Abstract
NMDA receptors play a crucial role in regulating synaptic plasticity and memory. Activation of NMDA receptors changes intracellular concentrations of Na+ and K+, which are subsequently restored by Na/K-ATPase. We used immunochemical and biochemical methods to elucidate the potential mechanisms of interaction between these two proteins. We observed that NMDA receptor and Na/K-ATPase interact with each other and this interaction was shown for both isoforms of α subunit (α1 and α3) of Na/K-ATPase expressed in neurons. Using Western blotting, we showed that long-term exposure of the primary culture of cerebellar neurons to nanomolar concentrations of ouabain (a cardiotonic steroid, a specific ligand of Na/K-ATPase) leads to a decrease in the levels of NMDA receptors which is likely mediated by the α3 subunit of Na/K-ATPase. We also observed a decrease in enzymatic activity of the α1 subunit of Na/K-ATPase caused by NMDA receptor activation. This effect is mediated by an increase in intracellular Ca2+. Thus, Na/K-ATPase and NMDA receptor can interact functionally by forming a macromolecular complex which can be important for restoring ionic balance after neuronal excitation. Furthermore, this interaction suggests that NMDA receptor function can be regulated by endogenous cardiotonic steroids which recently have been found in cerebrospinal fluid or by pharmacological drugs affecting Na/K-ATPase function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Na/K-ATPase (the sodium pump) is an integral membrane protein expressed in all mammalian cells. It maintains the asymmetric distribution of Na+ and K+ ions across the cell membrane by actively exporting three Na+ and importing two K+ while hydrolyzing one ATP molecule [1]. Na/K-ATPase is responsible for approximately 50 % of total brain energy consumption [2]. In neurons, the catalytic α subunit of Na/K-ATPase occurs in two isoforms, the ubiquitous α1 subunit and the neuron-specific α3 subunit [3, 4]. Mutations in the gene ATP1A3 encoding the α3 subunit are associated with two syndromes: rapid-onset dystonia-parkinsonism [5] and alternating hemiplegia of childhood [6].
Na/K-ATPase is a specific receptor for cardiotonic steroids (CTS), including ouabain [7]. Ouabain and at least two other CTS, digoxin and marinobufagenin, have been found in blood, adrenals, and hypothalamus. Circulating ouabain is bound by a specific binding globulin [8]. The concentration of CTS can be physiologically modulated through physical exercise and hypoxia [9]. In rodents, α3 is 103 times more sensitive to ouabain than α1.
Na/K-ATPase has been shown to interact with several proteins, and there is evidence that binding of CTS to Na/K-ATPase can modulate functions of its partners in protein-protein interaction [10–13]. Previous studies have suggested that there may be functional interactions between Na/K-ATPase and NMDA receptor [14]; however, the mechanism of this interaction remains unclear. NMDA receptor is an ionotropic glutamate receptor. Its distinctive feature in comparison to other ionotropic glutamate receptors is permeability to calcium (in addition to Na+ and K+), which is an important secondary messenger capable of modulating cellular response according to the external signal [15]. It forms a heterotetramer between two obligatory NR1 subunits and two NR2 subunits which occur in four isoforms (A–D) [16]. Recent studies have suggested that CTS can affect NMDA receptors [17–19], which participate in synaptic plasticity and molecular memory formation [15, 16]. It has been reported that administration of the CTS endobain E for 2 days leads to an increase in NMDA receptor expression in the cortex and hippocampus [20]. At the same time, mice heterozygous for the α3 subunit displayed a 40 % reduction in hippocampal NMDA receptor expression [21]. No proteomic studies have demonstrated direct interaction between Na/K-ATPase and NMDA receptor, but NMDA receptors have been shown to interact with PSD-95, PLC γ, PI3K, and tubulin [22], which are also known to interact with the α subunit of Na/K-ATPase [23, 12, 24–26]. Taken together, these observations prompted us to explore the mechanism of interaction between Na/K-ATPase and NMDA receptor and to study whether and to what extent such an interaction may be modulated by ouabain and/or by NMDA receptor ligands.
This study has been performed on rat primary cerebellar neurons. Protein-protein interaction was assessed with co-immunoprecipitation. Functional interaction was studied by immunochemical and biochemical methods in the presence and absence of NMDA or ouabain in concentrations that would discriminate between the effects on α3 and α1 isoforms.
Materials and Methods
Materials
Chemicals (≥95 % purity) were purchased from Sigma (St Louis, MO). Regents for cell culture, and culture media were purchased from Invitrogen (Carlsbad, CA). Antibodies against NR1 (sc-9058), NR2B (sc-9057) subunits of NMDA receptor, goat anti-mouse IgG-horseradish peroxidase, and goat anti-rabbit IgG-horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against tubulin (32–2500) were purchased from Invitrogen (Camarillo, CA). Antibodies against α1 subunit of Na/K-ATPase (α6F) were purchased from Developmental Studies Hybridoma Bank (Iowa City, IA). Antibodies against α3 subunit of Na/K-ATPase (MA3-915) were purchased from Pierce Biotechnology (Rockford, IL).
