecting cognitive function, especially in diseases such as Alzheimer’s disease (AD), is less clear. In order to evaluate the effects of JNK1 deficiency in an experimental model of familial Alzheimer’s disease, double transgenic APPswe/PS1dE9 mice were crossed with the JNK1 heterozygous deficient animals (jnk1+/−). As expected, a ∼50 % reduction in JNK1 protein levels was observed in the hippocampi of 9-month-old APPswe/PS1dE9/jnk1+/− mice, compared with the APPswe/PS1dE9 group. JNK1 deficiency resulted in reduced BACE1 expression, suggesting alterations in amyloidogenic pathway. However, no significant inter-group differences in the total number of β-amyloid plaques were observed in the hippocampal region. In addition, protein levels of PPAR gamma coactivator-1α (PGC-1α), a molecule involved in mitochondrial biogenesis and energy homeostasis, were decreased in 9-month-old APPswe/PS1dE9 mice but not in APPswe/PS1dE9/jnk1+/− animals. Furthermore, JNK1 deficiency did not have an effect on pro-inflammatory marker expression in the hippocampus. Heterozygous deficiency of JNK1 results in the decrease of BACE1 protein levels, which is not accompanied by the reduction in the total number of β-amyloid plaques in the hippocampi of APPswe/PS1dE9 mice. Moreover, PGC-1α expression is restored in APPswe/PS1dE9/jnk1+/− animals, which indicates a possible role of JNK1 in brain mitochondrial regulation. Nevertheless, our results suggest that partial inhibition of JNK1 is not sufficient to prevent the neuropathological processes in this model. It may be necessary to inhibit both the JNK1 and JNK3 simultaneously, especially as previous studies suggest that JNK3 contributes to AD neuropathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disorder histopathologically characterized by the presence of senile plaques and neurofibrillary tangles, as well as by the loss of neuronal cells and synapses [1–3]. AD is categorically divided into an age-related late-onset sporadic form of the disease, and an early-onset familial AD (FAD), caused by genetic mutations [4, 5]. Mutations in presenilin 1 (PS1), the catalytic subunit of gamma-secretase, are among the most common genetic defects observed in FAD which lead to an increased production of βA-42, a key component of senile plaques. Although the exact causes of the disease in its sporadic form are unclear, the rates of cognitive decline are correlated to the extent of senile plaque deposition [5–8].
The amyloid cascade hypothesis, proposed in the early 1990s, dominated the field until recently. The hypothesis identified β-amyloid (βA) peptide deposition in the brain as the root cause of AD [1–3]. Over the past 25 years, this hypothesis has been the most accepted and of vital importance to AD drug development. Unfortunately, none of the drugs targeting βA are sufficiently effective. Moreover, significant brain βA deposition has been reported in non-demented elderly population, suggesting that the amyloid peptide itself is unlikely to be the sole contributor to cognitive decline [9].
Thus, newer hypotheses and strategies taking into account potential involvement of βA-independent pathways as an early central pathogenic trigger of late-onset AD have been developed [4]. The “adaptive response hypothesis of AD” suggests that βA may accumulate in the brain as an adaptive response to continuous brain stress stimuli [10, 11]. These stimuli could be for example metabolic alterations such as insulin resistance mediated by obesity, alterations in cholesterol homeostasis, neuroinflammatory processes, and more [12–17].
Apart from the classical approaches to AD treatment, fundamental research may provide additional clues to disease pathogenesis, leading to the development of targeted pharmaceuticals focusing on βA-independent alternative molecular pathways [18]. For example, tau protein hyperphosphorylation has been linked to the overactivation of both the GSK3β and mitogen-activated protein kinase (MAPK) pathways [19–21]. One particular MAPK subfamily of proteins known as c-Jun NH2 terminal protein kinase (JNK) has received considerable attention in the context of neurodegenerative diseases and are also implicated in the pathophysiology of neuropsychiatric disorders [22, 23]. JNK pathway plays a critical role in the processes of cell death that occur naturally during development as well as in various pathologies. Recent studies have demonstrated the activation of the JNK signaling cascade in neurons in experimental models of neurodegeneration, suggesting its possible role in the processes of neuronal loss [24–28]. JNK proteins are derived from three related genes: Jnk1, Jnk2, and Jnk3. Initial research has shown that in mammals, the JNK1 and JNK2 proteins are ubiquitously expressed, whereas the JNK3 was found almost exclusively in the brain and testis [29–32]. However, it is now clear that all three JNK isoforms are expressed in the brain and may have a role in cognition [33]. The data concerning the effects of individual JNK isoforms in the brain are limited. The majority of recent studies have focused on JNK3. It was demonstrated that specific JNK3 deletion resulted in neuroprotection in diverse models including exposure to MPTP (a Parkinson’s disease neurotoxin), ischemia, and kainic acid-mediated excitotoxicity [25, 30, 31, 34, 35]. βA itself may also contribute to JNK activation [26–28, 36, 37]. Thus, βA-induced cell death was reduced in cultures of cortical neurons derived from JNK3 knockout mice [31]. Taken together, available data suggest that JNKs in general, and JNK3 in particular, may be potential targets for brain neuroprotection [30, 38].
