Abstract
Activation of microglia is a central event in the atypical inflammatory response occurring during prion encephalopathies. We report that the prion protein fragment encompassing amino acids 90–231 (PrP90-231), a model of the neurotoxic activity of the pathogenic prion protein (PrPSc), causes activation of both primary microglia cultures and N9 microglial cells in vitro. This effect was characterized by cell proliferation arrest and induction of a secretory phenotype, releasing prostaglandin E2 (PGE2) and nitric oxide (NO). Conditioned medium from PrP90-231-treated microglia induced in vitro cytotoxicity of A1 mesencephalic neurons, supporting the notion that soluble mediators released by activated microglia contributes to the neurodegeneration during prion diseases. The neuroinflammatory role of COX activity, and its potential targeting for anti-prion therapies, was tested measuring the effects of ketoprofen and celecoxib (preferential inhibitors of COX1 and COX2, respectively) on PrP90-231-induced microglial activation. Celecoxib, but not ketoprofen significantly reverted the growth arrest as well as NO and PGE2 secretion induced by PrP90-231, indicating that PrP90-231 pro-inflammatory response in microglia is mainly dependent on COX2 activation. Taken together, these data outline the importance of microglia in the neurotoxicity occurring during prion diseases and highlight the potentiality of COX2-selective inhibitors to revert microglia as adjunctive pharmacological approach to contrast the neuroinflammation-dependent neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation, a defense mechanism toward pathogens, traumatic injuries, and environmental toxins, is nowadays also recognized as a major mediator of neurotoxicity in various neurological and neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and transmissible spongiform encephalopathies (TSE, or prion diseases) [1].
TSE, including Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker disease (GSS), and fatal familiar insomnia (FFI) in humans, scrapie in sheep, and bovine spongiform encephalopathy (BSE) in cattle, are neurodegenerative disorders characterized by gray matter vacuolation, neuronal loss, and gliosis [2]. Prion protein (PrPC) misfolding into an aberrant isoform, called PrPSc, often, although not invariantly, deposited as amyloid aggregates in patients’ brain [3], is considered the causative event of TSE [2, 4]. PrPSc, isolated from scrapie-infected brains, induces neurotoxicity in primary neurons and brain organotypic cultures [5–7] indicating that, besides transmissibility, PrPSc accumulation is also responsible for neurodegeneration. However, a possible role of molecular intermediates along PrPC-PrPSc transition as effectors of neuronal death is still under debate [8].
Gliosis is a process that comprises proliferation and/or activation of astrocytes and microglia [9], representing a common hallmark in several neurodegenerative diseases such as AD, MS, and HIV-associated dementia [10]. As far as TSE, immunohistochemical analysis of scrapie- and CJD- affected brains demonstrated the presence of marked microglial response [11, 12]. Neuronal death and gliosis occurring during TSE can be partly mimicked in vitro by treating cell cultures with synthetic and recombinant polypeptides encompassing the crucial 106–126 amino acid PrP sequence [13–17]. The amyloidogenic peptide PrP106-126 is toxic for neurons in vitro and induces astrocytes proliferation via the activation of voltage-sensitive Ca2+ channels [18]; moreover, it activates a tyrosine kinase signaling cascade in microglial cells [19] and stimulates microglia to secrete soluble factors that exert trophic activity on astrocytes but induce apoptosis in neurons [20, 21]. However, one limitation in the use of small peptides is their intrinsic toxicity that does not allow structural studies [22].
A better structure-activity relationship characterization has been obtained after the synthesis of the recombinant fragment, encompassing the amino acids 90–231 of the human PrP (PrP90-231) [23]. This fragment corresponds to the so-called PrP27-30, the protease-resistant core of PrPSc, which is generated by a pathological metabolism of PrP [24] and accumulates in the brain of TSE-affected patients [25, 26]. PrP90-231 is a possible neurotoxic species derived from misfolded PrP [22, 27]. One of the most relevant features of PrP90-231 is its intrinsic plasticity that allows the transition from a soluble α-helix-rich into a β-sheet-prevalent and aggregation-prone conformer. This transition can be obtained in vitro inducing a partial thermal denaturation [28, 29]. The refolding process increases PrP90-231 hydrophobicity and resistance to protease K degradation and produces gain of toxicity in vitro [30]. We demonstrated that the neuronal death induced by PrP90-231 is mediated by several mechanisms, including unbalance of MAP kinase activity [31], impairment of lysosomal function [32], and potentiation of glutamatergic excitotoxicity [33]. The structure-effect relationship was further demonstrated using quinacrine and novel acridine derivatives, which prevent cell death interfering with the conformational transition [34], or using mutant peptides that are more prone to adopt a toxic three-dimensional structure [35]. Importantly, PrP90-231 in vitro neurotoxicity is comparable, as far as efficacy and mechanisms, to that induced by PrPSc partially purified form Hamster brain [6, 36], strongly validating the use of this peptide to study in vitro PrPSc toxicity. Finally, PrP90-231 in vitro neurotoxic effects are accompanied by strong activation of astrocytes and microglia, indicating its relevance also to characterize the glial reaction to PrPSc and glial-mediated neurotoxicity [37, 38].
Microglia, the CNS resident macrophages, is readily activated by different stimuli, including pathogens and particulate matter (e.g. axonal debris), acquiring phagocytic and secretive activity. Although forming the first line of brain defense, uncontrolled microglia activation may directly cause neurotoxicity through the release of inflammatory cytokines, prostaglandins (PGs), and nitric oxide (NO) radicals [39–41]. Abnormal microglial activation is observed in TSE patients [42], regulating a complex cross-talk between neurons and glial cells in brain areas characterized by PrPSc deposition [43]. In AD, the presence of numerous inflammatory mediators (PGs, cyto- and chemokines, free radicals, etc.) within neurodegenerative lesions led to the hypothesis of the causative role of neuroinflammation in neuronal injury and that anti-inflammatory drugs may act as protective agents [44], also in line with epidemiological studies showing that classical nonsteroidal anti-inflammatory drugs (NSAIDs) might prevent or retard AD progression [45].
Experimental pharmacological approaches for TSE have been, so far, mainly targeted to prion replication through the blockade of PrPSc interaction with its physiological counterpart PrPC [46–48]; less effort has been produced to prevent neuron apoptosis, acting on the pro-apoptotic activity of prions, including neuroinflammation led by glial cell activation. It is, however, conceivable to envisage that the control of excessive microglial activation could be beneficial to contrast neuronal death also in prion diseases.
In the present work, we analyzed the effects of PrP90-231 on microglia activity, focusing on cell proliferation, prostaglandin release and nitric oxide production, as mechanism of neurodegeneration, and the possible antagonism by in vivo treatment with NSAID, ketoprofen and celecoxib, with different COX selectivity.
Materials and Methods
Chemicals, Antibodies, and Dyes
Celecoxib (used at concentration of 0.5–2.5 μM), ketoprofen (used at concentration of 0.5–5 μM), and lipopolysaccharide (LPS) (used at concentration of 1 and 10 μg/ml) were purchased from Sigma-Aldrich (Milano, Italy); murine prostaglandin E2 (PGE2) was purchased by Cayman Chemical Company. High Sensitivity PGE2 EIA kit was from Arbor Assays (Ann Arbor, MI USA). All other compounds were from Sigma-Aldrich, unless otherwise specified. 3F4 mouse monoclonal anti-PrP antibody was from Covance (Princeton, NJ, USA), dilution 1:50,000; anti-iNOS antibody (C11) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA), dilution 1:1,000 for Western blot and 1:100 for immunofluorescence; anti-COX2 from Abcam (Cambridge, UK), dilution 1:1,000 for Western blot and 1:100 for immunofluorescence; Alexa Fluor 488 anti-rabbit antiserum and Alexa Fluor 594 anti-mouse antiserum were purchased by Molecular Probes (Life Technologies, Monza, Italy). 4′,6-Diamidino-2-phenylindole (DAPI) was from Sigma-Aldrich.
Synthesis and Refolding of PrP90-231
PrP90-231 was obtained from transformed E. coli and purified, as previously described [23]. To induce structural refolding, the protein was incubated for 1 h in NaCl-free 10 mM phosphate buffer, pH 7.2 at 53 °C [28]. As previously demonstrated, controlled thermal denaturation of PrP90-231 induces a three-dimensional refolding toward a β-sheet-rich structure and provokes gain of toxicity in vitro [29]. Microglial treatment was performed adding the recombinant peptide directly to the culture medium.