All research on rats were done in USA according to procedures and guidelines of the National Institutes of Health, and the protocols were approved by the Institutional Animal Care and Use Committee of the University of Toledo, College of Medicine and Life Sciences. Animal experiments in Russia were approved by the Institutional Committee for Ethics in Animal Experimentation of Biological Faculty, Moscow State University, Moscow, Russia.
Primary Culture of Cerebellar Neurons
Primary cultures of cerebellar neurons were prepared as follows [27]. Cerebella from 3–5-day-old Wistar rats of both sexes were excised, washed in cold HBSS (Invitrogen), and cut into small pieces with a scalpel. Tissue was suspended in 0.05 % Trypsin-EDTA and incubated at 37 °C for 20 min. This reaction was stopped by fresh MEM with 10 % FBS. Next, tissue was washed two times by warm HBSS and then transferred to 5 ml of Neurobasal-A Medium and triturated with a series of flame-polished glass Pasteur pipettes of decreasing tip diameter. The cell suspension was centrifuged at 300 g for 2 min, and the pellet was resuspended in culture medium consisting of Neurobasal-A Medium supplemented with 2 % B-27, 0.5 mM GlutaMax, 20 mM KCl, and 100 U/ml penicillin/streptomycin. Neurons were plated at a density of 5·105 cells/cm2 onto 6-well or 12-well plates pretreated with poly-d-lysine. Cultures were incubated in culture medium and maintained in a humidified atmosphere at 37 °C in 5 % CO2. Experiments were performed after 11 days.
Immunoprecipitation
For immunoprecipitation experiments, neurons were treated with ice-cold lysis buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 % Triton ×-100, 60 mM octylglucoside, 1 mM PMSF, 1 mM Na3VO3, 1 mM NaF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. After 30 min at 4 °C, the lysate was clarified by centrifugation at 24000g for 15 min. The supernatant (0.25–1 mg protein) was precleared and incubated with the appropriate antibody and then with protein A plus agarose beads. The proteins bound to the collected beads were subjected to SDS-PAGE and probed with appropriate antibodies as indicated below.
Western Blotting Analysis
Samples were subjected to 8 or 10 % SDS-PAGE, transferred to PVDF membrane, and probed with appropriate antibodies by standard procedures. The immunoreactive bands were developed and detected using enhanced chemiluminescence. For quantitative comparisons, images were scanned with a densitometer. Different dilutions of samples were subjected to SDS-PAGE, and multiple exposures of the films were used to ensure that quantifications were made within the linear range of the assays.
Na/K-ATPase Activity
Na/K-ATPase activity was measured as described previously [28]. Liberated inorganic phosphate was measured after the enzymatic reaction. This assay was performed in the presence and absence of 1 mM ouabain, and the ouabain-sensitive component was considered Na/K-ATPase activity. After treatment, cells were permeabilized by five freeze-thaw cycles, the enzymatic reaction was conducted, and the Rathbun and Betlach method was used for measuring inorganic phosphate. The Lowry protein assay was used to normalize the samples.
Fluorescence Microscopy
Treated cells were fixed in 3.7 % paraformaldehyde for 10 min at RT or in 10 % TCA for 5 min at 4 °C. Cells were then washed three times with PBS and permeabilized with 0.05 % Triton ×-100 for 5 min at RT. Following three more washes with PBS, cells were blocked with 10 % BSA and incubated with primary antibodies overnight at 4 °C. After incubation, cells were washed three times with PBS and incubated with secondary antibodies for 1 h in the dark at RT. Coverslips were mounted with ProLong Gold antifade reagent (Molecular Probes). Images were obtained using a Leica TCS SP5 broadband confocal microscope system equipped with Argon and HeNe lasers coupled to a DMI 6000CS inverted microscope with a ×63 oil immersion objective. Alexa Fluor 488 was excited using 488 spectral laser lines. The Leica Confocal Microscope System software was used for visualization and analysis.
MTT Test
Neuronal viability was assessed using MTT test. The method is based on reduction in the living cells of yellow 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to blue formazan. Cultures used in experiments were planted into 96-well plates with a density of 4×104 cells per well. Following experimental procedures, a solution of MTT in NBM was added to each well to obtain a final MTT concentration of 0.5 mg/ml. Wells without cells were used as negative control. After incubation with MTT for 2–3 h, the medium was completely removed from the wells and 100 μl of DMSO were added to each well. Sample absorbance was measured at wavelengths 570 and 660 nm using plate reader Synergy H4 (BioTek). Absorbance values at 660 nm as well as that in the negative control were subtracted from absorbance values at 570 nm. Data were presented as a percentage of the signal in the control wells (without ouabain or NMDA).
