Abstract
Imidazoline receptor antisera-selected (IRAS) protein, the mouse homologue named Nischarin, was found to target to early endosomes with properties of sorting nexins in vitro. Recently, we generated IRAS knockout mice and found IRAS deficiency exacerbated the analgesic tolerance and physical dependence caused by opioids, suggesting that IRAS plays a role in regulating μ opioid receptor (MOR) functions. In the present study, we found that IRAS interacts with MOR and regulates MOR trafficking in vitro. In the CHO or HEK293 cells co-expressing MOR and IRAS, IRAS, through its PX domain, interacted with MOR. The interaction facilitated the recycling of internalized MOR and prevented MOR downregulation induced by DAMGO, the MOR agonist. Functionally, IRAS accelerated MOR resensitization and attenuated DAMGO-induced MOR desensitization, which is believed as one of mechanisms mediating opioid tolerance and dependence. Taken together, we propose that IRAS is a new MOR interacting protein and regulates agonist-induced trafficking of MOR via sorting internalized MOR to the recycling pathway, which may be a molecular mechanism underlying IRAS modulating opioid tolerance and dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Opioid analgesics are commonly used in clinic to treat acute and chronic pain, while the development of profound tolerance and dependence caused by long-term use of opioids limits analgesic efficacy and causes the side effect. Mu opioid receptor (MOR) is a member of G protein-coupled receptors (GPCRs), mediates the rewarding and analgesic effect of opioids; on the other hand, MOR plays a central role in mediating the side effects of opioids dependence and tolerance when repeatedly being exposed to the drugs. Like many other GPCRs, MOR undergoes multiple trafficking steps by agonist activation. By the treatment of opioid agonists (e.g., DAMGO), MOR is phosphorylated by GPCR kinases and then binds to β-arrestins, which in turn leads to the receptor desensitization and internalization. The fates of the internalized MOR are diverse: recycling of internalized MOR helps resensitization and restores the functions, while the sorting to lysosomes of the MOR leads to downregulation and further attenuates cellular functions [1]. The trafficking processes, particularly the desensitization and degradation, are considered to be involved in the development of tolerance and dependence induced by opioids [2, 3].
Imidazoline receptor antisera-selected (IRAS) protein, a candidate for I1 imidazoline receptor, was cloned from a human hippocampus cDNA expression library by Piletz et al. [4]. Our lab previously reported that IRAS activated by agmatine, the endogenous ligand for I1 imidazoline receptor [5, 6], attenuated the dependence and tolerance to opioids. Recently, we generated IRAS knockout mice and found IRAS deficiency exacerbated the development of analgesic tolerance and physical dependence caused by MOR agonist methadone [7], indicating that IRAS might serve as a regulator for opioid functions. However, the mechanism for IRAS regulating opioid tolerance and dependence remains unclear.
There are lines of evidence suggesting that IRAS might play a regulating role in the trafficking by interacting with other protein. Lim and Hong reported that IRAS targeted to early endosomes with the properties of sorting nexins (SNXs) [8]. Nischarin, the mouse homologue of IRAS, could interact with α5 subunit of integrin [9] and regulate the trafficking of salmonella-containing vacuole by interacting with members of Rac and Rab GTPase [2]. In the recent years, a growing number of studies suggested that trafficking or signaling of GPCRs are affected by the interaction with various receptors or proteins. For example, synaptophysin enhances the MOR endocytosis and attenuates agonist-induced receptor desensitization by interacting with MOR [10]. The heterodimerization of somatostatin receptor and MOR cross-modulates phosphorylation, internalization, and desensitization of these receptors [11]. Regarding the property of IRAS, we hypothesized that modulating of MOR trafficking might be involved in the molecular mechanism of IRAS regulating opioid tolerance and dependence. In the present study, therefore, we first checked whether IRAS and MOR interacted with each other, and then investigated the role of IRAS in MOR trafficking, including internalization, recycling, and downregulation, as well as opioid-induced desensitization and resensitization.
Materials and Methods
Plasmids and Recombinant Protein
The rat MOR coding sequences without the stop codon were amplified from the receptor expression plasmids using sense and antisense primers harboring unique cloning sites (EcoRI and BglII). The PCR fragments were then inserted in-frame into p3 × FLAG (Sigma Chemical Company, MO, USA), pDsRed, and peGFP-N1 vectors (Clontech, Mountain View, CA, USA) to yield constructs named FLAG-MOR, RFP-MOR, and eGFP-MOR. Likewise, the full-length hIRAS was amplified by PCR and subcloned into pCMV-Myc vector or peGFP-N1 vectors (Clontech, Mountain View, CA, USA). The truncated IRAS with the deletion of 1–244 amino acids (ΔPX-IRAS) were constructed by PCR with sense primer 5′-GGGAATTCGGGAGCTCTTTGAGAAAGGAGAACAG-3′ and anti-sense primer 5′-GGGGTACCCTAAGGCCGGTGAGCTCGACAGGCAGCT-3′. Then, the fragments were subcloned into pCMV-Myc utilizing EcoRI/KpnI restriction sites.
Cell Culture
Human embryonic kidney 293 (HEK293) cells were cultured in DMEM (Life Technologies, CA, USA) supplemented with 10 % FBS (Gibco, Grand Island, NY, USA) at 37 °C with 5 % CO2. Chinese hamster ovary (CHO) cells stably expressing MOR (CHO-MOR) or co-expressing MOR and IRAS (CHO-MOR/IRAS) were cultured in DMEM supplemented with 10 % FBS and 50 μg/ml hygromycin B or 50 μg/ml hygromycin B and 200 μg/ml geneticin (Roche, Mannheim, Germany), respectively.