During the last decades, several studies have demonstrated the presence of oxidative metabolism abnormalities in AD [39]. Pathological changes in mitochondria, the main organelle involved in cellular bioenergetics and oxidative stress, have been reported [5]. The mitochondrial cascade hypothesis postulates that mitochondrial dysfunction may promote brain amyloidosis, thus suggesting that mitochondrial defects may be a primary event in sporadic AD. Because of heteroplasmy and the fact that mitochondrial DNA is maternally inherited, the actual percentage of mutated mitochondria may vary greatly in the offspring, leading to non-Mendelian patterns of inheritance. If a particular threshold of mitochondrial mutations is reached in the cells of the nervous system, the oxidative capacity of an entire organ may become severely diminished, which could result in increased β-amyloid deposition, the formation of neurofibrillary tangles and synaptic failure [5, 6, 8].
JNKs play a crucial role in the regulation of systemic glucose and lipid metabolism by modulating hormones involved in growth and energy homeostasis [40–43]. For example, JNK1 has been implicated in the pathogenesis of metabolic syndrome and type 2 diabetes mellitus (T2DM), and the inhibition of JNK signaling in the central nervous system (CNS) could act as a negative regulator of central insulin sensitivity [41–45]. A growing body of evidence suggests that metabolic hormone imbalances and cerebral insulin signaling deficiencies contribute to cognitive decline observed in AD. In addition, pro-inflammatory MAPK signaling plays a role in neuronal mechanisms of inflammation characteristic of neurodegenerative diseases [46].
Thus, the apparent relationship between insulin resistance, AD, and diabetes may be partially explained by perturbations in MAPK signaling [14], especially as the JNKs regulate both the apoptosis and metabolism pathways [47]. JNKs specifically have a wide range of functions, which include the regulation of various aspects of the inflammatory response and cytokine production, as well as stress-induced apoptosis and cellular transformation [46, 47]. In an extrinsic death-receptor-initiated apoptotic pathway, JNKs induce transcriptional activation of pro-apoptotic genes via the transcription factor c-Jun. However, in an intrinsic mitochondria-dependent pathway, JNKs modulate the activity of both the pro- and anti-apoptotic molecules, adding to the complexity of MAPK regulation [27, 28, 48].
The purpose of this study was to identify the role of JNK1 in the hippocampus of a FAD model. For this reason, APPswe/PS1dE9/jnk1+/− mice were generated by crossing double transgenic APPswe/PS1dE9 animals with the JNK1 heterozygous-deficient mice (jnk1+/−). Our results demonstrate that JNK1 is involved in amyloidogenesis and mitochondrial function in the brain.
Materials and Methods
Animals
Amyloid precursor protein (APP)/PS1 double transgenic mice were crossed with JNK1 heterozygous transgenic animals to generate novel triple APPswe/PS1dE9/Jnk1+/− mutants. APP/PS1 animals co-express a Swedish (K594M/N595L) mutation of a chimeric mouse/human APP (Mo/HuAPP695swe), together with the human exon-9-deleted variant of PS1 (PS1-dE9), allowing these mice to secrete elevated amounts of human Aβ peptide. Both mutations are associated with AD, are under control of the mouse prion protein promoter, directing both mutated proteins mainly to the CNS neurons, which results in age-dependent amyloid plaque depositions in mouse brain. APPswe-mutated APP is a favorable substrate for β-secretase, whereas the PS1dE9 mutation alters β-secretase cleavage, thereby promoting overproduction of Aβ42. The experiments were carried out on 9-month-old animals because previous studies have demonstrated that this age corresponds to AD-like symptomatic phase in APP/PS1 mice [24]. We evaluated between 7 and 13 animals per group, depending on the colony availability. To eliminate gender-related differences, only males were chosen for the present study. Animals were maintained under standard animal housing conditions with a 12-h dark–light cycle with free access to food and water. Animal procedures were conducted according to ethical guidelines (European Communities Council Directive 86/609/EEC) and approved by the local ethical committee (UB). Every effort was made to minimize animal suffering and to reduce the number of animals used.
RNA Extraction and Quantification
Total RNA was isolated from the hippocampi of wild-type, APPswe/PS1dE9 double transgenic and APPswe/PS1dE9/Jnk1+/− mutant mice utilizing Trizol-based extraction (Life Technologies Corporation) as previously described [24]. RNA pellet was reconstituted in RNAse-free water, with the RNA integrity determined by Agilent 2100 Bioanalyzer (Agilent Technologies, Inc).