Analysis of Data
Values are means ± SE of the results of a minimum of three experiments. Student’s t test was used and significance was accepted at p < 0.05.
Results
Analysis of Structural Interaction Between Na/K-ATPase and NMDA Receptor
First, we observed that the primary culture of rat cerebellar neurons we used contained both ouabain-sensitive α3 and ouabain-resistant α1 subunits of Na/K-ATPase by immunostaining (Fig. 1a-b), which is consistent with the previous literature [4]. Also, we showed that the primary culture of rat cerebellar neurons contained both NR1 and NR2B subunits of NMDA receptor by Western blots (Fig. 1c, d).
Presence of α1 and α3 subunits of Na/K-ATPase and NR1 and NR2B subunits of NMDA receptor in primary culture of cerebellar neurons. Neurons were stained with antibodies against α1 (a) and α3 (b) subunits of Na/K-ATPase and NR1 subunit of NMDA receptor (c). d Representative Western blots showing the presence of α1 and α3 subunits of Na/K-ATPase and NR1 and NR2B subunits of NMDA receptor in cerebellar neurons
Then, we evaluated possible protein interactions using co-immunoprecipitation. In a series of experiments, we incubated cerebellar neurons with 1 μM ouabain for 10 min, then lysed the cells and performed co-immunoprecipitation using antibodies against either NR1 or NR2B subunits. Na/K-ATPase and NMDA receptor molecules were solubilized under the conditions similar to the literature [23, 29]. As shown as Fig. 2, both α1 and α3 subunits co-immunoprecipitate with NMDA receptor. Thus, both subunits form a complex with NMDA receptor and potentially can participate in glutamate exchange regulation mechanisms. However, the specific ligand of Na/K-ATPase, ouabain [7, 30], did not cause significant changes in the interaction of NMDA receptor and Na/K-ATPase α subunits, indicating this interaction does not affect by ouabain binding of Na/K-ATPase.
Co-immunoprecipitation of NR1 and NR2B subunits of NMDA receptor with α1 and α3 subunits of Na/K-ATPase. Cell lysates of primary culture of cerebellar neurons were immunoprecipitated (IP) with monoclonal antibodies against NR1 or NR2B subunits of NMDA receptor and then probed by Western blotting with antibodies against α1 and α3 of Na/K-ATPase (a) and NR1 and NR2B subunits of NMDA receptor (b). One representative blots from three independent experiments
The Effects of Long-Term Exposure of Neurons to Ouabain
We tested whether exposure of cerebellar neurons to ouabain affects the amount of NMDA receptor. We incubated cells with 1 μМ ouabain for periods of time from 0.5 to 6 h (Fig. 3a), then lysed the cells and analyzed the amount of NR1 and NR2B subunits of NMDA receptor as well as α1 and α3 subunits of Na/K-ATPase.
Treatment with ouabain causes a decrease in the amount of NMDA receptor. Primary cultures of cerebellar neurons were incubated with 1 μM ouabain for different periods of time (a) or with different concentrations of ouabain for 6 h (b). Representative Western blots showing reduced levels of NR1 and NR2B in primary culture of cerebellar neurons. The optical density of the control was taken for 100 %; data were normalized to tubulin as a loading control. c Primary cultures of cerebellar neurons were incubated with ouabain for 24 h, then MTT tests were conducted as described in “Materials and Methods”. The viability in the control wells was taken for 100 %. *p < 0.05 compared to control, **p < 0.01 compared to control, ***p < 0.001 compared to control
The results showed that long-term incubation with ouabain (1 h or more) causes about 40 % decrease in the amount of NMDA receptor subunits. Furthermore, the level of NR2B subunit decreases faster than that of NR1 subunit. Thus, we can assert that ouabain causes a decrease in the number of NMDA receptors because NR1 is an obligatory subunit of NMDA receptor [16]. Notably, after 6 h incubation with 1 μМ ouabain, the amount of NMDA receptors did not change further (data not shown). We did not observe significant change in the levels of α1 and α3 subunits of Na/K-ATPase at 1 μМ ouabain (data not shown). To identify which Na/K-ATPase isoforms is involved in this process, we tested different ouabain concentration (ranging from 1 nM to 10 μM) after 6 h incubation (Fig. 3b). We did not see changes in the amount of Na/K-ATPase α subunits by lower than 10 μM ouabain (data not shown).