Flow Cytometric Analysis
Internalization and recycling of MOR were assessed using fluorescence-activated cell sorting analysis (FACS) as described previously [12]. To estimate internalization, cells were incubated with 5 μM DAMGO with or without 50 μM monensin (Sigma, St. Louis, MO, USA) for the indicated intervals at 37 °C. To measure recycling, cells were first exposed to DAMGO for 1 h to induce internalization and then washed at 37 °C for indicated time. After internalization or recycling, cells were harvested and washed twice with ice-cold PBS by centrifugation (400g × 5 min, 4 °C), and subsequently blocked in 5 % BSA for 1 h. Detection of cell surface MOR using N-terminal MOR antibody (AOR-011, Alomone Labs, Alomone Labs Ltd., Israel) at 4 °C for 4 h. Cells were then washed twice with ice-cold PBS and incubated with FITC-conjugated secondary antibody (1:100, BD Biosciences, CA, USA) for 1 h at 4 °C. After being washed twice with ice-cold PBS and fixed in 2 % paraformaldehyde, cells were subjected to flow cytometry and analyzed using FACSVantage™ SE system (BD Biosciences, CA, USA) equipped with a 488-nm argon laser.
Immunofluorescence Microscopy
The cells transfected with Myc epitope-tagged IRAS (Myc-IRAS) and/or FLAG-tagged MOR (FLAG-MOR) were washed three times with cold PBS, and fixed in acetone for 10 min at −20 °C. Then, cells were washed three times and blocked in 10 % FBS for 1 h at room temperature. Primary antibody incubation was done overnight at 4 °C. Anti-Myc (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MOR (AOR-011), anti-FLAG, anti-rab5, and anti-rab7 antibodies (Cell Signaling Technology, Beverly, MA, USA) were used at a dilution 1:100. For detecting the endogenous localization of IRAS and MOR, the rat was perfused with 4 % paraformaldehyde in 0.1 M PBS. Brains were post-fixed overnight in the same solution and transferred to 30 % sucrose until they were on the bottom. Thirty-micrometer-thick sections were cut and then processed for immunofluorescence. The brain sections were blocked in 10 % FBS for 1 h at room temperature, followed by overnight incubation with a mixture of an anti-MOR antibody (AOR-01, 1:100 dilution) and anti-IRAS antibody (established by our laboratory [13], 1:100 dilution). The next day, the cell slides or brain sections were incubated by goat anti-mouse FITC conjugated secondary antibody (1:100) and goat anti-rabbit TRITC conjugated secondary antibody (1:100) for 1 h at room temperature. The immunofluorescence stainings were viewed using a confocal laser-scanning microscope (Nikon, Japan) attached to a Radiance 2000 imaging system (BioRad, Hercules, CA, USA).
Assays of cAMP Accumulation
Receptor desensitization and resensitization was determined by measurement of cAMP levels. Approximately 1.0 × 105 cells per well were plated onto 24-well plates overnight. The level of cAMP was measured by Cisbio cAMP kits (Cisbio Bioassays, France). For desensitization assay, cells were incubated with 5 μM DAMGO for the indicated intervals at 37 °C. For resensitization assay, cells were first exposed to 5 μM DAMGO for 4 h to induce desensitization and then washed at 37 °C for indicated time. Then, the cells were harvested at the concentration of 1.6 × 106/ml in stimulation buffer (HBSS containing 5 mM HEPES, 0.1 % BSA, 0.05 mM IBMX) at 4 °C and then stimulated by 2 μM forskolin in the presence or absence of 5 μM DAMGO for 20 min at 37 °C. Then, the extracted cAMP content was measured according to the instruction of Cisbio cAMP kits.
Co-Immunoprecipitation Experiments
Transfected HEK293 cells or CHO cells were washed twice with PBS and lysed for 30 min in a radioimmunoprecipitation assay buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 0.5 % Nonidet P-40 and complete protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Immunoprecipitation was carried out overnight at 4 °C with 2 μg of anti-Myc, anti-FLAG, or anti-MOR antibody (Santa Cruz Biotechnology, CA, USA) in the presence of Protein A and G magnetic beads (Millipore Corporation, Billerica, MA, USA). The beads were then washed four times with RIPA buffer and analyzed by Western blotting.
Western Blotting Analysis
Western blotting was used for analysis of total MOR. After treatment by DAMGO, cells were washed with ice-cold PBS and then lysed for 30 min at 4 °C in the lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 % NP-40, 0.01 % SDS, 0.5 % sodium deoxycholate, 1 mg/L PMSF, 1 mM DTT, and complete protease inhibitor cocktail). The lysate was then centrifuged at 12,000g for 20 min at 4 °C. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Primary antibodies incubated at 4 °C overnight with the anti-MOR antibody (Santa Cruz Biotechnology, CA, USA). Bound primary antibodies were detected with peroxidase-labeled secondary antibodies, and immunoreactive proteins were visualized using the ECL kit.
Radioligand Binding Assay
Radioligand binding assay was used for measuring the MOR in the membrane surface. Cells were treated with 5 μM DAMGO for 16 h at 37 °C. After incubation, cells were rinsed three times with phosphate-buffered saline to remove DAMGO and then harvested to prepare membrane for measuring [3H]diprenorphine (NEN, Boston, MA, USA) binding. Membrane preparation and [3H]diprenorphine binding assays were done according to the previous report [14].