Real-Time-PCR
First-strand complementary DNA (cDNA) was reverse transcribed from 2 μg of total RNA using the High Capacity cDNA Reverse Transcription kit, according to manufacturer’s protocol (Applied Biosystems). 15 ng of RNA equivalent was used per reaction. Each sample was analyzed in triplicate for each target. The following TaqMan probes (Applied Biosystems) were used to determine transcription levels of individual genes: inos-Mm00440502_m1, il1b-Mm00434228_m1, il6-Mm00446190_m1, prnp-Mm00448389_m1, tnf-Mm00443260_g1, and tbp-Mm00446971_m1. Reaction was performed on the StepOnePlus Real-Time PCR system (Applied Biosystems) and the values were normalized to tbp.
Immunofluorescence Staining
Slides were allowed to defrost at room temperature and then were rehydrated with phosphate-buffered saline (PBS) for 5 min. Later, the brain sections were incubated with 0.3 % thioflavin S (Sigma-Aldrich) for 20 min at room temperature and with primary antibodies anti-GFAP, Iba1, and 12F4 in the dark. Subsequently, these were submitted to washes in 3-min series, specifically with 80 % ethanol (two washes), 90 % ethanol (one wash), and three washes with PBS. Finally, the slides were mounted using Fluoromount (EMS), allowed to dry overnight at room temperature in the dark, and stored at 4 °C. Image acquisition was performed with an epifluorescence microscope (BX41; Olympus, Germany). For plaque quantification, similar and comparable histological areas were selected, focusing on having the hippocampus and the whole cortical area positioned adjacently [24].
Immunoblot Analysis
Aliquots of hippocampal homogenates containing 15 μg protein per sample were analyzed using the Western blot method. In brief, samples were placed in a sample buffer (0.5 m Tris–HCl, pH 6.8, 10 % glycerol, 2 % (w/v) SDS, 5 % (v/v), 2-mercaptoethanol, 0.05 % bromophenol blue) and denatured by boiling at 95–100 °C for 5 min. Samples were separated by electrophoresis on 10–15 % acrylamide gels. Following this, the proteins were transferred to PVDF sheets using transblot apparatus. Membranes were blocked overnight with 5 % non-fat milk dissolved in TBS-T buffer (50 mM Tris; 1.5 % NaCl, 0.05 % Tween 20, pH 7.5). They were then incubated with primary antibodies, as detailed in Table 1. After O/N incubation, blots were washed thoroughly in TBS-T buffer and incubated for 1 h with a peroxidase-conjugated IgG secondary antibody (1:2000). Immunoreactive protein was detected using a chemiluminescence-based detection kit. Protein levels were determined by densitometry, using Chemidoc XRS+ Molecular Imager detection system (Bio-Rad), with ImageLab image analysis software. Measurements are expressed as arbitrary units. All results are normalized to GAPDH, unless stated otherwise.
Data Analysis
All data are presented as means ± SEM, and differences are considered significant at p < 0.05. Differences between samples were evaluated using one-way ANOVA, with Tukey’s post hoc test. Both the statistical analyses and the graphs presented here were created with the GraphPad InStat software 6.01 (GraphPad Sofware Inc., San Diego, CA, USA).
Results
Molecular Characterization of APPswe/PS1dE9/jnk1+/− Mice
Heterozygous deficiency of JNK1 resulted in a ∼50 % reduction in JNK1 protein levels in the hippocampus of APPswe/PS1dE9/jnk1+/− and jnk1+/− mice (Fig. 1). APPswe/PS1dE9 mice incorporate a non-protein-coding portion of the prnp promoter in the 5′-UTR region of the inserted sequence, which allows for targeted expression of app and ps1 transgenes primarily to the brain. By selecting a probe specific for this region of the prnp, we can indirectly determine the expression levels of the mutated app and ps1 messenger RNA (mRNA). A significant upregulation of the prnp probe mRNA confirms hippocampal expression of APPswe and PS1dE9 molecules in APPswe/PS1dE9 and APPswe/PS1dE9/jnk1+/− groups (Fig. 1). The presence of the prnp transcripts in the non-transgenic tissues is indicative of the expression levels of native prnp mRNA.
Protein expression levels of JNK1 and mRNA expression profile of prnp and in the hippocampus of 9-month-old WT, APPswe/PS1dE9, APPswe/PS1dE9/Jnk1+/−, and Jnk1+/− mice (n = 4–6 independent biological replicates per group). Statistical analysis was performed with regular one-way ANOVA, with Tukey’s post hoc test: *p < 0.05; **p < 0.01; ***p < 0.001
JNK1 Deficiency and Mitochondrial Signaling
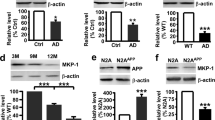
PGC-1α is a transcriptional coactivator involved in mitochondrial biogenesis, oxidative metabolism and energy homeostasis [49]. It regulates transcription of genes involved in mitochondrial genome replication and cellular respiration, including nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) [49–51]. Figure 2 shows a significant decrease in the expression of PGC-1α, NRF1, and TFAM proteins in APPswe/PS1dE9 mice, compared with wild-type (WT) animals. It is of great interest that the introduction of the heterozygous deficiency of JNK1 to APPswe/PS1dE9 animals appears to rescue the PGC-1α and NRF1 phenotypes, but does not have an effect on TFAM protein expression levels.