Data show the reduction of NR1 subunit decreases at only 1 nM ouabain, and further increase in ouabain concentration does not intensify this effect. The amount of NR2B subunit is dose-dependent in the range of ouabain concentrations from 1 nM to 1 μM, reaching the maximum effect of less than 50 % of the control value at 1 μM ouabain. Further increase in ouabain concentration does not significantly intensify the decrease in NR2B amount.
Apart from that, we tested whether ouabain in concentrations used in our experiments caused neuronal death. We analyzed the effect of ouabain on neuronal viability using MTT test after 24-h incubation with various ouabain concentrations (Fig. 3c). We found that ouabain in concentrations of 1 μM or less did not cause cell death. Thus, the decrease in NMDA receptor levels caused by 1 nM–1 μM ouabain cannot be explained to be induced due to neuronal death.
We conclude that ouabain binding to the ouabain-sensitive α3 subunit of Na/K-ATPase (ouabain concentrations ranging from 1 to 100 nM) leads to a decrease in NMDA receptor level, while ouabain binding to the ouabain-resistant α1 subunit (1–100 μM ouabain) has no effect on this process because ouabain in concentrations 100 nM–100 μM does not significantly enhance the effect.
NMDA Receptor Regulates Na/K-ATPase Enzymatic Activity
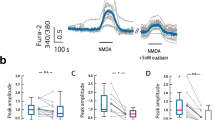
Previously, it has been shown that NMDA receptor activation can cause a decrease in Na/K-ATPase activity in cell lysates [14]. To further test this mechanism, we first studied the dose-dependent effects of NMDA ranging from 5 to 750 μM on Na/K-ATPase’s enzymatic activity in primary neuronal cultures (Fig. 4a). When neurons were incubated with different concentrations of NMDA for 30 min, neurons were permeabilized and Na/K-ATPase enzymatic activity was measured. While previous literature shows that NMDA receptor activation by NMDA in concentrations above 0.1 mM leads to a dose-dependent decrease in Na/K-ATPase activity [14, 31], we observed a small increase in Na/K-ATPase activity at 10–25 μM NMDA with significant decrease at higher doses (more than 0.1 mM).
Effect of NMDA treatment on Na/K-ATPase activity and cell viability in primary cultures of cerebellar neurons. a Effect of NMDA on Na/K-ATPase activity. Primary cultures of cerebellar neurons were incubated with different NMDA concentrations (30 min), lysed, and used to conduct the enzymatic reaction as described in “Materials and Methods”. The activity of the control was taken for 100 %; data were normalized to a measured amount of total protein. b Effect of NMDA on cell viability. Neurons were either incubated for 24 h with different NMDA concentrations, or incubated with NMDA for 30 min, then washed and incubated until 24 h in culture medium; then, MTT tests were conducted as described in “Materials and Methods”. The viability in the control wells was taken for 100 %. c NMDA-induced decrease in Na/K-ATPase activity was prevented by BAPTA. Neurons were pre-incubated with BAPTA (10 μM, 10 min), then incubated with NMDA (500 μM, 30 min), and then lysed and used to measure Na/K-ATPase activity. d D-AP5 blocked NMDA inhibition on ouabain curves of Na/K-ATPase activity. Primary cultures of cerebellar neurons were pre-incubated with or without D-AP5 (10 μM, 10 min), then incubated with NMDA (500 μM, 30 min). Cells were lysed and used to conduct the enzymatic reaction. *p < 0.05 compared to control, **p < 0.01 compared to control, ***p < 0.001 compared to control
To ensure that 30-min incubation with chosen NMDA concentrations did not affect cell viability, we conducted MTT test. Primary cultures of cerebellar neurons were incubated with NMDA for 30 min, then washed and incubated until 24 h in culture medium. Cells were incubated with NMDA for 24 h as positive control. As shown in Fig. 4b, 30-min incubation with different NMDA concentrations up to 1 mM did not affect cell viability, while 24-h incubation with NMDA caused a significant decrease in cell viability starting from 50 μM NMDA.
To test Ca2+ dependence of the effect of NMDA on Na/K-ATPase activity, we preincubated cells with BAPTA (Ca2+ chelator), then exposed the neurons to NMDA. As shown in Fig. 4c, the effect of NMDA on Na/K-ATPase activity is prevented by preincubation with BAPTA. Importantly, BAPTA itself does not affect Na/K-ATPase activity.