Living Cell Fluorescence and FRET Imaging
HEK293 cells transiently-expressing various eGFP/RFP-tagged proteins were grown on glass coverslips mounted in a microscope chamber and placed on a microscope stage. For experiments at 37 °C, a microscopy chamber was mounted onto the temperature-controlled adapter on the microscope stage. The fluorescence imaging workstation consisted of an inverted fluorescence microscope equipped with a ×60 oil immersion objective lens (Nikon Eclipse Ti-E, Japan) with an UltraView spinning disk confocal scanner unit (Perkin Elmer, Boston, MA, USA). Time-lapse images were taken at 5-min intervals. The method of normalization of fluorescence resonance energy transfer (FRET) measurement has been described previously [15]. Briefly, images were acquired sequentially through eGFP, RFP, and FRET filter channels. Filters were RFP (excitation, 561 nm; emission, 615/70 nm), eGFP (excitation, 488 nm; emission, 527/55 nm), and FRET (excitation, 488 nm; emission, 615/70 nm). FRET images are presented in sensitized emission mode. Because the normalized FRET (NFRET) values are functions of both the FRET efficiency (distant dependence) and the ratio of donor-acceptor complex to total donors or acceptors (concentration and affinity dependence), it is more reliable to quantify NFRET in cells to reflect the interactions between proteins independently of their protein expression levels. NFRET values for individual cellular compartments were calculated according to the following equation: NFRET = (IFRET − (a × IRFP) − (b × IeGFP) / Sqrt(IRFP × IeGFP). IFRET, IRFP, and IeGFP are intensities in each region of interest, where FRET, RFP, and eGFP correspond to background-subtracted images of cells co-expressing RFP and eGFP acquired through the FRET, RFP, and eGFP channels, respectively. The values for the bleed-through varied with different imaging systems, which were determined by analyzing images of cells expressing only eGFP or RFP and quantifying the relative intensity ratio under the FRET/eGFP or FRET/RFP filter sets. All calculations were performed using the volocity 6.0 software.
Statistical Analysis
Data were expressed as mean ± SEM. Statistical analyses were performed by two-way ANOVA followed by Bonferroni test or t test. A p value of <0.05 was considered significant.
Results
Co-localization of IRAS and MOR
In order to investigate the role of IRAS in agonist-induced MOR trafficking, we first observed the distribution of MOR and IRAS in both transfected cells and cortex neurons. In HEK293 cells co-transfected with Myc-IRAS and FLAG-MOR, some of IRAS uniformly localized in the cytosol and some of them clustered in the cytoplasm (Fig. 1a). MOR mainly localized on the plasma membrane, and some of them were constitutively internalized into the cytoplasm. Co-localization of IRAS and MOR was detected in the inner plasma membrane surface and in the intracellular puncta (Fig. 1a). Then, the distribution of IRAS and MOR was investigated in the rat cerebral cortex by immunofluorescence using antibodies against IRAS and MOR. Some of endogenous IRAS was found uniformly in the cytosol and also distributed as puncta around the cell nucleus, consistent with the distribution of IRAS in the transfected cells. IRAS and MOR co-localized mainly in the inner plasma membrane surface in many neurons of the cerebral cortex (Fig. 1b).
Distribution and partial co-localization of IRAS and MOR. a Distribution of IRAS and MOR in the HEK293 cells transiently transfected with Myc-IRAS and FLAG-MOR. The cells were fixed and subjected to immunofluorescence staining with anti-FLAG antibody followed by TRITC-conjugated anti-rabbit IgG for MOR (red) and anti-Myc antibody followed by FITC conjugated anti-mouse IgG for IRAS (green). Co-localization of IRAS and MOR was observed mainly in the inner plasma membrane, and some in the cytoplasm. b Distribution of IRAS and MOR in the neurons of rat cerebral cortex. The distribution of endogenous MOR and IRAS was determined by immunofluorescence with anti-MOR followed by TRITC conjugated anti-rabbit IgG for MOR (red) and anti-IRAS antibody followed by FITC conjugated anti-mouse IgG for IRAS (green). MOR and IRAS are co-localized in the inner plasma membrane in many neurons of the cortex. Scale bar 20 μm (color figure online)
Then, we further examined the subcellular distribution of IRAS indicated by the following markers: rab5 (early/recycling endosomes), rab4 (short recycling endosomes), rab11 (late recycling endosomes), and rab7 (late endosomes). Figure 2 showed that IRAS mostly co-localized with the early/recycling endosome compartment, as indicated by rab5, and in short recycling endosomal compartments marked by rab4. Little co-localization was observed with the late recycling endosome marker rab11 and the late endosome marker rab7. Therefore, the observations suggested that IRAS was associated with the early/recycling endosomes and may participate in regulating the process of receptor trafficking.
Association of IRAS with Rab5 and Rab4-containing endosomes in CHO cells transiently transfected with Myc-IRAS. Forty-eight hours after transfection with IRAS, the cells were fixed and subjected to immunofluorescence staining with anti-Myc antibody followed by FITC conjugated anti-mouse IgG (green) and anti-rab5, rab4, rab7, or rab11 antibody followed by TRITC conjugated anti-rabbit IgG (red) (color figure online)
Interaction of IRAS and MOR
To examine whether MOR interacts with IRAS, the co-immunoprecipitation was tested in HEK293 cells transiently transfected with Myc-IRAS and FLAG-MOR. MOR and IRAS showed as protein bands of Mr ~70 and ~210 kDa, respectively. Anti-FLAG-tagged MOR immunoprecipitated materials contained Myc-IRAS from the lysates of the cells co-expressing Myc-IRAS and FLAG-MOR, but not in the cells expressing Myc-IRAS and FLAG vector (Fig. 3a). Conversely, immunoprecipitation of IRAS with anti-Myc antibody co-immunoprecipitated FLAG-MOR in the cells co-expressing Myc-IRAS and FLAG-MOR, but not in the cells expressing FLAG-MOR and Myc vector (Fig. 3a). The unrelated protein GFP bound neither MOR nor IRAS under identical conditions from cells expressing FLAG-MOR or Myc-IRAS and GFP in these assays (data not shown). In order to preclude the artifact of IRAS/MOR interaction which might be due to the high transfection in the specific cells, the co-immunoprecipitation was tested in the CHO cells stably transfected with IRAS and MOR (CHO-MOR/IRAS cells). CHO-MOR/IRAS cells expressed relative lower levels of MOR and IRAS (Bmax and Kd values of MOR were 1.83 pmol/mg protein and 0.24 nM, Bmax and Kd of IRAS were 184.0 fmol/mg protein and 15.75 nM) [6]. CHO-MOR/IRAS cells were lysed and immunoprecipitated with rabbit anti-MOR antibody. The results showed the band of IRAS was detected in the MOR immunoprecipitate, but not in the rabbit normal IgG control, indicating that the co-immunoprecipitation of IRAS and MOR was formed in the cell line (Fig. 3b).