a Representative immunoblot images and b quantification of key molecules involved in mitochondrial regulation in the hippocampus of 9-month-old WT, APPswe/PS1dE9, APPswe/PS1dE9/Jnk1+/−, and Jnk1+/− mice (n = 4–6 independent biological replicates per group). Statistical analysis was performed with regular one-way ANOVA, with Tukey’s post hoc test: *p < 0.05; **p < 0.01
Amyloid Precursor Protein and Aβ Peptide Processing
Both the amyloidogenic and non-amyloidogenic pathways were analyzed by Western blot. As expected, total APP protein levels were significantly higher in mice with APPswe/PS1dE9 mutations. Transgenic APPswe/PS1dE9 animals also exhibited elevated hippocampal protein levels of both the beta-secretase 1 (BACE1) enzyme and its product sAPPβ (sAPPβ expression did not reach statistical significance). BACE1 cleaves APP, which results in the production of a secreted and soluble N-terminal APP fragment (sAPPβ), an intermediate step in Aβ peptide generation. Surprisingly, the levels of BACE1 were reduced to nearly WT levels in APPswe/PS1dE9/jnk1+/− group (sAPPβ expression demonstrated significant intra-group variability) (Fig. 3).
Representative immunoblot images and quantification of key molecules involved in amyloid peptide generation and processing in the hippocampus of 9-month-old WT, APPswe/PS1dE9, APPswe/PS1dE9/Jnk1+/−, and Jnk1+/− mice (n = 4–6 independent samples per group). Statistical analysis was performed with regular one-way ANOVA, with Tukey’s post hoc test: *p < 0.05; **p < 0.01
α-Secretases cleave APP within the Aβ peptide-containing sequence of the molecule and are a part of the non-amyloidogenic pathway. Protein levels of ADAM10, a major enzyme implicated in physiological proteolytic processing of APP, were decreased in hippocampal extracts of 9-month-old APPswe/PS1dE9 and APPswe/PS1dE9/jnk1+/− mice. sAPPα expression, which is an N-terminal (extracellular) ADAM10 cleavage product of the APP, was not significantly altered (Fig. 3).
JNK1 Heterozygous Deficiency Does Not Have an Effect on β-Amyloid Deposition in the Brain
The presence of β-amyloid plaques was analyzed by thioflavin S staining. As can be seen in Fig. 4a (1), β-amyloid depositions were readily detectable in cortex and hippocampus of 9-month-old APPswe/PS1dE9 and APPswe/PS1dE9/jnk1+/− mice but not in wild-type animals. JNK1 heterozygous deficiency did not result in statistically significant differences in plaque counts.
a Representative 1 thioflavin S and 2 β42 immunofluorescence in the brains of 9-month-old WT, APPswe/PS1dE9 and APPswe/PS1dE9/Jnk1+/− mice (n = 4 independent biological replicates per group). b Beta amyloid plaque counts in similar and comparable histological areas focusing on having the hippocampus and the whole cortical area positioned adjacently (n = 4–6 areas counted per sample, with at least three independent samples per biological group). No plaques were detectable in WT and Jnk1+/− groups
Moreover, we have evaluated the hippocampal deposits of human β42 with a specific antibody by immunohistochemistry and neither observed differences (Fig. 4a (2)).
JNK1 Heterozygous Deficiency Does Not Influence Hippocampal Inflammatory Response
Since previous studies have shown that JNK activation is involved in the βA-induced inflammatory response, we investigated whether decreased hippocampal JNK1 expression had an effect on neuroinflammation in APPswe/PS1dE9/jnk1+/− mice. Real-time PCR analysis revealed significantly elevated mRNA levels of interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNF-α), and inducible nitric oxide synthase (iNOS) in APPswe/PS1dE9 group, compared with wild-type mice. JNK1 heterozygous deficiency did not result in a significantly different response (Fig. 5). These findings indicate that a partial loss of JNK1 is not sufficient in reducing expression of pro-inflammatory markers in the hippocampus of APPswe/PS1dE9/jnk1+/− mice.
mRNA expression profile of pro-inflammatory modulators in hippocampus of 9-month-old WT, APPswe/PS1dE9, APPswe/PS1dE9/Jnk1+/−, and Jnk1+/− mice (n = 4–6 independent biological replicates per group). Statistical analysis was performed with regular one-way ANOVA, with Tukey’s post hoc test: *p < 0.05; **p < 0.01; ***p < 0.001
The analysis of astrocytes at 9 months in APPswe/PS1dE9 and APPswe/PS1dE9/jnk1+/− groups was done using an antibody that detects the glial acidic fibrilar protein (GFAP). The results obtained, revealed an accumulation of GFAP-positive activated cells around amyloid deposits/aggregates in both groups, when compared with the control group (Fig. 6a). The activation of astrocytes was detected in close proximity to amyloid aggregates (Fig. 6a). Thus, the spatial pattern of gliosis in APPswe/PS1dE9 and APPswe/PS1dE9/jnk1+/− brain closely followed the distribution of amyloid deposits (Fig. 6a). The same pattern of response was detected in both transgenic mice using the iba1 (ionized calcium binding adaptor molecule 1) antibody which immunostains microglial cells, meaning that the number of activated microglial cells accumulated around the myeloid aggregates (Fig. 6b), consistent with previous data indicating that APPswe/PS1dE9 mice display characteristics of neuroinflammation similar to those observed in AD brain.