Finally, to identify the isoform of Na/K-ATPase that was affected by NMDA, we conducted a dose-response curve for ouabain inhibition of Na/K-ATPase enzymatic activity in cell lysates. We also examined the changes in the curve caused by preincubation of NMDA, and with NMDA + D-AP5 (a selective NMDA receptor antagonist) before the lysis (Fig. 4d). Ouabain inhibition curve showed two phases. As the same as the previous report [32], the α3 subunits of Na/K-ATPase in rat neurons were inhibited by ouabain in concentrations ranging from 1 nM to 1 μM, while the α1 subunits were inhibited by ouabain in concentrations ranging from 1 μM to 1 mM. In our case, the activity from α3 subunit accounts for about 30 % of total Na/K-ATPase activity in the primary rat cerebellar neurons. As seen in Fig. 4d, the action of NMDA (either with or without D-AP5) did not cause significant changes in α3 subunits’ activity, because the activity still showed two phases. Ouabain-resistant α1 subunit activity accounts for about 70 % of total Na/K-ATPase activity. After preincubation with NMDA at 500 μM, the activity was reduced to 25 % of the total Na/K-ATPase activity compared to the control samples (without NMDA). D-AP5 prevented the effects of NMDA on α1 activity, so it returned to 70 % of the total Na/K-ATPase activity like it did in the control samples. Thus, we conclude that NMDA causes the inhibition of α1 subunit and does not affect α3 subunits.
Discussion
Previous studies have suggested that Na/K-ATPase and NMDA receptor can participate in functional interaction: changes in Na/K-ATPase activity can affect the properties of the NMDA receptor [18–21, 33], and NMDA receptor activation can mediate Na/K-ATPase activity [14, 34, 35]. The present study was devoted to molecular mechanisms of this interaction. First, we showed that our model system contained both the ouabain-sensitive α3 subunit and the ouabain-resistant α1 subunit of Na/K-ATPase, as well as a functional NMDA receptor. The latter was confirmed by showing the presence of both NR1 and NR2B subunits, since NR1 subunit and one of four NR2 subunits (A–D) are needed to form a functional NMDA receptor [16]. According to the literature, NR2B subunit is predominantly expressed in the cerebellum [36]; therefore, we chose this subunit for our research.
Na/K-ATPase is involved in protein-protein interaction with various membrane and cytoplasmic proteins and is capable of affecting their properties. Such interactions could represent a general principal of regulation of various proteins by CTS binding to Na/K-ATPase [13, 37–39]. Existing data has shown that ouabain binding to Na/K-ATPase can affect the properties of proteins participating in glutamate exchange in nervous tissue, such as ionotropic glutamate AMPA receptors in neurons [40] and glutamate transporters GLT-1 and GLAST in glial cells [41]. Using co-immunoprecipitation, we demonstrated that Na/K-ATPase forms a functional complex with NMDA receptor. However, we observed that ouabain does not affect the amount of NMDA receptor molecules associated with Na/K-ATPase α subunits, indicating that the proteins interact regardless of ouabain binding to the α subunit.
This interaction was shown in both α1 and α3 subunits of Na/K-ATPase, which may indicate that a conserved domain present in both isoforms is responsible for the interaction. Thus, likely, both isoforms can functionally interact with the NMDA receptor. Previously, it has been shown that Na/K-ATPase interacts with another ionotropic glutamate receptor—AMPA receptor [40] which shows structural homology with NMDA receptor. Co-immunoprecipitation of AMPA receptor was found only with the α1 but not the α3 subunit of Na/K-ATPase. These data allow us to believe that different sites of the α subunit of Na/K-ATPase are responsible for interaction with AMPA and NMDA receptors. Taken together, these data indicate that both α1 and α3 subunits of Na/K-ATPase are co-localized with NMDA receptors and form a functional complex either by interacting directly or through some intermediate proteins.
Furthermore, we demonstrated that long-term ouabain exposure induced regulation of NMDA receptor expression. We found that exposure of the cells to low ouabain concentrations (1 nM and higher) causes a decrease in the levels of NMDA receptor subunits (NR1 and NR2B), but these changes do not lead to the cell death. According to the literature, the ouabain-sensitive α3 subunit of Na/K-ATPase in rat neurons is inhibited by ouabain in concentrations ranging from 1 nM to 1 μM, while the ouabain-resistant α1 subunit is inhibited by ouabain in concentrations ranging from 1 μM to 1 mM [32]. We concluded that ouabain binding to the ouabain-sensitive α3 subunit of Na/K-ATPase but not the α1 subunit leads to a decrease in the level of NMDA receptors because this effect was caused by nanomolar concentrations of ouabain. Notably, it has been reported that endobain E injection into the lateral cerebral ventricle of rats resulted to an increase in the expression of NMDA receptor subunits in the cerebral cortex and hippocampus [20]. However, in our study, we observed a decrease in NMDA receptor levels after exposure to ouabain, which is in agreement with observations reported in Na/K-ATPase heterozygous mice [21]. It is possible that CTS may cause different effects in various regions of the brain. Another explanation could be that endobain E and ouabain simply act differently. In light of high sensitivity of NMDA receptor in response to low ouabain concentrations, we suggest that this mechanism may represent one of the ways of regulating the glutamatergic system in the brain, since recent evidence indicates that CTS could appear as endogenous steroid hormones [8, 9]. Recently, it has been shown that 2 nM ouabain is present in human cerebrospinal fluid [42] which correlates with our data on the effect of nanomolar concentrations of ouabain. Taken together, these data indicate that ouabain can play a crucial role in regulating the amount of NMDA receptor via α3 subunit of Na/K-ATPase. Thus, it is possible that ouabain might function as an endogenous regulator of the glutamatergic system. Intriguingly, one side effect of CTS-based drugs is psychosis [43] that is often associated with decreased NMDA receptor function [44]. Besides that, it has previously been demonstrated that ouabain induces glutamate exitotoxicity [17]. Thus, the decrease in the amount of NMDA receptor caused by CTS may have important physiological consequences.