Co-immunoprecipitation of MOR and IRAS. a Co-immunoprecipitation of MOR and IRAS in HEK293 cells transfected with Myc-IRAS and FLAG-MOR. HEK293 cells transiently transfected with Myc-IRAS, FLAG-MOR, or both were lysed and subjected to immunoprecipitation with anti-FLAG or anti-Myc antibodies. Immunoprecipitated materials were subjected to SDS-PAGE and immunoblotting was performed with anti-Myc or anti-FLAG antibody, respectively. n = 3, ***p < 0.001, t test. b Co-immunoprecipitation of MOR and IRAS in CHO cells stably transfected with IRAS and MOR (CHO-MOR/IRAS cells). Immunoprecipitation was performed with rabbit polyclonal anti-MOR or a control IgG and immunoblotting was performed with mouse monoclonal anti-IRAS. c The effect of DAMGO on the interaction of MOR and IRAS. CHO-MOR cells transiently transfected with Myc-IRAS were treated with DAMGO (5 μM) for 30 min at 37 °C; then, immunofluorescence and co-immunoprecipitated experiments was performed. n = 3, **p < 0.01, DAMGO treatment cells versus vehicle treatment cells, t test. d The effect of PX domain of IRAS on the interaction of MOR and IRAS. CHO-MOR cells were transfected with full-length Myc-IRAS or Myc-ΔPX-IRAS. Forty-eight hours after transfection, immunofluorescence for detecting the distribution of IRAS and MOR was performed by using anti-Myc antibody and anti-FLAG antibody. For co-immunoprecipitation experiment, the cells lysates were immunoprecipitated with anti-Myc antibody and immunoblotted with anti-FLAG antibody. n = 4, ***p < 0.001, cells with co-expressing MOR and Myc-IRAS versus cells with co-expressing MOR and Myc-ΔPX-IRAS, t test
To examine whether the interaction of IRAS and MOR was affected by agonist stimulation, CHO-MOR cells transfected with Myc-IRAS were treated by DAMGO (5 μM) for 30 min. As shown in Fig. 3c, the immunofluorescence pictures demonstrated that FLAG-MOR was internalized into the cytosol induced by DAMGO, where FLAG-MOR was co-localized with Myc-IRAS. Co-immunoprecipitation assay was used to quantify the effect of DAMGO on the interaction. Figure 3c shows DAMGO treatment (5 μM) for 30 min significantly increased the amount of co-immunoprecipitation of FLAG-MOR and Myc-IRAS, suggesting that stimulation of MOR led to an increasing interaction between MOR and IRAS.
For investigating which region is necessary to mediate the interaction of MOR/IRAS, a mutant of IRAS with deletion of PX domain (1–244 amino acids) was generated. Full-length Myc-IRAS or the mutant of IRAS (Myc-ΔPX-IRAS) was transfected into the CHO cells stably expressing MOR. As shown in Fig. 3d, Myc-ΔPX-IRAS was distributed uniformly in the cytosol (Fig. 3d, see immunofluorescence pictures), which was different from the punctate distribution of Myc-IRAS shown above (see Fig. 1a). Notably, in contrast to full-length Myc-IRAS, Myc-ΔPX-IRAS did not co-immunoprecipitate with MOR (Fig. 3d, see co-immunoprecipitation), indicating that PX domain of IRAS was a key region for the interaction between IRAS and MOR. Immunofluorescence showed DAMGO treatment induced the internalization of MOR with IRAS, without affecting the localization of Myc-ΔPX-IRAS (Fig. 3d, see immunofluorescence pictures).
The living cell fluorescence combined with FRET analysis was used to further confirm the interaction of MOR/IRAS. RFP-MOR and eGFP-MOR were co-expressed in HEK293 cells as the positive control since MOR was reported to form homodimers and/or higher-order oligomers [16]. HEK293 cells co-transfected with RFP-MOR and eGFP was used as a negative control. Consistent with the previous reports, the present data demonstrated a strong NFRET signal between RFP-MOR and eGFP-MOR (Fig. 4a). In contrast, no NFRET signal was seen in RFP-MOR and eGFP (Fig. 4c). In the HEK293 cells co-transfected with eGFP-IRAS and RFP-MOR, significant NFRET signals were detected between RFP-MOR and eGFP-IRAS (Fig. 4b), which localized on the inner surface of plasma membrane and in the cytoplasm.
Interaction between the MOR and IRAS by FRET assay. a HEK293 cells transiently transfected with RFP-MOR and eGFP-MOR were used as the positive control. Forty-eight hours later, RFP, eGFP, FRET, and N-FRET images were acquired as described in “Materials and Methods.” RFP-MOR and eGFP-MOR showed the interaction with each other as indicated by a strong NFRET signal. b Interaction and co-localization of IRAS and MOR. RFP-MOR and eGFP-IRAS were transiently co-transfected into HEK293 cells. Forty-eight hours later, RFP, eGFP, FRET, and NFRET images were acquired. The significant NFRET signal between RFP-MOR and eGFP-IRAS suggested the interaction between them. c HEK293 cells transfected with RFP-MOR and eGFP were used as the negative control. RFP-MOR and eGFP showed no N-FRET signal
IRAS Attenuated DAMGO-Induced Reduction of Cell Surface MOR by Facilitating MOR Recycling
To investigate whether the interaction of MOR/IRAS would affect MOR trafficking, HEK293 cells co-transfected with eGFP-IRAS/RFP-MOR were stimulated with DAMGO (5 μM) for 30 min. The movement of eGFP-IRAS or RFP-MOR and their interaction were visualized at 5-min intervals by living cell FRET imaging. As shown in Fig. 5a, DAMGO promoted the internalization of RFP-MOR. Interestingly, the complex of RFP-MOR/eGFP-IRAS as indicated by the NFRET signals was also moved to the cytoplasm gradually along with the internalized RFP-MOR by prolonged treatment of DAMGO (Fig. 5a, N-FRET with DAMGO treatment 0–30 min). By contrast, in HEK293 cells co-transfected with RFP-MOR and eGFP-MOR, eGFP or RFP-tagged MOR was internalized by DAMGO stimulation, but most of NFRET signal in RFP-MOR/eGFP-MOR still stayed in the plasma membrane (Fig. 5b).
