Abstract
Realgar is a type of mineral drug containing arsenic. The nervous system toxicity of realgar has received extensive attention. However, the underlying mechanisms of realgar-induced neurotoxicity have not been clearly elucidated. To explore the mechanisms that contribute to realgar-induced neurotoxicity, weanling rats were exposed to realgar (0, 0.3, 0.9, 2.7 g/kg) for 6 weeks, and cognitive ability was tested using the Morris water maze (MWM) test and object recognition task (ORT). The levels of arsenic in the blood and hippocampus were monitored. The ultrastructures of hippocampal neurons were observed. The levels of glutamate (Glu) and glutamine (Gln) in the hippocampus and hippocampal CA1 region; the activities of glutamine synthetase (GS) and phosphate-activated glutaminase (PAG); the mRNA and protein expression of glutamate transporter 1 (GLT-1), glutamate/aspartate transporter (GLAST), and N-methyl-d-aspartate (NMDA) receptors; and the level of intracellular Ca2+ were also investigated. The results indicate that the rats developed deficiencies in cognitive ability after a 6-week exposure to realgar. The arsenic contained in realgar and the arsenic metabolites passed through the blood-brain barrier (BBB) and accumulated in the hippocampus, which resulted in the excessive accumulation of Glu in the extracellular space. The excessive accumulation of Glu in the extracellular space induced excitotoxicity, which was shown by enhanced GS and PAG activities, inhibition of GLT-1 mRNA and protein expression, alterations in NMDA receptor mRNA and protein expression, disturbance of intracellular Ca2+ homeostasis, and ultrastructural changes in hippocampal neurons. In conclusion, the findings from our study indicate that exposure to realgar induces excitotoxicity and that the mechanism by which this occurs may be associated with disturbances in Glu metabolism and transportation and alterations in NMDA receptor expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Realgar (known as Xionghuang in China) is a type of mineral drug containing arsenic [1]. In recent years, a number of realgar-induced arsenic poisoning events have been reported in China, some of which were due to abuse by children [2, 3]. Therefore, much interest has been paid to the toxic effects of realgar or realgar-containing traditional Chinese medicines (TCMs) on humans and especially children.
A large number of studies show that arsenic is neurotoxic [4]. Long-term exposure to arsenic can cause cognitive impairment, mental retardation, and learning and memory decline. Children are particularly sensitive to the neurotoxicity induced by arsenic [5, 6]. The metabolites of arsenic have been detected in blood and brain tissues of animals treated with realgar and realgar-containing medicines [7, 8], indicating that the arsenic contained in realgar can pass through the blood-brain barrier (BBB) and accumulate in the brain tissue [9, 10]. Thus, the long-term use of realgar or realgar-containing TCMs may cause nervous system defects and impair intelligence in children. Therefore, clarifying the neurotoxic effects and mechanisms of realgar would prevent nervous system impairment and protect children’s health.
The hippocampus is a part of the limbic system that is crucial to memory function and spatial navigation. The Morris water maze (MWM) is devised for the study of hippocampal-dependent spatial learning and memory in rodents [11]. The object recognition task (ORT) has proven to be very useful for the assessment of memory functions in animals [12]. This test is the most frequently used laboratory tests in behavioral neuroscience.
Glutamate (Glu) is the main excitatory neurotransmitter in the central nervous system (CNS) and is also an excitotoxin. Extracellular Glu concentrations are maintained within physiological levels exclusively by members of a family of sodium-dependent glutamate transporters (also known as excitatory amino acid transporters or EAAT). Glutamate transporters, located on both neurons and astrocytes, are responsible for the uptake of extracellular Glu and permit normal excitatory transmission. Up to 80 % of the Glu released into the synaptic cleft is taken up by astrocytes by the glutamate/aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1). In astrocytes, through the effects of glutamine synthetase (GS), Glu is converted to glutamine (Gln), which can then be excreted from astrocytes. After being taken up by neurons, Gln is converted to Glu by phosphate-activated glutaminase (PAG) [13]. The “Glu-Gln” cycle plays an important role in maintaining normal neuronal functions. However, once the cycle is disrupted, changes in Glu metabolism and transportation may lead to an excessive extracellular Glu concentration, resulting in the overstimulation of neurons (excitotoxicity), leading to cell death and ultimately learning and memory impairment. However, until now, no work has addressed the effects of realgar on Glu level in the synaptic cleft and Glu metabolism in the hippocampus.
N-Methyl-d-aspartate (NMDA) receptors, which are glutamate-gated ion channel receptors with high calcium permeability, are widely expressed in the CNS and play key roles in learning and memory. NMDA receptors are composed of three distinct classes of heteromeric subunits: NR1 and its eight splice variants, NR2A-NR2D, and NR3; each subunit is encoded by a distinct gene. The NR1 subunit is assumed to have functional roles, and the NR2 subunit is assumed to have modulatory roles on channel activities. Glu is the agonist of the NMDA receptors. The effect of Glu excitotoxicity induced by realgar on NMDA receptors has not been reported.
In this study, weanling Wistar rats were treated with 0, 0.3, 0.9, or 2.7 g/kg realgar for 6 weeks. The MWM and ORT tests were used to investigate the effect of realgar on spatial learning and memory and novel object recognition ability, respectively. The arsenic levels in the blood and hippocampal tissues as well as ultrastructural changes in the hippocampal neurons were measured. The mechanisms that contribute to realgar-induced neurotoxicity, alterations in hippocampal Glu metabolism and transportation, and NMDA receptor activity were also investigated by determining the effects of realgar on Glu and Gln levels in the hippocampus and the hippocampal CA1 region, the activities of PAG and GS, and the mRNA and protein expression of GLAST, GLT-1, NR1, NR2A, and NR2B. The study improves our understanding of the mechanism of realgar-induced impairment in learning and memory and provides a theoretical basis for preventing realgar-induced arsenic poisoning.