a Immunohistochemistry against GFAP and b against iba-1, in 9-month-old mice. Brain sections were obtained from wild-type, transgenic APPswe/PS1dE9 mice, and heterozygote APPswe/PS1dE9/Jnk1+/− mice. a In wild type, the astrocytes display a non-reactive morphology (arrows). b, c In APPswe/PS1dE9 and APPswe/PS1dE9/Jnk1+/− mice, a reactive astrocytes (arrow) are located around β-amyloid plaques (Aβ depositions) (arrowhead). Double immunostaining of glial cells and plaque by thioflavin S: show amyloid plaque (in green) colocalized with astrocytes and microglia (GFAP and Iba 1 in red). Scale bar in (a–c) represents 100 μm
Discussion
Three members of the JNK protein family (JNK1, JNK2, and JNK3) were identified in mammals, and all three are expressed in cerebral tissues [33]. JNK3 is most abundant in the brain and we and others have shown that its activation is clearly pro-apoptotic [27, 34, 35]. Recent studies have demonstrated that pan-JNK-inhibitors are neuroprotective in various experimental models known to produce neurotoxicity [47, 52]. Unfortunately, due to the lack of specificity of these compounds, off-target effects are likely. For example, while treatment with JNK-inhibitor SP600125 results in neuroprotection in an APP/PS1 mouse model of AD, it also increases plasma cortisol levels, which may lead to cognitive impairment [52]. For this reason, the understanding of the role of each individual JNK isoform is important for the development of more specific drugs, which would presumably have more favorable therapeutic profiles.
The role of JNKs in neurodegenerative diseases is undisputed. However, JNK signaling also affects peripheral insulin resistance and obesity [30, 42, 43, 50]. Obesity increases JNK activity in adipose tissue and liver of mice, and JNK1 is involved in cell signaling cascades that sense metabolic stress [40, 42]. Moreover, mice genetically deficient in Jnk1 were protected from weight gain in both dietary and genetic mouse models of obesity [40]. JNK1 may directly contribute to insulin resistance because it phosphorylates IRS1 at an inhibitory site that can block signal transduction by the insulin receptor [44]. Therefore, JNK inhibition may be a suitable drug target for the treatment of metabolic syndrome and diabetes.
In recent years, metabolic syndrome and the risk of AD development were closely linked [53, 54]. Since JNK1 plays a known role in metabolic regulation, we have generated triple APPswe/PS1dE9/jnk1+/− mutants by crossing APPswe/PS1dE9 mice with jnk1+/− animals. The reason for breeding and crossing jnk1+/− mutants, instead of the full germline jnk1 knockouts, is that the birth rate of jnk1−/− mice is rather low. Confirmative Western-blot analysis demonstrated that the newly generated APPswe/PS1dE9/Jnk1+/− mice do indeed show about 50 % downregulation in JNK1 protein.
Previous studies suggest that mitochondrial dysfunction can play a role in early stages of AD development [5, 6, 8, 55]. Thus, research into mitochondrial function is considered a key element in understanding the molecular mechanisms of AD. Recent evidence points towards the importance of transcriptional coactivator PGC-1α [51]. This molecule is an integrator of the molecular regulatory circuitry involved in the transcriptional control of cellular energy metabolism, including mitochondrial function and biogenesis. In fact, PGC-1α levels are reduced in the brain of sporadic AD patients when compared with healthy controls [56]. In addition, Qin and colleagues demonstrated a correlation between reduced expression of PGC-1α and dementia in human AD patients [51]. In light of these observations, some authors have suggested that PGC-1α may be considered as a potential biomarker of AD [51, 55, 56]. In the current paper, we observed decreases in PGC-1α in the hippocampi of 9-month-old APPswe/PS1dE9 mice compared with age-matched WT controls.
PGC-1α activates mitochondrial biogenesis through a group of transcription factor targets including NRF1 and mitochondrial transcription factor A (TFAM), key transcriptional regulators of mitochondrial DNA transcription and replication [56]. PGC-1α also forms a part of antioxidant defense system by regulating the expression of enzymes involved in ROS detoxification [57, 58]. In our study, PGC-1α downregulation in APPswe/PS1dE9 brains was accompanied by reductions in both the TFAM and NRF1, compared with WT mice. Interestingly, introduction of jnk1 heterozygous deficiency to the double transgenic mice resulted in a significant recovery in the hippocampal protein levels of PGC-1α and NRF1, but not of TFAM (APPswe/PS1dE9/Jnk1+/− vs APPswe/PS1dE9 vs WT). This strongly suggests an important regulatory role of JNK1 in hippocampal mitochondrial function, including biogenesis and respiration, as well as oxidative metabolism and energy homeostasis maintenance. This result is of particular significance, given the number of studies which consider mitochondria among the key players in neurodegenerative disease development and progression [59].