After characterizing the effect of CTS binding to Na/K-ATPase on NMDA receptor function, we attempted to study the effect of NMDA receptor activation on Na/K-ATPase activity. We showed that NMDA receptor activation leads to a dose-dependent inhibition of Na/K-ATPase, which corresponds to previously published data [33, 14]. Using inhibitor analysis, we attempted to establish a possible mechanism of Na/K-ATPase inhibition. These experiments demonstrated that this effect of NMDA is prevented by D-AP5 (NMDA receptor inhibitor) and BAPTA (Ca2+ chelator). It has been reported that NMDA receptor activation causes Ca2+ to enter the cells, leading to protein kinase C activation [45] and PKC phosphorylates Na/K-ATPase, thereby reduces its activity [46]. Therefore, we conclude that Na/K-ATPase inhibition by NMDA receptor activation depends on an increase in intracellular Ca2+. This probably leads to activation of protein kinase C, which can inhibit Na/K-ATPase through the aforementioned phosphorylation [47]. In this manner, the effect is likely possibly through a number of intermediates rather than through direct protein-protein interaction between Na/K-ATPase and NMDA receptor.
Analyzing the dose-response curves of ouabain inhibition of Na/K-ATPase under the simultaneous action of NMDA and D-AP5, we observed that NMDA causes inhibition of the α1 subunit of Na/K-ATPase while D-AP5 prevents this effect. Why activation of NMDA receptors by high concentrations of NMDA causes inhibition of the α1 subunit of Na/K-ATPase? We assume this allows the α3 subunit in restoring the gradient of Na+ and K+ after cell excitation and reduces the role of the α1 subunit in the process. This is perhaps an adaptive response, as it is known that different isoforms of Na/K-ATPase have different affinity to Na+ ions [48] which leads to different pump function in neurons [49] and differ in the way they are involved in cell signaling [50].
In summary, we have shown for the first time the existence of a functional complex formed by Na/K-ATPase and NMDA receptor. Moreover, both α1 and α3 subunits of Na/K-ATPase can be involved in this interaction.
References
Skou JC, Esmann M (1992) The Na, K-ATPase. J Bioenerg Biomembr 24(3):249–261
Erecinska M, Silver IA (1994) Ions and energy in mammalian brain. Prog Neurobiol 43(1):37–71
Dobretsov M, Stimers JR (2005) Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci: J Virtual Libr 10:2373–2396
Lingrel JB, Williams MT, Vorhees CV, Moseley AE (2007) Na, K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr 39(5–6):385–389. doi:10.1007/s10863-007-9107-9
de Carvalho AP, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ (2004) Mutations in the Na+/K + −ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43(2):169–175. doi:10.1016/j.neuron.2004.06.028
Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmuller J, Frommolt P, Zirn B, Ebinger F, Siemes H, Nurnberg P, Brockmann K, Gartner J (2012) Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol 11(9):764–773. doi:10.1016/S1474-4422(12)70182-5
Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z (1998) Multiple signal transduction pathways link Na+/K + −ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273(24):15249–15256
Antolovic R, Bauer N, Mohadjerani M, Kost H, Neu H, Kirch U, Grunbaum EG, Schoner W (2000) Endogenous ouabain and its binding globulin: effects of physical exercise and study on the globulin’s tissue distribution. Hypertens Res: Off J Japan Soc Hypertens 23(Suppl):S93–S98
Schoner W (2002) Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem / FEBS 269(10):2440–2448
Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A (1999) Intracellular reactive oxygen species mediate the linkage of Na+/K + −ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem 274(27):19323–19328
Aizman O, Uhlen P, Lal M, Brismar H, Aperia A (2001) Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A 98(23):13420–13424. doi:10.1073/pnas.221315298
Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z (2005) Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16(9):4034–4045. doi:10.1091/mbc.E05-04-0295
Xie Z, Cai T (2003) Na + −K + −−ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 3(3):157–168. doi:10.1124/mi.3.3.157