Cell Cultures
Primary Microglia
Cortical microglia was prepared from 7 day-old rats (Sprague Dawley, Charles-River Italia, Como, Italy) as previously reported [49]. Briefly, tissue was minced and digested with trypsin-EDTA 0.25 % (EuroClone, Milano, Italy); mixed astrocytes-microglia cell dispersion was cultured in Dulbecco’s modified Eagle’s medium (DMEM, EuroClone) supplemented with 10 % fetal bovine serum (FBS), penicillin/streptomycin (100 IU/ml), glutamine (2 mM), and maintained in 5 % CO2 atmosphere at 37 °C. When cells reached confluence, floating or barely adherent microglial cells were separated from astrocytes by shaking flasks on rotational plates for 2 h [37]. Microglial cells were cultured in RPMI medium (EuroClone) containing glutamine 2 mM, 100 U/ml penicillin, 100 μg/ml streptomycin (EuroClone), and supplemented with 10 % FBS and maintained in 5 % CO2 atmosphere at 37 °C. Experimental procedures and animal care complied with the European Communities Parliament and Council Directive of 22nd September 2010 (2010/63/EU) and were approved by the Italian Ministry of Health (protocol number 3470–1) in accordance with D.M. 116/1992. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable results.
N9 Cells
The murine immortalized microglial cell line N9 was cultured in RPMI supplemented with 5 % FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol at 37 °C in humidified 5 % CO2 incubator [38].
A1 Cell Cultures
Neuronal Mes-c-myc A1 (hereafter A1) cell line was generated from mouse embryonic mesencephalic primary culture. Phenotypical characterization and the demonstration that A1 cells, upon differentiation, retain neuronal features was described elsewhere [50–52]. A1 cells were cultured in MEM/F12 (Gibco-BRL, Milan, Italy) supplemented with 10 % FBS (Invitrogen, USA) and were induced to differentiate by serum withdrawal and stimulation with 1 mM cAMP (Sigma-Aldrich) and N2 supplement (Invitrogen).
Microglial-Mediated PrP90-231 Toxicity of A1 Cells
Microglia cells were treated with vehicle (saline phosphate buffer, PBS) or PrP90-231 (100 nM). Every 12 h, conditioned medium was collected and replaced with fresh medium containing PrP90-231. Conditioned media were centrifuged for 30 min at 16,000×g at 5 °C and then added to A1 neuronal cells (1:1 ratio with their medium). Medium conditioned by dead microglia (dried and UV-irradiated for 15 min) was also added to A1 cells in order to have an index of the cytotoxicity of residual PrP90-231 contained in the microglial-conditioned medium.
Cell Proliferation Assays
MTT Assay
Mitochondrial function, as index of cell number and viability, was evaluated by measuring the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich). The reduction of MTT to a purple formazan product by mitochondrial dehydrogenase was quantified spectrophotometrically, as previously reported [53]. Briefly, cells were incubated for 1 h with 0.25 mg/ml MTT, in serum-free DMEM at 37 °C; after medium removal, formazan crystals were dissolved in dimethylsulfoxide and absorbance was spectrophotometrically measured at 570 nm.
Cell Proliferation Assay CyQUANT®
DNA synthesis, as index of cell proliferation, was determined by fluorescence kit CyQUANT® (Life Technologies, USA) [54]. After treatments, cells were incubated with CyQUANT® for 1 h, following manufacturer’s instructions. The dye-DNA complexes were read using a microplate fluorescence reader (Tecan® Infinite 200 Pro, Switzerland) ex/em 485/530 nm.
Cell Counting
To directly evaluate the effects of PrP90-231 and LPS on the proliferation of N9 cells, we treated these cells for 4 days and counted them with an automated cell counting test. N9 cells were harvested by trypsin-EDTA and the cell suspension diluted 1:10 in sterile DPBS and mixed with an equal volume of 0.4 % Trypan Blue solution to evaluate the number of live/dead cells using TC20 Cell Counter (Bio-Rad Laboratories, Inc., Hercules, CA).
mRNA Expression
Total RNA was prepared from N9 cells by using the Pure Link® RNA Mini Kit (Life Technologies) according to the manufacturer’s protocol. Total RNA was reverse-transcribed using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) with 1 μg of total RNA and oligodT. The following primers (from Tib-Molbiol, Genova, Italy) were used: iNOS: forward 5′-TCCTACACCACACCAAAC-3′; reverse 5′-CTCCAATCTCTGCCTATCC-3′ (NM_010927.3), β-actin: forward 5′-TGTGATGGTGGGAATGGGTCAG-3′; reverse 5′-TTTGATGTCACGCACGATTTC-3′) [55]. Reactions were performed with I-Cycler PCR system (Bio-Rad) for one cycle (1 min at 94 °C) followed by 30 cycles (15 s at 94 °C, 30 s 63 °C, and 30 s at 72 °C) ended by 2 min at 72 °C. PCR products were separated on 1.5 % agarose gels and visualized by ethidium bromide staining. Reactions amplified fragments of 198 bp for NOS and 514 bp for β-actin.
Determination of NO Release (Griess Reaction)
Accumulation of nitrites (NO2−) in culture medium, as an indicator of NO release, was measured by the Griess reaction [56]. Briefly, cells were treated in serum-free DMEM without phenol red; after treatments, 50-μl culture supernatant and Griess reagent (1:1) were incubated at room temperature for 15 min. Nitrite amount, proportional to color development, was determined as absorbance at 540 nm (Tecan® Infinite 200 Pro).
PGE2 Secretion Assay
PGE2 release from N9 cell line and microglial primary cultures were measured by competitive enzyme immunoassay kit (High Sensitivity PGE2 EIA kit; Arbor Assays). After 24 h of treatment with PrP90-231 with or without celecoxib, the amount of PGE2 in the supernatants was spectrophotometrically detected using a microtiter plate reader (Tecan® Infinite 200 Pro, abs. 450 nm).
Western Blot
Cells were lysed in NaCl 150 mM, Tris/EDTA 20 mM pH 8, glycerol 10 %, NP-40 1 %, protease inhibitor cocktail (Complete mini, Roche Diagnostic, Mannheim, Germany), PMSF 1 mM, orthovanadate 1 mM. Cell debris and nuclei were discarded by brief centrifugation, and protein content was determined by Bradford assay. Proteins (30 μg/lane) were size-fractionated by SDS-PAGE, blotted into PVDF membrane and probed with primary antibodies diluted in PBS-TWEEN 20 0.1 %. Immunoreactivity was detected with peroxidase-linked anti-rabbit IgG (dilution 1:5,000) (GE-Healthcare) followed by ECL (Millipore) [57] and quantified by densitometry using a Chemidoc-MP-4.0.1 image lab software (Bio-Rad).
Indirect Immunofluorescence Detection of COX2 and iNOS Expression
N9 cells were grown on glass coverslips, fixed in 4 % paraformaldehyde, rinsed in PBS (pH 7.4), and treated with 0.1 M glycine and Triton X-100 (0.1 % in PBS). Non-specific sites were blocked in 10 % normal goat serum for 30 min. After incubation with primary antibodies for 1 h at r.t., secondary Alexa Fluor 488 anti-rabbit and AlexaFluor 594 anti-mouse antisera (1:200; Molecular Probes, Invitrogen, Milano, Italy) were added for 1 h at r.t. After neuclear counterstain with DAPI (Sigma-Aldrich), coverslips were mounted with Mowiol (Calbiochem). Immunofluorescent staining was visualized and photographed with a DM2500 microscope (Leica Microsystems, Wetzlar, Germany) equipped with a DFC350FX digital camera (Leica Microsystems). Quantification of the level of cellular fluorescence from microscopy images was performed using the NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Statistics
Data were obtained by three independent experiments conducted in quadruplicate, unless otherwise specified. Statistical analysis was performed by means of one-way ANOVA.
p < 0.05 was considered statistically significant.