APPswe/PS1dE9 animals overexpress APP and, as expected, we observed significant increases in APP protein in the hippocampi of APPswe/PS1dE9 and APPswe/PS1dE9/Jnk1+/− mice. It is widely known that BACE1 is a key beta secretase involved in APP processing, ultimately resulting in generation of amyloidogenic βA peptides [60]. During amyloidogenesis, APP is cleaved by BACE1 leading to generation of soluble APP-beta (sAPPβ), an intermediate step in Aβ peptide production. We have detected significantly elevated levels of BACE1 (sAPPβ exhibited significant intra-group variability) in the hippocampi of 9-month-old APPswe/PS1dE9 mice. This result demonstrates that amyloidogenic route is clearly upregulated in this model. In a recent study, Gong and colleagues reported that PGC-1α may enhance BACE1 degradation in the brain [49]. Reduced expression of PGC-1α coupled with BACE1 upregulation in APPswe/PS1dE9 mice in our model is consistent with this observation. Furthermore, our results demonstrate that partial loss of JNK1 in triple mutants (APPswe/PS1dE9/Jnk1+/−) is sufficient to rescue hippocampal expression of PGC-1α and BACE1 to levels similar to that of WT mice. Our data suggest that JNK1 may have a dual role in AD pathophysiology, affecting both the mitochondria and amyloidogenesis.
In a non-amyloidogenic pathway, soluble APP-alpha (sAPPα) is generated by cleavage of APP by enzymes with α-secretase activity, most notably ADAM10. Hippocampal expression of ADAM10 was significantly reduced in both APPswe/PS1dE9 and APPswe/PS1dE9/Jnk1+/− groups. Taken together, our results indicate an imbalance between amyloidogenic and non-amyloidogenic signaling in the hippocampus of 9-month-old mice in this experimental model of FAD. In the case of ADAM10 expression however, we did not detect any differences between the triple mutants and the double transgenic animals. Thus, we can conclude that JNK1 is unlikely to play a role in regulation of hippocampal non-amyloidogenic route.
Previous reported data have demonstrated a clear implication of JNKs in the pathogenesis of AD, in particular JNK3 the main isoform present in the brain hippocampus that phosphorylate APP, enhance βA production and increase BACE1 activity [32, 61–63]. Despite of the observed inter-group differences in molecules involved in βA generation, immunofluorescence data indicate that partial inhibition of JNK1 does not have significant effects on βA plaque numbers in the brain in our model. Since our results indicate no significant changes in the number of brain plaques in triple mutants, these data could be interpreted as partial deletion of JNK1 is not sufficient to decrease the plaque production. Thus, it would be necessary the inhibition of both brain isoforms (JNK1 and JNK3), as demonstrated with the drug SP600125 or perhaps a selective deletion of JNK3, by itself, would be sufficient to inhibit plaque formation.
Moreover, pro-inflammatory phenotype was previously described in APPswe/PS1dE9 mice [64]. Accordingly, we have detected elevated hippocampal expression in mRNA levels of iNOS, IL-1β, IL-6, and TNF-α in a double transgenic versus control group. JNK1 heterozygous deficiency did not influence the results.
In conclusion, JNK3 has been considered as one of the principal JNK protein family targets for neurodegenerative disease treatment. In fact, previous studies have implicated JNK3 in the pathogenesis of AD, suggesting that it may increase βA production by affecting both APP processing and β-secretase expression [62–69]. Pan-JNK-inhibitors such as SP600125 offer neuroprotection but are associated with significant adverse events. Independent assessments of individual JNK members are still lacking. The purpose of this study was to analyze the role of JNK1 in a mouse model of FAD. Our results indicate that JNK1 heterozygous deficiency may be beneficial in an APPswe/PS1dE9 model. Selective JNK1 inhibition may not be sufficient to completely protect against AD pathology. Nevertheless, our results suggest that JNK1 is involved in both the mitochondrial metabolism and the pro-amyloidogenic route of AD development.
AD Alzheimer’s disease, JNKs c-Jun N-terminal kinases, MAP mitogen-activated protein kinases, FAD familial Alzheimer’s disease.