Bulygina ER, Lyapina LY, Boldyrev AA (2002) Activation of glutamate receptors inhibits Na/K-ATPase of cerebellum granule cells. Biochem Biokhim 67(9):1001–1005
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51(1):7–61
VanDongen AM (2009) Biology of the NMDA receptor. Frontiers in neuroscience. CRC Press, Boca Raton
Stelmashook EV, Weih M, Zorov D, Victorov I, Dirnagl U, Isaev N (1999) Short-term block of Na+/K + −ATPase in neuro-glial cell cultures of cerebellum induces glutamate dependent damage of granule cells. FEBS Lett 456(1):41–44
Reines A, Pena C, de Rodriguez LAG (2001) [3H]dizocilpine binding to N-methyl-D-aspartate (NMDA) receptor is modulated by an endogenous Na+, K + −ATPase inhibitor. Comparison with ouabain. Neurochem Int 39(4):301–310
Bersier MG, Miksztowicz V, Pena C, de Lores RAG (2005) Modulation of aspartate release by ascorbic acid and endobain E, an endogenous Na+, K + −ATPase inhibitor. Neurochem Res 30(4):479–486
Bersier MG, Pena C, de Lores RAG (2008) The expression of NMDA receptor subunits in cerebral cortex and hippocampus is differentially increased by administration of endobain E, a Na+, K + −ATPase inhibitor. Neurochem Res 33(1):66–72. doi:10.1007/s11064-007-9412-z
Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB (2007) Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27(3):616–626. doi:10.1523/JNEUROSCI. 4464-06.2007
Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3(7):661–669. doi:10.1038/76615
Blom H, Ronnlund D, Scott L, Spicarova Z, Widengren J, Bondar A, Aperia A, Brismar H (2011) Spatial distribution of Na + −K + −ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy. BMC Neurosci 12:16. doi:10.1186/1471-2202-12-16
Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM (2000) Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K + −ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A 97(12):6556–6561. doi:10.1073/pnas.100128297
Liu L, Zhao X, Pierre SV, Askari A (2007) Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 293(5):C1489–C1497. doi:10.1152/ajpcell.00158.2007
Zampar GG, Chesta ME, Carbajal A, Chanaday NL, Diaz NM, Casale CH, Arce CA (2009) Acetylated tubulin associates with the fifth cytoplasmic domain of Na(+)/K(+)-ATPase: possible anchorage site of microtubules to the plasma membrane. Biochem J 422(1):129–137. doi:10.1042/BJ20082410
Khodorov BI, Storozhevykh TP, Surin AM, Iuriavichus AI, Sorokina EG, Borodin AV, Vinskaia NP, Khaspekov LG, Pinelis VG (2001) The leading role of mitochondrial depolarization in the mechanism of glutamate-induced disorder in Ca (2+)-homeostasis. Rossiiskii Fiziologicheskii Zh Imeni IM Sechenova / Rossiiskaia Akademiia nauk 87(4):459–467
Petrushanko I, Bogdanov N, Bulygina E, Grenacher B, Leinsoo T, Boldyrev A, Gassmann M, Bogdanova A (2006) Na-K-ATPase in rat cerebellar granule cells is redox sensitive. Am J Physiol Regul Integr Comp Physiol 290(4):R916–R925. doi:10.1152/ajpregu.00038.2005
Zhang Q, Xu X, Li T, Lu Y, Ruan Q, Lu Y, Wang Q, Dong F, Yang Y, Zhang G (2014) Exposure to bisphenol-A affects fear memory and histone acetylation of the hippocampus in adult mice. Horm Behav 65(2):106–113. doi:10.1016/j.yhbeh.2013.12.004
Yatime L, Laursen M, Morth JP, Esmann M, Nissen P, Fedosova NU (2011) Structural insights into the high affinity binding of cardiotonic steroids to the Na+, K + −ATPase. J Struct Biol 174(2):296–306. doi:10.1016/j.jsb.2010.12.004
Boldyrev A, Bulygina E, Gerassimova O, Lyapina L, Schoner W (2004) Functional relationship between Na/K-ATPase and NMDA-receptors in rat cerebellum granule cells. Biochem Biokhim 69(4):429–434
Sweadner KJ (1985) Enzymatic properties of separated isozymes of the Na, K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem 260(21):11508–11513
Zhang L, Guo F, Su S, Guo H, Xiong C, Yin J, Li W, Wang Y (2012) Na(+)/K(+)-ATPase inhibition upregulates NMDA-evoked currents in rat hippocampal CA1 pyramidal neurons. Fundam Clin Pharmacol 26(4):503–512. doi:10.1111/j.1472-8206.2011.00947.×
Inoue N, Soga T, Kato T (1999) Glutamate receptors mediate regulation of Na pump isoform activities in neurons. Neuroreport 10(16):3289–3293