Results
PrP90-231 Effects on Microglia Activation
N9 microglia cell line reproduces in vitro many functional features of primary microglia cultures [58]; remarkably, both Aβ and prion peptides induce in these cells a complex pattern of response including chemotaxis, phagocytosis, and production of soluble pro-inflammatory factors comparable to what was observed in vivo during AD and TSE [37, 59–61]. To characterize the role of microglia activation in PrP90-231 neurotoxicity, we first analyzed the morphological and proliferative response of N9 cells to this prion fragment, using concentrations (0.1 and 1 μM) that we previously reported to affect microglia activity [37]. In comparison to control cells (Fig. 1a), PrP90-231 (1 μM) treatment caused a time-dependent morphological alteration that was detectable already after 2 days, with an evident increase in ramified cells (Fig. 1c). This effect was associated with a significant reduction of cell proliferation but not with cell death. In particular, N9 treatment with PrP90-231 reduced cell growth by 27 and 59 %, after 2 and 4 days, respectively (Fig. 2a). As positive control, to outline the sensitivity of microglial cells to pro-inflammatory stimuli, we assessed the activity of the pro-inflammatory bacterial endotoxin LPS on N9 morphology and proliferation. LPS elicited morphological change of microglial cells, which showed a ramified phenotype (Fig. 1b) and reduced proliferation in a statistically significant manner, although at a lower extent when compared to PrP90-231 (Fig. 2a). Importantly, PrP90-231 and LPS effects on N9 cell proliferation were confirmed using the CyQuant labeling assay, which detects double strand nucleic acids and represents a more direct index of cell number. Four days treatment with PrP90-231 (100 nM) or LPS (10 μg/ml) produced a highly significant reduction of cell growth, that reached almost 50 % in PrP90-231-treated N9 cells (Fig. 2b). Similar effects were also obtained in microglial primary cultures, using the CyQuant proliferation assay, in which growth arrest was induced after treatment with both PrP90-231 (reduction of 30 and 42 % in cell number with 0.1 and 1 μM PrP90-231, respectively) and LPS (−38 and −55 % with 1 and 10 μg/ml LPS, respectively) (Fig. 2c). This effect was mainly cytostatic as further demonstrated by the cell growth recovery occurring after PrP90-231 wash-out (data not shown), and by automated cell counting and Trypan blue exclusion test that showed a reduction in cell number without inducing cell death (Fig. 2d). These different approaches fully validated the results obtained using the MTT assay, excluding that the observed results are dependent on reduced mitochondrial activity rather than modification in cell number or on increased exclusion of formazan crystals from the cells, induced by PrP90-231 as described for Aβ1-42 [62].
PrP90-231 inhibits proliferation rate of microglial cells. a PrP90-231 (100 nM) and LPS (10 μg/ml) reduced N9 cell proliferation in a time-dependent manner. Values are obtained by MTT reduction assay after 2 and 4 days of treatment and are expressed as percentage on untreated controls. b Four days of treatment with PrP90-231 (100 nM) and LPS (10 μg/ml) produced a significant inhibition of N9 cell proliferation. Values are obtained by CyQUANT® test and expressed as percent of untreated control. c PrP90-231 (0.1–1 μM) and LPS (1–10 μg/ml) treatment of microglial primary cultures reduced cell proliferation in a concentration-dependent manner. Values are obtained by CyQUANT® test and expressed as percent of untreated control. Data are the mean values ± SEM of three independent experiments performed in quadruplicate. d Inhibition of proliferation rate of N9 cells treated with PrP90-231 or LPS. Treatment of N9 cells with PrP90-231 (100 nM) or LPS (10 μg/ml) inhibited cell proliferation, as determined by live cell counting and Trypan Blue exclusion test, using a TC20 automated cell counter. Cell number was evaluated at t0 (soon after cell plating) and after 4 days of treatment with vehicle, PrP90-231, or LPS. Values are expressed as percentage of cell number at t0. A significant decrease in cell number was observed after both treatments, without inducing cell death. Live cells: light gray bars; dead cells: dark gray bars. Data are the mean values ± SEM of three independent experiments performed in quadruplicate. *p < 0.05 and **p < 0.01 vs. control
PrP90-231 Stimulates Microglial Cell to Produce Nitric Oxide and PGE2
Microglia activation is associated with the secretion of several cytokines and pro-inflammatory molecules that participated to the atypical neuroinflammation occurring during neurodegenerative disease. Thus, we investigated whether PrP90-231 in vitro modulation of pro-inflammatory factor release by microglia was responsible for neuronal death. We mainly focused our study on microglial release of both NO and PGE2. PrP90-231, concentration-dependently, increased NO release from both N9 (Fig. 3a) and microglia primary cultures (Fig. 3c) resulting in a 3-fold increase over the baseline for the concentration of 1 μM. PrP90-231 also induced a statistically significant PGE2 secretion in both cell cultures (Fig. 3b, d), showing an increase of about 2-fold over basal production. Similarly, LPS stimulates NO (Fig. 3a, b) and PGE2 secretion (Fig. 3c, d) in both N9 and primary microglia.
PrP90-231 induces NO and PGE2 secretion by microglial cells. Treatment of N9 cells (a) and microglial primary cultures (b) with PrP90-231 (100 nM–1 μM) or LPS (10 μg/ml) stimulates NO release. NO production, was determined by Griess assays after 24 h of treatment and expressed as percent of untreated controls. PGE2 secretion was measured, by immunoassay, in N9 (c) and microglial primary cultures (d) after 24 h of treatment with PrP90-231 (100 nM) and LPS (10 μg/ml). PGE2 concentration is expressed in pg/ml. Data are the mean values ± SEM of three independent experiments done in quadruplicate. **p < 0.01 vs. control
The effects of PrP90-231 on NO and PGE2 release were related to increased expression of both inducible NO synthase (iNOS) and COX2, evaluated in N9 cells by indirect fluorescence labeling (Fig. 4a) and in microglial primary cultures by immunoblotting (Fig. 5a, b). iNOS and COX2 expression was also induced by LPS (Figs. 4 and 5), enforcing the assumption that, under our in vitro experimental conditions, both N9 and microglial primary cells respond to PrP90-231 treatment with the same enzymatic activation than typical pro-inflammatory stimuli.
Detection of iNOS and COX2 expression in N9 cells by indirect immunofluorescence staining. a Cells were treated for 24 h with PrP90-231 (100 nM) or LPS (10 μg/ml) and immunostained with specific antibodies directed against COX2 and iNOS. Antisera conjugated with Alexa Fluor® 594 or Alexa Fluor® 488 dyes were used to detect iNOS and COX2 expression, respectively. PrP90-231 and LPS induced a significant expression of both iNOS and COX2. Nuclear counterstaining was obtained by fluorescent DAPI. Scale bar 50 μm. COX2 (b) and iNOS (c) fluorescent staining of each microscopy image was quantified using Image J software. COX2 (green) and iNOS (red) expression was estimated as percentage of DAPI (blue) fluorescence, representing total cell number. Values represent the mean ± SEM of three independent experiments performed in quadruplicate. **p < 0.01 vs. vehicle
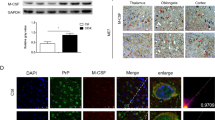
PrP90-231 induces the expression of COX2 and iNOS in microglial cells. COX2 (a) and iNOS (b) expression in microglial primary cultures in vehicle-, PrP90-231- or LPS-treated cells, was evaluated by immunoblotting and quantified by densitometric analysis. Treatment with PrP90-231 (100 nM) and LPS (10 μg/ml) significantly increased the expression of both COX2 and iNOS. Equal protein loading of each lane is showed by α-tubulin expression. Values represent the mean ± SEM of three independent experiments performed in quadruplicate and represent the ratio between COX2 or iNOS band intensity and their respective α-tubulin expression.**p < 0.01 vs. control
Conditioned Media from Activated Microglia Are Toxic to Neuronal A1 Cell Line
To ascertain the relevance of the PrP90-231-dependent microglia activation as mediator of neurotoxicity, we investigated whether in vitro-cultured microglia is able to produce functionally active amounts of toxic species upon treatment with this peptide. To this purpose, we evaluated the survival of A1 immortalized mesencephalic neurons by MTT test after incubation with conditioned medium derived from microglial cells treated for 2 days with PrP90-231 (100 nM). Since PrP90231 is per se neurotoxic for A1 cells, to minimize direct cytotoxic effects of the residual peptide derived from microglia treatments, conditioned media were high-speed centrifuged before being added to A1 cells, to clear up the PrP90-231. In fact, PrP90-231 undergo to spontaneous aggregation after 48 h incubation at 37 °C [30], favoring its precipitation as macroaggregates. Furthermore, to exclude the possibility that A1 cell death was induced by spontaneous release of neurotoxic factors from microglia, independently from the activation induced by PrP90-231, we repeated the experiment using conditioned media derived from microglia treated with vehicle (PBS) or media conditioned by dead microglia (dried and exposed to UV rays for 30 min before the treatment with PBS or PrP90-231). While conditioned medium from PBS-treated microglia did not affect A1 cells viability, conditioned medium from PrP90-231-treated microglia cultures caused a strong reduction of A1 viability (Fig. 6a). PrP90-231 conditioned medium derived from UV-treated microglia cells was modestly effective, although some residual A1 toxicity was detected (Fig. 6a). These data strongly suggest that, in this experimental setting, neuronal toxicity is determined by soluble molecules secreted by microglia cells after PrP90-231 treatment. In order to delve deeper in the possible mediators of neuronal death produced by PrP90–231-activated microglia, we measured NO content in the same conditioned media used for cell toxicity experiments (Fig. 6b). High levels of NO were detected only in the medium derived from microglial cells treated with PrP90-231, the experimental condition that caused the highest toxicity of A1 cells. Conversely, the medium of cells subjected to UV treatment contained minimal concentrations of NO (Fig. 6b). These data excluded that the low toxicity observed after PrP90-231 treatment of UV-treated microglia was due to the presence of toxic species, and allowed us to hypothesize that in this experimental condition, a small amount of residual PrP90-231 could be present in the conditioned medium, thus exerting direct toxicity. Thus, we performed immunoblotting analysis to detect the presence of PrP90-231 in the culture media conditioned by live and dead cells (Fig. 6c). To this purpose, media were centrifuged at 16,000×g for 30 min and subjected to immunoblotting using the anti-PrP 3F4 antibody. In the conditioned medium derived from living cells, no 3F4-immunoreactive bands were detected (Fig. 6c), unlike with the significant amounts of PrP90-231 identified in the conditioned medium by UV-treated microglia. Further, we show that live microglia exert a highly efficient phagocytosis of PrP90-231, as revealed by indirect immunostaining that identify the presence of large amounts of 3F4-positive clusters in the majority of microglia, after PrP90-231 exposure (Fig. 6d). This result indicated that microglia activation by PrP90-231 is associated to (or caused by) a phagocytic activity able to remove the peptide from the culture medium (Fig. 6c). Conversely, this PrP fragment persists in the medium when added to dead microglia: this residual soluble PrP90-231 could exert a direct toxicity on A1 cells, accounting for the low toxicity observed using this conditioned medium.