References
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hardy JA, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12:383–388
Karran E, Hardy J (2014) A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol 76:185–205
Drachman DA (2014) The amyloid hypothesis, time to move on: amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement 10:372–380
Swerdlow RH, Khan SM (2004) A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses 63:8–20
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 20(Suppl 2):S265–S279
Nisbet RM, Polanco JC, Ittner LM, Götz J (2015) Tau aggregation and its interplay with amyloid-β. Acta Neuropathol 129:207–220
Swerdlow RH (2012) Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid Redox Signal 16:1434–1455
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA et al (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65:1509–1517
Castello MA, Soriano S (2014) On the origin of Alzheimer’s disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev 13:10–22
Castello MA, Jeppson JD, Soriano S (2014) Moving beyond anti-amyloid therapy for the prevention and treatment of Alzheimer’s disease. BMC Neurol 14:169
Demetrius LA, Driver J (2013) Alzheimer’s as a metabolic disease. Biogerontology 14:641–649
Kaminsky YG, Tikhonova LA, Kosenko EA (2015) Critical analysis of Alzheimer’s amyloid-beta toxicity to mitochondria. Front Biosci (Landmark Ed) 20:173–171
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63:2262–2272
de Lemos ML, de la Torre AV, Petrov D, Brox S, Folch J, Pallàs M, Lazarowski A, Beas-Zarate C et al (2013) Evaluation of hypoxia inducible factor expression in inflammatory and neurodegenerative brain models. Int J Biochem Cell Biol 45:1377–88
Di Scala C, Chahinian H, Yahi N, Garmy N, Fantini J (2014) Interaction of Alzheimer’s β-amyloid peptides with cholesterol: mechanistic insights into amyloid pore formation. Biochemistry 53:4489–502
Ramos-Rodriguez JJ, Ortiz-Barajas O, Gamero-Carrasco C, de la Rosa PP, Infante-Garcia C, Zopeque-Garcia N et al (2014) Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology 48:123–135
Hardy J, Bogdanovic N, Winblad B, Portelius E, Andreasen N, Cedazo-Minguez A, Zetterberg H (2014) Pathways to Alzheimer’s disease. J Intern Med 275:296–303
Bain J, McLauchlan H, Elliott M, Cohen P (2003) The specificities of protein kinase inhibitors: an update. Biochem J 371:199–204
Braithwaite SP, Schmid RS, He DN et al (2010) Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer’s disease. Neurobiol Dis 39:311–317
Clarke M, Pentz R, Bobyn J, Hayley S (2012) Stressor-like effects of c-Jun N-terminal kinase (JNK) inhibition. PLoS ONE 7, e44073
Gourmaud S, Paquet C, Dumurgier J, Pace C, Bouras C, Gray F, Laplanche JL, Meurs EF et al (2014) Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: links to cognitive decline. J Psychiatry Neurosci 39(6):140062
Sclip A, Tozzi A, Abaza A et al (2014) c-Jun N-terminal kinase has a key role in Alzheimer disease synaptic dysfunction in vivo. Cell Death Dis 5, e1019
de Lemos L, Junyent F, Verdaguer E, Folch J, Romero R, Pallàs M, Ferrer I, Auladell C et al (2010) Differences in activation of ERK1/2 and p38 kinase in Jnk3 null mice following KA treatment. J Neurochem 114:1315–1322
Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA (2004) JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A 101:665–670
Ma QL, Yang F, Rosario ER et al (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29:9078–9089
Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y et al (2001) Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21:7551–7560
Okuno S, Saito A, Hayashi T et al (2004) The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci 24:7879–7887
Lee JK, Park J, Lee YD, Lee SH, Han PL (1999) Distinct localization of SAPK isoforms in neurons of adult mouse brain implies multiple signaling modes of SAPK pathway. Brain Res Mol Brain Res 70:116–124
Resnick L, Fennell M (2004) Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov Today 9:932–939
Kimberly WT, Zheng JB, Town T, Flavell RA, Selkoe DJ (2005) Physiological regulation of the beta-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J Neurosci 25:5533–5543
Lagalwar S, Guillozet-Bongaarts AL, Berry RW et al (2006) Formation of phospho-SAPK/JNK granules in the hippocampus is an early event in Alzheimer disease. J Neuropathol Exp Neurol 65:455–464
Reineckea K, Herdegena T, Eminela S, Aldenhoff JB, Schiffelholz T (2013) Knockout of c-Jun N-terminal kinases 1, 2 or 3 isoforms induces behavioural changes. Behav Brain Res 245:88–95
Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ et al (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389:865–70
Qi SH, Liu Y, Hao LY, Guan QH, Gu YH, Zhang J, Yan H, Wang M et al (2010) Neuroprotection of ethanol against ischemia/reperfusion-induced brain injury through decreasing c-Jun N-terminal kinase 3 (JNK3) activation by enhancing GABA release. Neuroscience 167:1125–1137
Wang Q, Walsh DM, Rowan MJ et al (2004) Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24:3370–3378
Sclip A, Antoniou X, Colombo A et al (2011) c-Jun N-terminal kinase regulates soluble Aβ oligomers and cognitive impairment in AD mouse model. J Biol Chem 286:43871–43880