Analysis of the MOR internalization by live cell fluorescence and FRET imaging. a The interaction of RFP-MOR/eGFP-IRAS and the MOR internalization was influenced by DAMGO treatment. RFP-MOR and eGFP-IRAS were transiently co-transfected in HEK293 cells for 48 h. The cells were then incubated with DAMGO (5 μM) for 30 min and were photographed at every 5 min. b Effect of DAMGO stimulation on the interaction of RFP-MOR/eGFP-MOR and the MOR internalization. RFP-MOR and eGFP-MOR were transiently co-transfected in HEK293 cells for 48 h. The significant internalization of MOR, but little internalization of the complex of RFP-MOR/eGFP-MOR, was observed during DAMGO treatment for 30 min. c The effect of monensin on the interaction of IRAS and MOR. HEK-RFP-MOR/eGFP-IRAS cells were pretreated with monensin (50 μM), and then incubated with DAMGO (5 μM) for 30 min. With the pretreatment of monensin, the internalization of RFP-MOR or eGFP-IRAS was increased; however, monensin decreased the interaction of RFP-MOR/eGFP-IRAS and blocked the recycling of MOR
To further determine the effect of interaction of IRAS/MOR on MOR trafficking, HEK293 cells expressing eGFP-IRAS/RFP-MOR was pretreated with the recycling inhibitor monensin (50 μM) for 1 h. Intriguingly, the NFRET signal of eGFP-IRAS/RFP-MOR interaction almost disappeared by monensin pretreatment, suggesting that the interaction of IRAS and MOR might associate with MOR recycling (Fig. 5c, see NFRET of eGFP-IRAS/RFP-MOR). The images also clearly showed that pretreatment of monensin led to the faster internalization of RFP-MOR induced by DAMGO, which was supposed to be due to the poor recycling of MOR back to the plasma membrane (Fig. 5c, see internalized RFP-MOR).
Then flow cytometry analysis was used to measure the internalization and recycling of MOR in CHO-MOR or CHO-MOR/IRAS cells. Results showed that treatment of DAMGO (5 μM) produced a decreased level of MOR in the cell surface of CHO-MOR cells. By contrast, MOR in the cell surface decreased at a lower speed in the presence of IRAS (Fig. 6a). Two-way ANOVA revealed a significant difference between groups (effect of IRAS: F(1,50) = 43.07, p < 0.001; time of treatment: F(4,50) = 24.03, p < 0.001; interaction: F(4,50) = 4.45, p < 0.01). Bonferroni post hoc analyses revealed that there was significant difference in the decreased MOR in the surface by treatment of DAMGO for 10 and 30 min between CHO-MOR and CHO-MOR/IRAS cells (p < 0.001, CHO-MOR vs CHO-MOR/IRAS).
Effect of IRAS on MOR internalization and recycling. a Time course of MOR internalization induced by DAMGO with or without the pretreatment of monensin. CHO-MOR or CHO-MOR/IRAS cells were incubated by DAMGO (5 μM) for 0–60 min with or without monensin (50 μM) pretreatment at 37 °C. Cell surface MOR was labeled with anti-N terminal MOR antibody followed by FITC conjugated anti-rabbit IgG and measured by FACS analysis with being quantified as % of control cell surface MOR (without DAMGO treatment). n = 5, ***p < 0.001, versus CHO-MOR, two-way ANOVA followed by Bonferroni test. b Time course of MOR recycling. CHO-MOR or CHO-MOR/IRAS cells were exposed to DAMGO (5 μM) for 1 h to induce internalization and then washed at 37 °C for 0–60 min. Cell surface MOR was measured by FACS analysis and quantified as % of control cell surface MOR (without DAMGO treatment). n = 5, *p < 0.05, versus CHO-MOR, two-way ANOVA followed by Bonferroni test
Monensin, the recycling inhibitor, however, diminished the effect of IRAS on regulating the internalization of MOR. As Fig. 6a shows, the cell surface MOR decreased at a similar rate in CHO-MOR cells and CHO-MOR/IRAS cells after monensin pretreatment (Fig. 6a). These results suggested IRAS might regulate the plasma membrane number of MOR by modulating its recycling. To test this hypothesis, we then examined the effect of IRAS on recycling of MOR by removal of DAMGO after internalization. As we expected, MOR recycled back to cell surface at a faster rate in CHO-MOR/IRAS cells comparing with that in CHO-MOR cells. Two-way ANOVA revealed the effect of IRAS in MOR recycling (Fig. 6b) (effect of IRAS: F(1,45) = 24.22, p < 0.0001; time of treatment: F(4,50) = 6.25, p < 0.0001; interaction: F(4,50) = 0.5, p > 0.05). Bonferroni post hoc analyses revealed that there was significant difference in the MOR recycling by free of DAMGO for 5 and 10 min between CHO-MOR and CHO-MOR/IRAS cells (p < 0.05, CHO-MOR vs CHO-MOR/IRAS), indicating that IRAS increased the recycling of MOR.
IRAS Prevented DAMGO-Induced Downregulation of MOR
After agonist-induced receptor internalization, internalized receptors are either recycled to cell surface or sorted to lysosomes for degradation. We then examined whether IRAS affected the downregulation of MOR. Treatment of DAMGO (5 μM) for 16 h resulted in a significant downregulation of MOR in CHO-MOR cells. Notably, downregulation of total and cell surface level of MOR was attenuated in the presence of IRAS (Fig. 7a, b). Two-way ANOVA analysis showed IRAS played a role in regulating the down-regulation of total MOR (effect of IRAS: F(1,20) = 16.38, p < 0.001; treatment: F(3,20) = 8.15, p < 0.001; interaction: F(3,20) = 18.68, p < 0.001;). Bonferroni post hoc analyses revealed that IRAS expression significantly attenuated total MOR downregulation induced by DAMGO treatment (p < 0.01, CHO-MOR vs CHO-MOR/IRAS). In addition, monensin pretreatment eliminated the effect of IRAS on regulating MOR downregulation, suggesting that recycling of internalized MOR might contribute to the effect of IRAS on decreasing MOR downregulation (Fig. 7b).