Medium conditioned by microglial cells treated with PrP90-231 is toxic to A1 cells. a Conditioned medium (CM) from live or dead microglial cells, treated with either vehicle and PrP90-231 (100 nM), was added to A1 neuronal cell line. A1 viability was assessed after 48 h by MTT test. Data are the mean values ± SEM of three independent experiments performed in quadruplicate **p < 0.01 vs. vehicle; §§ p < 0.01 vs. PrP90-231-treated UV exposed microglia; b Analysis of NO release induced by PrP90-231 (100 nM) in live and UV-irradiated microglial cells. c Immunoblotting analysis of residual PrP90-231 contained in culture medium in the presence of live or dead microglia. Live microglial removes PrP90-231 from medium beyond detectable level. In contrast, medium from UV-irradiated cells show detectable amount of uncleaved PrP90-231. PrP90-231 aliquot used for cell treatment was also immunoblotted (first lane). d PrP90-231 is internalized in primary microglia cells. Cells were treated overnight with PrP90-231 (100 nM), fixed with paraformaldehyde (4 %) and immunostained with the specific anti-PrP antibody (clone 3F4). DAPI was used to counterstain cell nuclei. Images, collected by fluorescence microscopy reveals that primary microglia actively uptake high amount of PrP90-231. Scale bar 50 μm
On their whole, these data support the idea that, in this experimental setting, neurotoxic factors released by microglia in response to PrP90-231 are responsible for A1 cell death.
Effect of Cyclooxygenase Inhibitors on PrP90-231-Induced Microglia Activation
To investigate whether microglia activation could be pharmacologically regulated, we evaluated the efficacy of COX1 and COX2 preferred inhibitors, ketoprofen and celecoxib, respectively, to prevent PGE2 secretion induced by PrP90-231.
Four days of treatment with both ketoprofen (0.5–5 μM) and celecoxib (0.5–2.5 μM) did not significantly modify N9 cell proliferation rate (data not shown), suggesting that both COX isoforms are not primarily involved in the regulation of cell growth in resting microglia cells. Conversely, celecoxib was able to completely revert N9 growth inhibition induced by PrP90-231 (100 nM, for 2 days) and significantly reduced this effect after 4 days of treatment (Fig. 7), while ketoprofen was ineffective (Fig. 7). These results suggest that COX2, but not COX1, is primarily involved in microglia activation by PrP90-231.
Effect of celecoxib and ketoprofen on glial activation induced by PrP90-231. N9 cells were pretreated for 2 h with celecoxib (2.5 μM) or ketoprofen (5 μM) before being exposed to PrP90-231 (100 nM). Cell proliferation was evaluated by MTT assay after 2 and 4 days. Celecoxib, but not ketoprofen, significantly reverted PrP90-231 antiproliferative effects in a highly significant manner. **p < 0.01 vs. untreated control; §§ p < 0.01 vs. PrP90-231-treated samples
Microglia activation by PrP90-231 also caused release of NO that, transformed in highly reactive peroxynitrite radicals, is able to react and alter membrane phospholipids and proteins. For this reason, the intensity of NO release from microglia is considered a major index to determine microglia-mediated neurotoxicity during TSE, and a relevant biological parameter to evaluate the anti-prion therapeutic potential of anti-inflammatory drugs. Thus, we investigated the effect of celecoxib and ketoprofen on PrP90-231-induced iNOS expression and NO production from N9 cells. iNOS mRNA was examined by RT-PCR amplification and quantified by densitometric analysis (Fig. 8a). N9 cells, treated for 6 h with PrP90-231 (100 nM) or LPS (10 μg/ml) showed a marked increase in iNOS mRNA (+65 and +70 % vs. untreated control cells). Celecoxib (1 μM), but not ketoprofen (5 μM), while did not affect basal iNOS mRNA expression, caused a slight, but significant from a statistical point of view, inhibition of the increment in iNOS mRNA induced by PrP90-231 (Fig. 8a). Similar results were obtained analyzing by immunoblotting the effects of celecoxib on iNOS protein expression stimulated by PrP90-231 (Fig. 8b). PrP90-231 (100 nM) and LPS (10 μg/ml) treatment of N9 cells increased iNOS protein levels that were partly reduced, although reaching statistical significance, in the presence of celecoxib (−55 % vs. PrP90-231 treated cells).
Effects of celecoxib and ketoprofen on iNOS expression in N9 treated with PrP90-231. a N9 cells were pretreated with celecoxib (2.5 μM) and ketoprofen (5 μM) 2 h before being treated with PrP90-231 (100 nM). After 6 h of incubation, iNOS mRNAs was amplified by RT-PCR experiments. Amplification products were quantified as iNOS/β-actin ratio by densitometry, and expressed as percent on untreated controls. Values represents the average ± of three independent experiments. *p < 0.05 vs. control values; § p < 0.05 vs. PrP90-231 values. b N9 cells were pretreated with celecoxib (2.5 μM) before being exposed to PrP90-231 (1 μM) or LPS (1 μg/ml). iNOS expression was evaluated by immunoblotting, immunoreactivity was quantified by densitometry and expressed as percentage of iNOS expression in the untreated controls. Values represents are the mean ± SEM of three experiments *p < 0.05 vs. control
The different efficacy between celecoxib and ketoprofen in affecting iNOS expression in activated microglia prompted us to analyze whether COX1 and/or COX2 blockade reduces NO production by microglial cells activated by PrP90-231 or LPS. N9 cells were treated with PrP90-231 or LPS in the presence or absence of celecoxib or ketoprofen. NO release was measured after 24 h of treatment, as nitrate accumulation in the conditioned media, using the Griess reaction (Fig. 9a, b). PrP90-231 (0.1–1 μM) led to a highly significant increase of basal level of NO release (+178 and +326 %, respectively); similar effects were induced by LPS (+235 % of untreated controls, Fig. 9a). In the presence of celecoxib (2.5 μM), NO release, elicited by both concentrations of PrP90-231 and by LPS, was almost completely abolished. Conversely, ketoprofen had no significant inhibitory effect on NO secretion (Fig. 9b). Similar results, although less evident, were obtained treating primary microglia cultures with celecoxib before adding PrP90-231 (0.1–1 μM) (Fig. 9c). In these experiments, PrP90-231-induced NO production (+256 % vs. control) was significantly reduced by celecoxib (2.5 μM) pretreatment.