Thakur A, Wang X, Siedlak SL et al (2007) c-Jun phosphorylation in Alzheimer disease. J Neurosci Res 85:1668–1673
Zhou Q, Lam PY, Han D, Cadenas E et al (2008) c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem 104:325–335
Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS (2002) A central role for JNK in obesity and insulin resistance. Nature 420:333–336
Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS (2011) Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes 60:486–495
Belgardt BF, Mauer J, Brüning JC (2010) Novel roles for JNK1 in metabolism. Aging (Albany NY) 2:621–626
Sabio G, Davis RJ (2010) c-Jun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci 35:490–496
Lee YH, Giraud J, Davis RJ, White MF (2003) c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278:2896–2902
Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE (2004) Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem 279:35298–35305
Waetzig V, Czeloth K, Hidding U et al (2005) c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50:235–246
Wang Y, Zhang Y, Wei Z, Li H, Zhou H, Zhang Z, Zhang Z (2009) JNK inhibitor protects dopaminergic neurons by reducing COX-2 expression in the MPTP mouse model of subacute Parkinson’s disease. J Neurol Sci 285:172–177
Savage MJ, Lin YG, Ciallella JR et al (2002) Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci 22:3376–3385
Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M et al (2013) Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 34:1581–1588
Katsouri L, Parr C, Bogdanovic N, Willem M, Sastre M (2011) PPARγ co-activator-1α (PGC-1α) reduces amyloid-β generation through a PPARγ-dependent mechanism. J Alzheimers Dis 25:151–162
Qin W, Haroutunian KP, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM (2009) PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol 66:352–361
Zhou Q, Wang M, Du Y, Zhang W, Bai M, Zhang Z, Li Z, Miao J (2015) Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Ann Neurol 77:637–654
Ríos JA, Cisternas P, Arrese M, Barja S, Inestrosa NC (2014) Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol 121:125–146
Calvo-Ochoa E, Arias C (2015) Cellular and metabolic alterations in the hippocampus caused by insulin signalling dysfunction and its association with cognitive impairment during aging and Alzheimer’s disease: studies in animal models. Diabetes Metab Res Rev 31:1–13
Pedrós I, Petrov D, Allgaier M, Sureda F, Barroso E, Beas-Zarate C, Auladell C, Pallàs M et al (1842) Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim Biophys Acta 2014:1556–1666
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120:419–429
Godoy JA, Rios JA, Zolezzi JM, Braidy N, Inestrosa NC (2014) Signaling pathway cross talk in Alzheimer’s disease. Cell Commun Signal 12:23
Chaturvedi RK, Flint BM (2013) Mitochondrial diseases of the brain. Free Radic Biol Med 63:1–29
Swerdlow RH (2014) Bioenergetic medicine. Br J Pharmacol 171:1854–1869
Vassar R, Kuhn PH, Haass C, Kennedy ME, Rajendran L, Wong PC, Lichtenthaler SF (2014) Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem 130:4–28
Colombo A, Bastone A, Ploia C et al (2009) JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol Dis 33:518–525
Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, Lim TH, Pastorino L et al (2012) JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron 75:824–837
Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O et al (2008) Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 104:683–95
Zhang W, Bai M, Xi Y et al (2012) Multiple inflammatory pathways are involved in the development and progression of cognitive deficits in APPswe/PS1dE9 mice. Neurobiol Aging 33:2661–2677
Colombo A, Repici M, Pesaresi M et al (2007) The TAT-JNK inhibitor peptide interferes with beta amyloid protein stability. Cell Death Differ 14:1845–1848
Guglielmotto M, Monteleone D, Giliberto L, Fornarod M, Borghid R, Tamagnoa E, Tabaton M (2011) Amyloid-β42 activates the expression of BACE1 through the JNK pathway. J Alzheimers Dis 27:871–883
Coffey ET (2014) Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci 15:285–299
Ghasemi R, Zarifkar A, Rastegar K et al (2014) Repeated intra-hippocampal injection of beta-amyloid 25–35 induces a reproducible impairment of learning and memory: considering caspase-3 and MAPKs activity. Eur J Pharmacol 726C:33–40
Pesini A, Iglesias E, Garrido N, Bayona-Bafaluy MP, Montoya J, Ruiz-Pesini E (2014) OXPHOS, pyrimidine nucleotides, and Alzheimer’s disease: a pharmacogenomics approach. J Alzheimers Dis 42:87–96
Acknowledgments
This study was funded by grant 2009/SGR00853 Generalitat de Catalunya (autonomous government of Catalonia) and by grants SAF2011-23631 and SAF2012-39852-C02-01 from the Spanish Ministerio de Ciencia e Innovación.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Jaume Folch and Antoni Camins are senior co-authors.
Rights and permissions
About this article
Cite this article
Petrov, D., Luque, M., Pedrós, I. et al. Evaluation of the Role of JNK1 in the Hippocampus in an Experimental Model of Familial Alzheimer’s Disease. Mol Neurobiol 53, 6183–6193 (2016). https://doi.org/10.1007/s12035-015-9522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9522-6