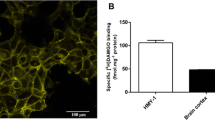
Effect of IRAS on MOR degradation. a IRAS inhibited the downregulation of MOR in the membrane as determined with [3H]diprenorphine binding. CHO-MOR or CHO-MOR/IRAS cells were treated by DAMGO (5 μM) for 16 h. Membranes were prepared, and [3H]diprenorphine binding assay (1 nM) was performed. n = 3, *p < 0.05, CHO-MOR/IRAS versus CHO-MOR, # P < 0.05, versus DAMGO-untreated group, two-way ANOVA followed by Bonferroni test. b IRAS inhibited the downregulation of MOR in the total cell as determined with immunoblotting, which was reversed by monensin pretreatment. CHO-MOR or CHO-MOR/IRAS cells were treated with DAMGO (5 μM) for 16 h with or without monensin pretreatment for (50 μM) for 1 h. Cells were lysed, and MOR was detected with immunoblotting with anti-MOR antibodies and quantified. n = 4, *p < 0.05, **p < 0.01, two-way ANOVA followed by Bonferroni test. c The effect of deletion off PX domain from IRAS on MOR downregulation. After CHO-MOR was transfected with full-length IRAS or IRAS lack of PX domain for 48 h, the cells were treated with DAMGO (5 μM) for 16 h, and then MOR was detected by immunoblotting. n = 3, *p < 0.05, two-way ANOVA followed by Bonferroni test
We then asked whether the effect of IRAS on regulating MOR depends on the interaction between IRAS and MOR. As shown above, the PX domain of IRAS mediates the interaction of IRAS/MOR. Figure 7c shows that the mutant of IRAS deleting off the PX domain (ΔPX-IRAS) failed to inhibit DAMGO-induced downregulation of MOR, indicating that the interaction between IRAS and MOR was necessary for IRAS regulating MOR downregulation.
IRAS Decreased MOR Desensitization and Facilitated Its Resensitization
Functionally, we examined whether IRAS affected DAMGO-induced desensitization of MOR. CHO-MOR and CHO-MOR/IRAS cells were pre-incubated with DAMGO (5 μM) for various time; then, DAMGO-induced inhibition of cAMP accumulation by forskolin stimulation was measured. As Fig. 8a shows, the desensitization of MOR in CHO-MOR/IRAS cells did not exhibit significant difference from that in CHO-MOR cells until the time of DAMGO treatment was prolonged to 4 h. About 40 and 60 % loss was observed in DAMGO-induced inhibition of cAMP in CHO-MOR cell after 4 and 16 h of DAMGO pretreatment, respectively, whereas 20 and 32 % loss of this function was observed in the CHO-MOR/IRAS cells (Fig. 8a), suggesting that IRAS expression decreased the long-term desensitization of MOR.
Effect of IRAS on desensitization and resensitization of MOR. a The effect of IRAS on DAMGO-induced desensitization of MOR. CHO-MOR or CHO-MOR/IRAS cells were incubated with DAMGO (5 μM) for 0–16 h at 37 °C to induce desensitization, and the effect of DAMGO on cAMP accumulation induced by 2 μM forskolin was measured. n = 4, ***p < 0.001, CHO-MOR/IRAS versus CHO-MOR, two-way ANOVA followed by Bonferroni test. b The effect of IRAS on MOR resensitization. CHO-MOR or CHO-MOR/IRAS cells were exposed to DAMGO (5 μM) for 4 h to induce desensitization, then were washed to remove DAMGO and recovered at 37 °C for 0–60 min. n = 4, values are normalized to the resensitization level of MOR at 0 min, which was considered to be 0 %. *p < 0.05, CHO-MOR/IRAS versus CHO-MOR, two-way ANOVA followed by Bonferroni test
We next investigated whether IRAS affected MOR resensitization induced by removal of DAMGO after desensitization. Figure 8b shows that MOR restored its function in decreasing cAMP accumulation in both CHO-MOR and CHO-MOR/IRAS. By comparison, MOR resensitization was higher in CHO-MOR/IRAS (the percentage of resensitization was about 17 %) than that in CHO-MOR cell (the percentage of resensitization was about 11 %) by 60 min of DAMGO removal.
Discussion
We previously showed that IRAS had a potential role in modulating opioid tolerance and dependence in vivo by using the pharmacological [5, 6] and genetic methods [7], while the underlying mechanism remained unknown. In the present study, for the first time, we demonstrate that IRAS interacts with MOR and regulates MOR trafficking in vitro. By interacting with MOR, IRAS facilitates the recycling of internalized MOR and prevents its downregulation, and hence functionally attenuates opioid-induced desensitization and accelerates resensitization of MOR. Thus, we proposed that IRAS might be a novel protein to interact with MOR and regulate MOR function activated by opioids.
In the present study, we observed that IRAS localized in the cytoplasma, where some of them were assembled as cluster and co-localized well with rab5 (the marker of early/recycling endosomes) and rab4 (the marker of short recycling endosomes). This indicated that IRAS was associated with early/recycling endosomes compartment, consistent with the results of Lim and Hong [8]. Since Rab family regulates the intracellular trafficking of membrane proteins, including GPCRs [17], the distribution of IRAS suggested it may be involved in the process of MOR trafficking. Additionally, the interaction of IRAS and MOR indicated as NFRET signal moved into the cytosol along with the internalization of MOR induced by DMAGO, further suggesting that IRAS may be associated with the process of MOR trafficking.