Effects of celecoxib or ketoprofen on NO release induced by PrP90-231 in microglial cells. N9 cells were pretreated with celecoxib (2.5 μM), ketoprofen (5 μM), for 2 h before being treated with PrP90-231 (100 nM and 1 μM) or LPS (10 μg/ml). After 24 h of treatment, NO release was detected by Griess assay. While celecoxib (a) almost completely abolished NO release induced by PrP90-231 or LPS, ketoprofen (b) was not effective. c Microglial primary cells were pretreated for 2 h with celecoxib (2.5 μM) before being treated with PrP90-231 (0.1–1 μM) or vehicle. After 24 h NO release was detected by Griess assay. Celecoxib significantly prevented NO release by microglial cells in response to PrP90-231. **p < 0.01 vs. vehicle; °°p < 0.01 vs. LPS; §§p < 0.01 vs. PrP90-231 100 nM; #p <0.05 and ##p < 0.01 vs. PrP90-231 1 μM
Altogether, these data suggest that the high NO production induced by PrP90-231 in microglial cells, possibly reaching neurotoxic levels, could be antagonized by celecoxib.
Inhibitory Effects of Celecoxib on PGE2 Production in Activated Microglial Cells
PrP90-231-mediated microglia activation is characterized by a significant increase in PGE2 secretion also representing a marker of the atypical inflammation occurring in course of TSEs [63]. Thus, we measured the inhibitory effects of celecoxib on PGE2 secretion, induced by 24 h of treatment with PrP90-231 (100 nM) or LPS (10 μg/ml) in N9 cells (Fig. 10a) and microglial primary cultures (Fig. 10b). PGE2 release by N9 cells increased from the basal level of 325 pg/ml to 490 and 470 pg/ml following PrP90-231 or LPS treatment, respectively (Fig. 10a). Similar results were obtained in microglia primary cultures, in which PGE2 secretion was strongly induced by PrP90-231 and LPS, which caused PGE2 levels to increase from 490 pg/ml (basal level) to 851 and 770 pg/ml, respectively (Fig. 10b). These effects were significantly reduced by celecoxib pretreatment that significantly inhibited PGE2 release in N9 cells, and completely abolished it in microglial primary cell cultures (Fig. 10a, b).
Celecoxib inhibits PGE2 secretion induced by PrP90-231 or LPS. a N9 cells and b microglial primary cultures were pretreated with vehicle or celecoxib (2.5 μM) 2 h before being treated with PrP90-231 (100 nM) or LPS (10 μg/ml). After 24 h of treatment, PGE2 release was measured by competitive enzyme immunoassay. **p < 0.01 vs. control; §§p < 0.01 vs. LPS; °p < 0.05 vs. PrP90-231 °°p < 0.01 vs. PrP90-231
Activation of iNOS and COX2 Are Independent Events
The dual effect of celecoxib that was able to revert both NO and PGE2 release induced by PrP90-231 in microglial cells prompted us to investigate whether the two events could be related with the increased PGE2 levels in the culture medium as a main stimulus for NO release. However, we observed that the treatment of the cells with PGE2 did not induce NO release (Fig. 11a), suggesting that PGE2 production does not influence iNOS activity, and, thus, that celecoxib inhibition of NO release is not dependent on the inhibition of COX2 activity. Similarly, we measured PGE2 release from microglia treated with sodium nitroprusside (SNP, 100 μM), a NO donor, to verify whether PGE2 release was dependent on NO levels in the extracellular space. We did not observe significant increase of PGE2 production in comparison with untreated controls, but conversely, we found that the high NO levels caused a reduction in PGE2 secretion (Fig. 11b). These results demonstrate that the activation of iNOS and COX2 by PrP90-231 are independent effects and that celecoxib is able to directly interfere with both enzyme activity.
PGE2 and NO production induced by PrP90-231 follows independent pathways. a NO release was measured, by Griess assay, in N9 cells treated for 24 h with PrP90-231 (100 nM) (used as positive control) or PGE2 (1 μM). PGE2 did not evoke significant stimulatory effects on NO production. b N9 cell were treated for 24 h with PrP90-231 (100 nM) or sodium nitroprusside (SNP, 100 μM), and PGE2 secretion was measured. Whereas PrP90-231 induced a significant increase of PGE2 production, sodium nitroprusside exerted an inhibitory effect. *p < 0.05 vs. control; **p < 0.01 vs. control. Data represent the mean values ± SEM, of three independent experiments performed in triplicate
Finally, we evaluated, by MTT assay, the possible direct toxicity of NO (using SNP) and PGE2 on A1 neurons viability. SNP, concentration-dependently (0.5–50 mM), caused a highly significant A1 cell death (max effect: 94 % reduction in viability), which was statistically significant already at the lower concentration tested. However, this effect that was not reproduced by treatment with exogenous PGE2 (0.5–500 μM), that even at the maximal concentration tested did not reached statistical significance (−14 %).
Discussion
In prion diseases, neuronal loss is associated with atypical neuroinflammation, characterized by microglial activation and production of both NO and PGE2 [12, 64]. Recruitment and activation of microglia are early events in TSE pathogenesis and often precedes the onset of neurological symptoms [11, 21]. Notably, the presence of phagocytic microglia has been described in the brain of prion-affected humans and animals where it co-localizes with spongiotic areas and PrPSc deposits [65]. PrPSc activates a functional cross-talk involving neurons, astrocytes and microglial cells, being the latter stimulated to release multiple soluble factors including prostaglandins, cytokines, and reactive species of oxygen and nitrogen [43, 66, 67]. In prion diseases, as well as in other neurodegenerative proteinopathies, microglia play a double role in modifying the progression of the disease. On one hand, microglial cells act as scavengers of amyloid material through either the secretion of amyloid-degradative metalloproteases [68] or by phagocytosis [69]. In particular, it was demonstrated that in course of prion-induced neuronal death, microglial activation is induced by neuronal apoptotic bodies, and that microglial phagocytosis is crucial to clear brain tissue from PrPSc amyloid, resulting in a delay of the neurodegenerative process [70]. On the other hand, microglia-induced neuroinflammation and oxidative stress play a major role in determining neuronal death. Immunohistochemical analysis of human and animal prion-infected brain tissues revealed the presence of oxidative stress markers suggesting that the significant release of pro-inflammatory cytokines and neurotoxic free radicals from activated microglial cells could contribute to neuronal loss [71–73]. Thus, PrPSc-dependent prolonged astrocyte and microglia activation results in high and sustained levels of reactive species with detrimental consequences for neuronal survival. So far, however, anti-inflammatory therapies have not proven beneficial to slow the progression of AD neurodegeneration and it has been hypothesized that the inhibition of microglial activation could represent a clinically useful goal only in the early stages of the disease, when initial amyloid deposition occurs [74, 75].
Among the therapeutic strategies pursued, so far unsuccessfully, to slow-down neurodegeneration in TSE, the most promising are targeted to prevent PrPSc replication to inhibit the interaction between PrPC-PrPSc and the formation of PrPSc neurotoxic oligomers [76]. However, multi-target strategies against prion neurotoxicity should also consider the control of microglial overreaction using anti-inflammatory drugs, in particular those tested against microglial activation in AD [77]. Glial cultures have been widely used to characterize molecular aspects of PrPSc-associated neuroinflammation because both astrocytes and microglia are highly responsive to PrPSc, as well as to synthetic peptides modeling its effects [18, 78–80]. Importantly, polypeptides corresponding to specific amyloidogenic portions of PrP are able to induce microglia to release prostaglandins, free radicals, including NO, and cytokines in vitro [20, 37, 81–83]. Moreover, significant evidence supports the neurotoxic role of NO in neuronal cell death, both in vivo [84, 85] and in vitro [86], thus validating our model of amyloid-driven neuroinflammation. The ability of microglia to potentiate the neurotoxic effects of amyloidogenic peptides has been described in microglia-neurons co-cultures demonstrating that Aβ1-40 and 1–42 cause neuronal death through the activation of microglial cells [87, 88].