Rambo LM, Ribeiro LR, Schramm VG, Berch AM, Stamm DN, Della-Pace ID, Silva LF, Furian AF, Oliveira MS, Fighera MR, Royes LF (2012) Creatine increases hippocampal Na(+), K(+)-ATPase activity via NMDA-calcineurin pathway. Brain Res Bull 88(6):553–559. doi:10.1016/j.brainresbull.2012.06.007
Popp RL, Reneau JC, Brotherton BJ (2008) Activation of protein kinase C enhances NMDA-induced currents in primary cultured cerebellar granule cells: effect of temperature and NMDA NR2 subunit composition. Eur J Pharmacol 599(1–3):1–10. doi:10.1016/j.ejphar.2008.08.007
Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A (2003) Cell signaling microdomain with Na, K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem 278(50):50355–50361. doi:10.1074/jbc.M305378200
Liu X, Spicarova Z, Rydholm S, Li J, Brismar H, Aperia A (2008) Ankyrin B modulates the function of Na, K-ATPase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J Biol Chem 283(17):11461–11468. doi:10.1074/jbc.M706942200
Illarionova NB, Gunnarson E, Li Y, Brismar H, Bondar A, Zelenin S, Aperia A (2010) Functional and molecular interactions between aquaporins and Na, K-ATPase. Neuroscience 168(4):915–925. doi:10.1016/j.neuroscience.2009.11.062
Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY (2009) Na, K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci : Off J Soc Neurosc 29(14):4498–4511. doi:10.1523/JNEUROSCI. 6094-08.2009
Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR (2009) Glutamate transporter coupling to Na, K-ATPase. J Neurosci : Off J Soc Neurosc 29(25):8143–8155. doi:10.1523/JNEUROSCI. 1081-09.2009
Dvela M, Rosen H, Ben-Ami HC, Lichtstein D (2012) Endogenous ouabain regulates cell viability. Am J Physiol Cell Physiol 302(2):C442–C452. doi:10.1152/ajpcell.00336.2011
Bagrov AY, Shapiro JI, Fedorova OV (2009) Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61(1):9–38. doi:10.1124/pr.108.000711
Farber NB (2003) The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci 1003:119–130
Young KW, Garro MA, Challiss RA, Nahorski SR (2004) NMDA-receptor regulation of muscarinic-receptor stimulated inositol 1,4,5-trisphosphate production and protein kinase C activation in single cerebellar granule neurons. J Neurochem 89(6):1537–1546. doi:10.1111/j.1471-4159.2004.02458.×
Cheng SX, Aizman O, Nairn AC, Greengard P, Aperia A (1999) [Ca2+] i determines the effects of protein kinases A and C on activity of rat renal Na+, K + −ATPase. J Physiol 518(Pt 1):37–46
Lopina OD (2001) Interaction of Na, K-ATPase catalytic subunit with cellular proteins and other endogenous regulators. Biochem Biokhim 66(10):1122–1131
Horisberger JD, Kharoubi-Hess S (2002) Functional differences between alpha subunit isoforms of the rat Na, K-ATPase expressed in Xenopus oocytes. J Physiol 539(Pt 3):669–680
Azarias G, Kruusmagi M, Connor S, Akkuratov EE, Liu XL, Lyons D, Brismar H, Broberger C, Aperia A (2013) A specific and essential role for Na, K-ATPase alpha3 in neurons co-expressing alpha1 and alpha3. J Biol Chem 288(4):2734–2743. doi:10.1074/jbc.M112.425785
Karpova L, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G (2010) Sodium pump alpha1 and alpha3 subunit isoforms mediate distinct responses to ouabain and are both essential for survival of human neuroblastoma. FEBS J 277(8):1853–1860. doi:10.1111/j.1742-4658.2010.07602.×
Acknowledgments
We dedicate this article to the memory of Professor Alexander Boldyrev, who passed away in 2012. Professor Boldyrev worked for more than 40 years in this field and created the Moscow school of Na/K-ATPase research at Moscow State University. We thank Professor Amir Askari for the opportunity to perform a part of this work in his Laboratory. We also thank Professor Anita Aperia for the opportunity to do a part of this work in her Laboratory and for her comments on the manuscript. This work was supported by RFBR grants 12-04-31468 and 14-04-32123, the Russian President Grant for 2010/2011, and NIH HL036573. We also received financial support from internal postdoctoral grant from St. Petersburg State University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akkuratov, E.E., Lopacheva, O.M., Kruusmägi, M. et al. Functional Interaction Between Na/K-ATPase and NMDA Receptor in Cerebellar Neurons. Mol Neurobiol 52, 1726–1734 (2015). https://doi.org/10.1007/s12035-014-8975-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8975-3