Our data revealed that the interaction of IRAS and MOR mediated by PX domain of IRAS played a role in trafficking of internalized MOR. PX domains, known to be involved in phosphoinositide binding, are found in a number of proteins involved in signaling and trafficking, such as SNXs [18]. PX domain also acts as a scaffolding protein to regulate the functions of other protein [19–21]. By analyzing the structure of IRAS, Piletz suggested that the PX domain of IRAS is one of the important domains which mediated protein-protein or protein-membrane interactions [4]. Lim and Hong also proposed that PX may contribute to the effect of IRAS in the trafficking since they found that PX domain (monomer) of IRAS has affinity for early/sorting and recycling endosomes [8]. The present study demonstrated PX domain of IRAS mediated its interaction with MOR, providing the direct evidence that PX domain participated in the functions of IRAS.
Following agonist treatment, internalized GPCRs are either recycled to plasma membranes or sorted to lysosomes for degradation. Some GPCRs are predominantly recycled, for example, the β2-adrenergic [22] and NK1 tachykinin receptors [23], while others are mainly trafficked for degradation, for example, the δ opioid receptor [24] and protease-activated receptor-1 [25]. We showed that IRAS regulated MOR trafficking induced by DAMGO that manifested as fasting the recycling of the internalized MOR and decreasing its downregulation. Moreover, IRAS promoted MOR resensitization and attenuated MOR desensitization. Several factors affect the development of desensitization. The rapid desensitization (within several minutes) is presumably due to the phosphorylation and arrestin binding to the receptor, and long-term loss of MOR function may be owing to the receptor degradation, loss of recycling, and cellular adaptation. We found that IRAS expression did not significantly change the rapid desensitization of MOR, but decreased the desensitization induced by prolonged treatment of DAMGO. We speculated that the possible mechanism that IRAS attenuated the desensitization of MOR function in the delayed period might be related to the facilitated recycling and decreased degradation of MOR mediated by interacting with IRAS.
The dynamic nature of receptor recycling and degradation contributes oppositely to the development of desensitization [26, 27], and desensitization is believed to be a key event leading to tolerance and dependence induced by opioid [28, 29]. Multiple evidence showed desensitization manifested as the impaired MOR-effector coupling that measured in isolated tissues, neurons, and membrane preparations after chronic morphine treatment [30, 29]. Since we found IRAS facilitated MOR recycling and resensitization and attenuated desensitization, it is reasonable to speculate that IRAS might play a role in modulating MOR tolerance to opioid agonist. Indeed, we recently found that IRAS knockout mice showed the increased analgesic tolerance and physical dependence induced by chronic exposure to methadone [7], which is consistent to our hypothesis that opioid tolerance and dependence was decreased in the presence of IRAS. Moreover, accumulating results showed that morphine dependence and tolerance was attenuated by administration of endogenous imidazoline receptor ligand, agmatine [5], as well as imidazoline I1/α2 adrenergic receptor agonist, clonidine [31], while aggravated by imidazoline receptor nonspecific antagonist, idazoxan [32], further supporting the effect of IRAS activation on regulating opioid function. Although we cannot preclude the other factors probably involved in the elevated tolerance and dependence in the absence of IRAS in vivo, such as the changed neurotransmitter or the downstream signal pathway, the change of MOR trafficking mediated by IRAS should be considered as one of the important cellular mechanisms underlying the development of opioid tolerance and dependence.
Some protein or receptor modification that determines the fate of internalized receptors has been identified so far. The proteins that are shown to be involved in recycling of GPCRs, such as N-ethylmaleimide-sensitive factor for the β2-adrenergic receptor [33], EBP50/NHERF-1 for the β2-adrenergic and κ opioid receptors [34], and GASP-1 for a number of receptors such as δ opioid receptors and cannabinoid 1 receptors [3, 35]. Now, IRAS is herein identified as a protein of which binding to MOR and causes MOR sorting to the recycling route, which provides one of the important cellular mechanisms of IRAS for decreasing opioid tolerance and dependence. By extension, since IRAS acts as a SNX to regulate MOR trafficking, whether the property of IRAS would generalize to the receptors other than MOR is needed for investigation in the future.
In conclusion, we demonstrate for the first time that IRAS interacts with MOR. Functionally, IRAS regulates the trafficking of MOR via sorting internalized MOR to the recycling pathway and hence attenuates the desensitization and facilitates the resensitization of MOR, indicating that IRAS is one of important proteins to modulate opioid tolerance and dependence.