The aim of the present work is to delve deep into the cellular mechanisms associated with prion neurotoxicity, focusing on the pathogenic relevance of diffusible molecules produced by microglial cells upon their exposure to PrPSc oligomers, using PrP90-231 as a model. To mimic PrPSc-induced neuroinflammatory response in vitro, we used microglial cells derived from primary cultures and the immortalized microglial cell line N9. Both cell types showed a robust activation by PrP90-231, that was comparable, or even higher to that induced by LPS, a known pro-inflammatory molecule. We demonstrate that prolonged treatment of microglial cells with PrP90-231 conditioned the culture medium leading, when transferred to neuronal A1 cells, to a significant reduction of cell viability.
We focused our investigation on NO production because its overproduction contribute to neuronal death in several neurodegenerative conditions, including TSE [82]. While nearly undetectable in basal conditions, iNOS expression and NO production were significantly induced after PrP90-231 treatment. Importantly, conditioned medium from PrP90-231-treated microglia was highly enriched in NO content, that significantly contributed to the toxic effects on A1 mesencephalic neurons. In fact, we demonstrate that these neurons are highly sensitive to elevated NO concentration, as shown by the neurotoxicity induced by the NO donor SNP. These effects were specific since conditioned medium from untreated cells or PrP90-231-treated dead microglia were virtually devoid of neurotoxic effects. We observed only a low level of cell death in the latter condition, likely due to residual PrP90-231 soluble oligomers persisting in the conditioned medium and exerting direct toxicity on A1 cells, independently from microglia secreted factors. In fact, in these experiments, live microglial cells actively remove PrP90-231 from the medium by phagocytic internalization and PrP90-231 was not present in the conditioned medium. This was not the case treating dead microglia, and, since the peptide cannot be uptaken by the cells, it was not completely removed after centrifugation, and partially still present in the conditioned medium. Thus, PrP90-231 persistence as soluble oligomers could be responsible for the low neurotoxic activity observed in these experimental conditions.
In addition to NO, PrP90-231-stimulated microglia also release high amounts of PGE2 due to a prolonged induction of COXs. Thus, we investigated whether COX inhibitors could counteract microglia activation induced by PrP90-231. We compared the effects of COX1- and COX2-preferential inhibitors, ketoprofen and celecoxib, respectively. Interestingly, our results demonstrated that celecoxib, but not ketoprofen, reverted all the effects elicited in microglial cells by PrP90-231, and that, besides small quantitative differences, this effect was observed in both microglia primary cultures and N9 cells. PrP90-231-induced microglia proliferation arrest was significantly reverted by celecoxib pretreatment. Moreover, celecoxib impaired microglial response to PrP90-231 causing a significant reduction of PGE2 production, but also powerfully inhibiting iNOS expression and NO release. Previous studies are concordant to indicate that the blockade of COX2 and iNOS activities provides with protection against neuroinflammation induced by amyloidogenic peptides [75, 89, 90]. More surprisingly, we found that the release of the two mediators were independent events rather than being reciprocally modulated. In fact, the presence of high PGE2 concentrations did not induce NO release, and the NO donor SNP did not evoke PGE2 release. These data suggests not only that iNOS activity is not directly stimulated by PGE2, and vice versa COX2 is not induced by NO, but also that celecoxib affects independently the activity of the two enzymes. In line with this COX-independent activity of celecoxib, recent studies identified a direct modulation of the endoplasmic reticulum stress response, via the inhibition of the sarcoendoplasmic reticulum Ca2+ ATPase, able to affect cell viability [91, 92].
Although the reciprocal modulation of iNOS and COX2 in activated microglia is still matter of controversy and did not represent a major goal of our work [93–95], it is possible that in this cell population COX2 and iNOS expression are both stimulated by PrP90-231 through the activation of a common inflammatory pathway, directly or indirectly inhibited by celecoxib. This hypothesis is in agreement with several reports in which the induction iNOS and COX2 passes through common enzymatic cascades and nuclear translocation factors [96–98]. Interestingly, although prostaglandin production by microglia was demonstrated in TSE patients and several experimental models (included this study), we did not observe a direct toxic effect of PGE2 on A1 neurons. Thus, we can speculate that the role of prostaglandins in the atypical neuroinflammation occurring during TSE is not related to a direct neurotoxic effect, as we propose for NO, but might contribute to sustain microglia activation, as observed in other tissues.
In conclusion, our study supports the possibility that the control of neuroinflammation by COX2 inhibitors represent a valuable approach within a multi-target pharmacological anti-prion therapy.
References
Skaper SD (2007) The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci 1122:23–34
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95(23):13363–13383
Corsaro A, Thellung S, Villa V, Nizzari M, Florio T (2012) Role of prion protein aggregation in neurotoxicity. Int J Mol Sci 13(7):8648–8669
Prusiner SB (1998) The prion diseases. Brain Pathol 8(3):499–513
Muller WE, Ushijima H, Schroder HC, Forrest JM, Schatton WF, Rytik PG, Heffner-Lauc M (1993) Cytoprotective effect of NMDA receptor antagonists on prion protein (PrionSc)-induced toxicity in rat cortical cell cultures. Eur J Pharmacol 246(3):261–267
Sorrentino S, Bucciarelli T, Corsaro A, Tosatto A, Thellung S, Villa V, Schinina ME, Maras B, Galeno R, Scotti L, Creati F, Marrone A, Re N, Aceto A, Florio T, Mazzanti M (2012) Calcium binding promotes prion protein fragment 90–231 conformational change toward a membrane destabilizing and cytotoxic structure. PLoS One 7(7):e38314. doi:10.1371/journal.pone.0038314
Falsig J, Julius C, Margalith I, Schwarz P, Heppner FL, Aguzzi A (2008) A versatile prion replication assay in organotypic brain slices. Nat Neurosci 11(1):109–117
Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302(5646):871–874
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50(4):427–434
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202(1–2):13–23
Williams AE, Lawson LJ, Perry VH, Fraser H (1994) Characterization of the microglial response in murine scrapie. Neuropathol Appl Neurobiol 20(1):47–55
Tribouillard-Tanvier D, Race B, Striebel JF, Carroll JA, Phillips K, Chesebro B (2012) Early cytokine elevation, PrPres deposition and gliosis in mouse scrapie: no effect on disease by deletion of cytokine genes, IL-12p40 and IL-12p35. J Virol 86(19):10377-10383
Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F (1993) Neurotoxicity of a prion protein fragment. Nature 362(6420):543–546
Brown DR (2000) Prion protein peptides: optimal toxicity and peptide blockade of toxicity. Mol Cell Neurosci 15(1):66–78
Thellung S, Florio T, Corsaro A, Arena S, Merlino M, Salmona M, Tagliavini F, Bugiani O, Forloni G, Schettini G (2000) Intracellular mechanisms mediating the neuronal death and astrogliosis induced by the prion protein fragment 106–126. Int J Dev Neurosci 18(4–5):481–492
Thellung S, Florio T, Villa V, Corsaro A, Arena S, Amico C, Robello M, Salmona M, Forloni G, Bugiani O, Tagliavini F, Schettini G (2000) Apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in rat cerebellar granule cells treated with the prion protein fragment 106–126. Neurobiol Dis 7(4):299–309
Florio T, Paludi D, Villa V, Principe DR, Corsaro A, Millo E, Damonte G, D’Arrigo C, Russo C, Schettini G, Aceto A (2003) Contribution of two conserved glycine residues to fibrillogenesis of the 106–126 prion protein fragment. Evidence that a soluble variant of the 106–126 peptide is neurotoxic. J Neurochem 85(1):62–72