References
von Zastrow M, Svingos A, Haberstock-Debic H, Evans C (2003) Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol 13(3):348–353
Kuijl C, Pilli M, Alahari SK, Janssen H, Khoo PS, Ervin KE, Calero M, Jonnalagadda S et al (2013) Rac and Rab GTPases dual effector Nischarin regulates vesicle maturation to facilitate survival of intracellular bacteria. EMBO J 32(5):713–727. doi:10.1038/emboj.2013.10
Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M (2002) Modulation of postendocytic sorting of G protein-coupled receptors. Science 297(5581):615–620. doi:10.1126/science.1073308
Piletz JE, Ivanov TR, Sharp JD, Ernsberger P, Chang CH, Pickard RT, Gold G, Roth B et al (2000) Imidazoline receptor antisera-selected (IRAS) cDNA: Cloning and characterization. DNA Cell Biol 19(6):319–329. doi:10.1089/10445490050043290
Li F, Wu N, Su R, Chen Y, Lu X, Liu Y, Li J (2012) Imidazoline receptor antisera-selected/Nischarin regulates the effect of agmatine on the development of morphine dependence. Addict Biol 17(2):392–408. doi:10.1111/j.1369-1600.2011.00373.x
Wu N, Su RB, Xu B, Lu XQ, Liu Y, Zheng JQ, Piletz JE, Li J et al (2005) IRAS, a candidate for I1-imidazoline receptor, mediates inhibitory effect of agmatine on cellular morphine dependence. Biochem Pharmacol 70(7):1079–1087. doi:10.1016/j.bcp.2005.07.006
Zhang L, Zhao TY, Hou N, Teng Y, Cheng X, Wang B, Chen Y, Jiang L et al (2013) Generation and primary phenotypes of imidazoline receptor antisera-selected (IRAS) knockout mice. CNS Neuroscience Therapeutics 19(12):978–981. doi:10.1111/cns.12192
Lim KP, Hong WJ (2004) Human nischarin/imidazoline receptor antisera-selected protein is targeted to the endosomes by a combined action of a PX domain and a coiled-coil region. J Biol Chem 279(52):54770–54782. doi:10.1074/jbc.M411315200
Alahari SK, Lee JW, Juliano RL (2000) Nischarin, a novel protein that interacts with the integrin alpha 5 subunit and inhibits cell migration. J Cell Biol 151(6):1141–1154. doi:10.1083/jcb.151.6.1141
Liang YJ, Wu DF, Yang LQ, Hoellt V, Koch T (2007) Interaction of the mu-opioid receptor with synaptophysin influences receptor trafficking and signaling. Mol Pharmacol 71(1):123–131. doi:10.1124/mol.106.026062
Pfeiffer M, Koch T, Schroder H, Laugsch M, Hollt V, Schulz S (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem 277(22):19762–19772. doi:10.1074/jbc.M110373200
Hanyaloglu AC, von Zastrow M (2007) A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem 282(5):3095–3104. doi:10.1074/jbc.M605398200
Wang B, Liu Y, Shan Y, Yao Z, Liu X, Su R, Sun Q, Cong Y et al (2009) Generation and characterization of novel human IRAS monoclonal antibodies. J Biomed Biotechnol 2009:973754. doi:10.1155/2009/973754
Li J, Huang P, Chen C, de Riel JK, Weinstein H, Liu-Chen LY (2001) Constitutive activation of the mu opioid receptor by mutation of D3.49(164), but not D3.32(147): D3.49(164) is critical for stabilization of the inactive form of the receptor and for its expression. Biochemistry 40(40):12039–12050
Xia ZP, Liu YH (2001) Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophy J 81(4):2395–2402
Wang DX, Sun XC, Bohn LM, Sadee W (2005) Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol 67(6):2173–2184. doi:10.1124/mol.104.010272
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2(2):107–117. doi:10.1038/35052055
Teasdale RD, Collins BM (2012) Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem J 441:39–59. doi:10.1042/Bj20111226
Jang IH, Lee S, Park JB, Kim JH, Lee CS, Hur EM, Kim IS, Kim KT et al (2003) The direct interaction of phospholipase C-gamma 1 with phospholipase D2 is important for epidermal growth factor signaling. J Biol Chem 278(20):18184–18190. doi:10.1074/jbc.M208438200
Lee CS, Kim IS, Park JB, Lee MN, Lee HY, Suh PG, Ryu SH (2006) The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol 8(5):477–U101. doi:10.1038/Ncb1401
Parks WT, Frank DB, Huff C, Renfrew Haft C, Martin J, Meng X, de Caestecker MP, McNally JG et al (2001) Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-beta family of receptor serine-threonine kinases. J Biol Chem 276(22):19332–19339. doi:10.1074/jbc.M100606200
Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW 2nd (2004) Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A 101(18):7082–7087. doi:10.1073/pnas.0308335101
Garland AM, Grady EF, Lovett M, Vigna SR, Frucht MM, Krause JE, Bunnett NW (1996) Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin2 receptors. Mol Pharmacol 49(3):438–446
Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN (2011) The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic 12(2):170–184. doi:10.1111/j.1600-0854.2010.01145.x
Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J (2002) Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell 13(6):1965–1976. doi:10.1091/mbc.E01-11-0131
Martini L, Whistler JL (2007) The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol 17(5):556–564. doi:10.1016/j.conb.2007.10.004
Grady EF, Bohm SK, Bunnett NW (1997) Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am J Physiol 273(3 Pt 1):G586–601
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ et al (2013) Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65(1):223–254. doi:10.1124/pr.112.005942
Christie MJ (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154(2):384–396. doi:10.1038/bjp.2008.100
Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ (2000) Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neuroscience 20(12):4555–4562
Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL (2014) Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLoS One 9(10):e109903. doi:10.1371/journal.pone.0109903
Su RB, Li J, Gao K, Pei G, Qin BY (2000) Influence of idazoxan on analgesia, tolerance, and physical dependence of morphine in mice and rats in vivo. Acta Pharmacol Sin 21(11):1011–1015
Cong M, Perry SJ, Hu LA, Hanson PI, Claing A, Lefkowitz RJ (2001) Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem 276(48):45145–45152. doi:10.1074/jbc.M106087200
Huang P, Steplock D, Weinman EJ, Hall RA, Ding Z, Li J, Wang Y, Liu-Chen LY (2004) kappa Opioid receptor interacts with Na(+)/H(+)-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na(+)/H(+) exchange independent of G(i)/G(o) proteins. J Biol Chem 279(24):25002–25009. doi:10.1074/jbc.M313366200
Martini L, Thompson D, Kharazia V, Whistler JL (2010) Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacol 35(6):1363–1373. doi:10.1038/npp.2010.6
Acknowledgments
The authors would like to express their gratitude to Dr. Lee-Yuan Liu-Chen (School of Medicine, Temple University, USA) for giving the advice and revising this manuscript. This work was supported by the Key Project of National Natural Science Foundation of China (No. 30930040), the National Basic Research Program of China (No. 2009CB522008 and No. 2015CB553504), and the Beijing Natural Science Foundation of China (No. 7122137).
Conflict of Interest
We declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, F., Ma, H., Wu, N. et al. IRAS Modulates Opioid Tolerance and Dependence by Regulating μ Opioid Receptor Trafficking. Mol Neurobiol 53, 4918–4930 (2016). https://doi.org/10.1007/s12035-015-9417-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9417-6