Florio T, Grimaldi M, Scorziello A, Salmona M, Bugiani O, Tagliavini F, Forloni G, Schettini G (1996) Intracellular calcium rise through L-type calcium channels, as molecular mechanism for prion protein fragment 106-126-induced astroglial proliferation. Biochem Biophys Res Commun 228(2):397–405
Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O (2000) Signal transduction through prion protein. Science 289(5486):1925–1928
Brown DR, Schmidt B, Kretzschmar HA (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380(6572):345–347
Giese A, Brown DR, Groschup MH, Feldmann C, Haist I, Kretzschmar HA (1998) Role of microglia in neuronal cell death in prion disease. Brain Pathol 8(3):449–457
Corsaro A, Thellung S, Villa V, Nizzari M, Aceto A, Florio T (2012) Recombinant human prion protein fragment 90–231, a useful model to study prion neurotoxicity. Omics: J Integ Biol 16(1–2):50–59. doi:10.1089/omi.2011.0038
Corsaro A, Thellung S, Russo C, Villa V, Arena S, D’Adamo MC, Paludi D, Rossi Principe D, Damonte G, Benatti U, Aceto A, Tagliavini F, Schettini G, Florio T (2002) Expression in E. coli and purification of recombinant fragments of wild type and mutant human prion protein. Neurochem Int 41(1):55–63
Checler F, Vincent B (2002) Alzheimer’s and prion diseases: distinct pathologies, common proteolytic denominators. Trends Neurosci 25(12):616–620
Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L (1995) Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem 270(32):19173–19180
Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, Chen SG (2003) Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem 278(42):40429–40436
Chiesa R, Harris DA (2001) Prion diseases: what is the neurotoxic molecule? Neurobiol Dis 8(5):743–763
Corsaro A, Paludi D, Villa V, D’Arrigo C, Chiovitti K, Thellung S, Russo C, Di Cola D, Ballerini P, Patrone E, Schettini G, Aceto A, Florio T (2006) Conformation dependent pro-apoptotic activity of the recombinant human prion protein fragment 90–231. Int J Immunopathol Pharmacol 19(2):339–356
Villa V, Corsaro A, Thellung S, Paludi D, Chiovitti K, Venezia V, Nizzari M, Russo C, Schettini G, Aceto A, Florio T (2006) Characterization of the proapoptotic intracellular mechanisms induced by a toxic conformer of the recombinant human prion protein fragment 90–231. Ann N Y Acad Sci 1090:276–291
Chiovitti K, Corsaro A, Thellung S, Villa V, Paludi D, D’Arrigo C, Russo C, Perico A, Ianieri A, Di Cola D, Vergara A, Aceto A, Florio T (2007) Intracellular accumulation of a mild-denatured monomer of the human PrP fragment 90–231, as possible mechanism of its neurotoxic effects. J Neurochem 103(6):2597–2609
Corsaro A, Thellung S, Chiovitti K, Villa V, Simi A, Raggi F, Paludi D, Russo C, Aceto A, Florio T (2009) Dual modulation of ERK1/2 and p38 MAP kinase activities induced by minocycline reverses the neurotoxic effects of the prion protein fragment 90–231. Neurotox Res 15(2):138–154
Thellung S, Corsaro A, Villa V, Simi A, Vella S, Pagano A, Florio T (2011) Human PrP90-231-induced cell death is associated with intracellular accumulation of insoluble and protease-resistant macroaggregates and lysosomal dysfunction. Cell Death Disease 2:e138
Thellung S, Gatta E, Pellistri F, Corsaro A, Villa V, Vassalli M, Robello M, Florio T (2013) Excitotoxicity through NMDA receptors mediates cerebellar granule neuron apoptosis induced by prion protein 90–231 fragment. Neurotox Res 23(4):301–314
Villa V, Tonelli M, Thellung S, Corsaro A, Tasso B, Novelli F, Canu C, Pino A, Chiovitti K, Paludi D, Russo C, Sparatore A, Aceto A, Boido V, Sparatore F, Florio T (2011) Efficacy of novel acridine derivatives in the inhibition of hPrP90-231 prion protein fragment toxicity. Neurotox Res 19(4):556–574
Corsaro A, Thellung S, Bucciarelli T, Scotti L, Chiovitti K, Villa V, D’Arrigo C, Aceto A, Florio T (2011) High hydrophobic amino acid exposure is responsible of the neurotoxic effects induced by E200K or D202N disease-related mutations of the human prion protein. Int J Biochem Cell Biol 43(3):372–382. doi:10.1016/j.biocel.2010.11.007
Paulis D, Maras B, Schinina ME, di Francesco L, Principe S, Galeno R, Abdel-Haq H, Cardone F, Florio T, Pocchiari M, Mazzanti M (2011) The pathological prion protein forms ionic conductance in lipid bilayer. Neurochem Int 59(2):168–174
Thellung S, Villa V, Corsaro A, Pellistri F, Venezia V, Russo C, Aceto A, Robello M, Florio T (2007) ERK1/2 and p38 MAP kinases control prion protein fragment 90-231-induced astrocyte proliferation and microglia activation. Glia 55(14):1469–1485
Thellung S, Corsaro A, Villa V, Venezia V, Nizzari M, Bisaglia M, Russo C, Schettini G, Aceto A, Florio T (2007) Amino-terminally truncated prion protein PrP90-231 induces microglial activation in vitro. Ann N Y Acad Sci 1096:258–270
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29(8):357–365
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69
Perry VH, Cunningham C, Boche D (2002) Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol 15(3):349–354
Marella M, Gaggioli C, Batoz M, Deckert M, Tartare-Deckert S, Chabry J (2005) Pathological prion protein exposure switches on neuronal mitogen-activated protein kinase pathway resulting in microglia recruitment. J Biol Chem 280(2):1529–1534
Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A (2002) Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40(2):232–239
Int’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH (2001) Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 345(21):1515–1521
Aguzzi A, Heikenwalder M, Miele G (2004) Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J Clin Invest 114(2):153–160
Forloni G, Iussich S, Awan T, Colombo L, Angeretti N, Girola L, Bertani I, Poli G, Caramelli M, Grazia Bruzzone M, Farina L, Limido L, Rossi G, Giaccone G, Ironside JW, Bugiani O, Salmona M, Tagliavini F (2002) Tetracyclines affect prion infectivity. Proc Natl Acad Sci U S A 99(16):10849–10854
Collinge J, Gorham M, Hudson F, Kennedy A, Keogh G, Pal S, Rossor M, Rudge P, Siddique D, Spyer M, Thomas D, Walker S, Webb T, Wroe S, Darbyshire J (2009) Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol 8(4):334–344
Ciccarelli R, Di Iorio P, D’Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP (2000) Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia 29(3):202–211
Colucci-D’Amato GL, Tino A, Pernas-Alonso R, ffrench-Mullen JM, di Porzio U (1999) Neuronal and glial properties coexist in a novel mouse CNS immortalized cell line. Exp Cell Res 252(2):383–391
Chambery A, Colucci-D’Amato L, Vissers JP, Scarpella S, Langridge JI, Parente A (2009) Proteomic profiling of proliferating and differentiated neural mes-c-myc A1 cell line from mouse embryonic mesencephalon by LC-MS. J Proteome Res 8(1):227–238. doi:10.1021/pr800454n
Gentile MT, Nawa Y, Lunardi G, Florio T, Matsui H, Colucci-D’Amato L (2012) Tryptophan hydroxylase 2 (TPH2) in a neuronal cell line: modulation by cell differentiation and NRSF/rest activity. J Neurochem 123(6):963–970
Paludi D, Thellung S, Chiovitti K, Corsaro A, Villa V, Russo C, Ianieri A, Bertsch U, Kretzschmar HA, Aceto A, Florio T (2007) Different structural stability and toxicity of PrP(ARR) and PrP(ARQ) sheep prion protein variants. J Neurochem 103(6):2291–2300
Jones LJ, Gray M, Yue ST, Haugland RP, Singer VL (2001) Sensitive determination of cell number using the CyQUANT cell proliferation assay. J Immunol Methods 254(1–2):85–98
Baker BJ, Park KW, Qin H, Ma X, Benveniste EN (2010) IL-27 inhibits OSM-mediated TNF-alpha and iNOS gene expression in microglia. Glia 58(9):1082–1093
Arena S, Pattarozzi A, Corsaro A, Schettini G, Florio T (2005) Somatostatin receptor subtype-dependent regulation of nitric oxide release: involvement of different intracellular pathways. Mol Endocrinol 19(1):255–267
Thellung S, Villa V, Corsaro A, Arena S, Millo E, Damonte G, Benatti U, Tagliavini F, Florio T, Schettini G (2002) p38 MAP kinase mediates the cell death induced by PrP106-126 in the SH-SY5Y neuroblastoma cells. Neurobiol Dis 9(1):69–81
Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP (2000) Antiinflammatory effects of estrogen on microglial activation. Endocrinology 141(10):3646–3656
Bonaiuto C, McDonald PP, Rossi F, Cassatella MA (1997) Activation of nuclear factor-kappa B by beta-amyloid peptides and interferon-gamma in murine microglia. J Neuroimmunol 77(1):51–56
Tiffany HL, Lavigne MC, Cui YH, Wang JM, Leto TL, Gao JL, Murphy PM (2001) Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J Biol Chem 276(26):23645–23652
Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM (2006) Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem 281(6):3651–3659
Weidner AM, Housley M, Murphy MP, Levine H 3rd (2011) Purified high molecular weight synthetic Abeta(1–42) and biological Abeta oligomers are equipotent in rapidly inducing MTT formazan exocytosis. Neurosci Lett 497(1):1–5
Minghetti L, Pocchiari M (2007) Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases. Int Rev Neurobiol 82:265–275
Rozemuller AJ, Jansen C, Carrano A, van Haastert ES, Hondius D, van der Vies SM, Hoozemans JJ (2012) Neuroinflammation and common mechanism in Alzheimer’s disease and prion amyloidosis: amyloid-associated proteins, neuroinflammation and neurofibrillary degeneration. Neurodegener Dis 10(1–4):301–304
Guiroy DC, Wakayama I, Liberski PP, Gajdusek DC (1994) Relationship of microglia and scrapie amyloid-immunoreactive plaques in kuru, Creutzfeldt-Jakob disease and Gerstmann-Straussler syndrome. Acta Neuropathol 87(5):526–530
Williams AE, van Dam AM, Man AHWK, Berkenbosch F, Eikelenboom P, Fraser H (1994) Cytokines, prostaglandins and lipocortin-1 are present in the brains of scrapie-infected mice. Brain Res 654(2):200–206
Brown DR, Schmidt B, Kretzschmar HA (1996) A neurotoxic prion protein fragment enhances proliferation of microglia but not astrocytes in culture. Glia 18(1):59–67
Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ (1997) Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J Biol Chem 272(10):6641–6646
Paresce DM, Ghosh RN, Maxfield FR (1996) Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron 17(3):553–565
Kranich J, Krautler NJ, Falsig J, Ballmer B, Li S, Hutter G, Schwarz P, Moos R, Julius C, Miele G, Aguzzi A (2010) Engulfment of cerebral apoptotic bodies controls the course of prion disease in a mouse strain-dependent manner. J Exp Med 207(10):2271–2281
Freixes M, Rodriguez A, Dalfo E, Ferrer I (2006) Oxidation, glycoxidation, lipoxidation, nitration, and responses to oxidative stress in the cerebral cortex in Creutzfeldt-Jakob disease. Neurobiol Aging 27(12):1807–1815
Kim JI, Ju WK, Choi JH, Choi E, Carp RI, Wisniewski HM, Kim YS (1999) Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res Mol Brain Res 73(1–2):17–27
Muhleisen H, Gehrmann J, Meyermann R (1995) Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol Appl Neurobiol 21(6):505–517
Lleo A, Galea E, Sastre M (2007) Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci 64(11):1403–1418
Imbimbo BP, Solfrizzi V, Panza F (2010) Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front Aging Neurosci 2
Forloni G, Artuso V, Roiter I, Morbin M, Tagliavini F (2013) Therapy in prion diseases. Curr Top Med Chem 13(19):2465–2476
Farooqui AA, Ong WY, Horrocks LA (2006) Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev 58(3):591–620
Brown DR, Herms JW, Schmidt B, Kretzschmar HA (1997) PrP and beta-amyloid fragments activate different neurotoxic mechanisms in cultured mouse cells. Eur J Neurosci 9(6):1162–1169
Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE (1999) Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci 19(3):928–939
Fabrizi C, Silei V, Menegazzi M, Salmona M, Bugiani O, Tagliavini F, Suzuki H, Lauro GM (2001) The stimulation of inducible nitric-oxide synthase by the prion protein fragment 106–126 in human microglia is tumor necrosis factor-alpha-dependent and involves p38 mitogen-activated protein kinase. J Biol Chem 276(28):25692–25696
Bate C, Boshuizen RS, Langeveld JP, Williams A (2002) Temporal and spatial relationship between the death of PrP-damaged neurones and microglial activation. Neuroreport 13(13):1695–1700
Gibbons HM, Dragunow M (2006) Microglia induce neural cell death via a proximity-dependent mechanism involving nitric oxide. Brain Res 1084(1):1–15
Bate C, Kempster S, Williams A (2006) Prostaglandin D2 mediates neuronal damage by amyloid-beta or prions which activates microglial cells. Neuropharmacology 50(2):229–237
Iravani MM, Kashefi K, Mander P, Rose S, Jenner P (2002) Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience 110(1):49–58
He Y, Imam SZ, Dong Z, Jankovic J, Ali SF, Appel SH, Le W (2003) Role of nitric oxide in rotenone-induced nigro-striatal injury. J Neurochem 86(6):1338–1345
Lee P, Son D, Lee J, Kim YS, Kim H, Kim SY (2003) Excessive production of nitric oxide induces the neuronal cell death in lipopolysaccharide-treated rat hippocampal slice culture. Neurosci Lett 349(1):33–36
Schutze S, Loleit T, Zeretzke M, Bunkowski S, Bruck W, Ribes S, Nau R (2012) Additive microglia-mediated neuronal injury caused by amyloid-beta and bacterial TLR agonists in murine neuron-microglia co-cultures quantified by an automated image analysis using cognition network technology. J Alzheimers Dis 31(3):651–657
Skaper SD, Facci L, Giusti P (2013) Intracellular ion channel CLIC1: involvement in microglia-mediated beta-amyloid peptide(1–42) neurotoxicity. Neurochem Res 38(9):1801–1808
Riemer C, Gultner S, Heise I, Holtkamp N, Baier M (2009) Neuroinflammation in prion diseases: concepts and targets for therapeutic intervention. CNS Neurol Disord Drug Targets 8(5):329–341
Hochstrasser T, Hohsfield LA, Sperner-Unterweger B, Humpel C (2013) beta-Amyloid induced effects on cholinergic, serotonergic, and dopaminergic neurons is differentially counteracted by anti-inflammatory drugs. J Neurosci Res 91(1):83–94
White MC, Johnson GG, Zhang W, Hobrath JV, Piazza GA, Grimaldi M (2013) Sulindac sulfide inhibits sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic reticulum stress response, and exerts toxicity in glioma cells: relevant similarities to and important differences from celecoxib. J Neurosci Res 91(3):393–406. doi:10.1002/jnr.23169
Johnson GG, White MC, Wu JH, Vallejo M, Grimaldi M (2014) The deadly connection between endoplasmic reticulum, Ca2+, protein synthesis, and the endoplasmic reticulum stress response in malignant glioma cells. Neuro-Oncology 16(8):1086–1099
Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR (1995) Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol 114(7):1335–1342
Salvemini D, Manning PT, Zweifel BS, Seibert K, Connor J, Currie MG, Needleman P, Masferrer JL (1995) Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest 96(1):301–308
Hamilton LC, Warner TD (1998) Interactions between inducible isoforms of nitric oxide synthase and cyclo-oxygenase in vivo: investigations using the selective inhibitors, 1400W and celecoxib. Br J Pharmacol 125(2):335–340
Huang Y, Liu J, Wang LZ, Zhang WY, Zhu XZ (2005) Neuroprotective effects of cyclooxygenase-2 inhibitor celecoxib against toxicity of LPS-stimulated macrophages toward motor neurons. Acta Pharmacol Sin 26(8):952–958
Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, Shyur LF (2005) Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br J Pharmacol 146(3):352–363
Choi Y, Lee MK, Lim SY, Sung SH, Kim YC (2009) Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br J Pharmacol 156(6):933–940
Acknowledgments
We thank Prof. Giulia Menozzi (Department of Pharmacy, University of Genova) for providing us with reagents at the beginning of the study. The study was supported by grants from Italian Ministry of University and Research (Accordi di Programma FIRB 2011, project num. RBAP11HSZS) and Compagnia di San Paolo (2013).
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Valentina Villa and Stefano Thellung equally contributed and should both be considered as first authors.
Rights and permissions
About this article
Cite this article
Villa, V., Thellung, S., Corsaro, A. et al. Celecoxib Inhibits Prion Protein 90-231-Mediated Pro-inflammatory Responses in Microglial Cells. Mol Neurobiol 53, 57–72 (2016). https://doi.org/10.1007/s12035-014-8982-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8982-4