Experimental
Reagents
Realgar (>90 % As4S4) was purchased from Shenyang Medicine Company. Glu and Gln standards and the derivatization reagent (dansyl chloride) were obtained from Sigma Chemical Corporation (St. Louis, MO, USA). The standard reference materials of inorganic arsenic (iAs) (GBW 08611), methyl arsine (MMA), and dimethyl arsine (DMA) were obtained from Tri Chemical Laboratories Inc. (Yamanashi, Japan). The TRIzol reagent and kits for real-time PCR and DNA marker analysis were purchased from TaKaRa Biotechnology Company (Dalian, China). The rabbit polyclonal antibodies against GLAST, GLT-1, NR1, NR2A, NR2B, and β-actin and the horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody were bought from Santa Cruz Biotechnology, Inc. (CA). The enhanced chemiluminescence (ECL) plus kit was obtained from Amersham Life Science (Buckinghamshire, UK). The Fluo-3/AM reagent, trypsin, and bovine serum albumin were purchased from Sigma Chemical Corporation (St. Louis, MO, USA). All other chemicals were obtained from local chemical suppliers. All chemicals were of analytical grade, high-performance liquid chromatography (HPLC) grade or the best pharmaceutical grade available.
Animal Treatments
A total of 120 Wistar rats (4 weeks old, equal numbers of male and female) with initial body weights of 80–100 g were obtained from the Experimental Animal Center, China Medical University. All rats were housed at 24 ± 1 °C with a relative humidity of 50 ± 5 %, under artificial lighting (12-h light/dark cycle). The rats were randomly divided into four groups by weight with 30 rats in each group (15 male and 15 female, housed separately). The first group was designated the control group and was intragastrically (i.g.) treated with 0.5 % (w/v) sodium carboxymethylcellulose (CMC-Na); the second, third and fourth groups were exposed to realgar daily for six consecutive weeks at concentrations of 0.3 g/kg (low dosage), 0.9 g/kg (middle dosage) or 2.7 g/kg (high dosage) body weight suspended in CMC-Na. During the experiment, all rats were allowed free access to food and water and were observed for symptoms of toxicity every day. All animal treatments were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the China Medical University (Publication No. 85-23, revised 1985). All efforts were made to minimize the number and suffering of the animals.
Sample Collection
One week before the exposure period, eight rats from each group were trained in the MWM and tested on the last day of exposure. At the end of the MWM testing, microdialysis (MD) on the right hippocampus was performed for the extracellular Glu and Gln determination. After the MD, the rats were anesthetized and sacrificed by decapitation prior to the removal of the hippocampus. The right-side hippocampus was used for the determination of Glu and Gln levels, and the left-side hippocampus was removed for arsenic determination. Eight rats from each group performed the ORT test 4 days before the exposure period and were tested on the last day of exposure. At the end of the ORT test, the rats were anesthetized and sacrificed; subsequently, the hippocampus was removed for the determination of GS and PAG activities. The hippocampus of four rats in each group was removed for the measurement of mRNA and protein expression of GLAST, GLT-1, NR1, NR2A, and NR2B; the hippocampus of two rats in each group was used to observe the ultrastructural feature of the hippocampal neurons; and the hippocampus of the last eight rats in each group was dissected for Ca2+ determination.
MWM Test
The MWM test was performed according to Vorhees and Williams [14] with some modifications. The water maze consisted of a circular, 170-cm diameter, 50-cm deep, black-painted tank filled with 22 ± 1 °C water to a depth of 23 cm. The area of the water maze was divided into four quadrants: northeast (NE), northwest (NW), southeast (SE), and southwest (SW). In the hidden platform test, the animals were trained to locate a hidden plexiglass platform placed 2 cm below the water surface. The pool was surrounded by different cues for spatial coordination. The animals from each group were given one session of four trials daily. During each trial, the rat was placed in the water with its face turned to the wall of the pool at one of three equally spaced starting points. These points were situated in quadrants that did not contain the platform and changed on each trial and day. The hidden platform was located in the SE quadrant. In each trial, the animal was given 60 s to find the hidden platform. When the animal found the platform within 60 s, it was given further 10 s to stay on the platform. If it did not find the platform within 60 s, it was guided to the platform and then allowed to stay on the platform for 10 s. The interval between two trials was 5 min. After 4 days of training, the MWM test was conducted on the seventh day (a 3-day interval). A probe trial was conducted on the eighth day of the MWM test. There was no platform in the SE sector during the probe trial, and the rats were allowed to swim for 30 s before the end of the session. Data from the water maze (latencies to locate the platform, distance traveled, swimming speed, number of crossings in a target area, and time spent in the goal quadrant) were recorded by the ANY-maze™ software (Stoelting, USA).
ORT Test
The ORT test was performed according to Heyser and Ferris [15] with some modifications. The behavioral task was performed in a 75 × 75-cm open field surrounded by 100-cm-high walls. The training (5 min) was conducted by placing each individual rat into the field, in which two identical objects were positioned 10 cm from the walls in two adjacent corners. After 3 days of training, the rats explored the open field for 5 min in the presence of one familiar and one novel object. All objects presented similar textures, colors, and sizes but had distinctive shapes. The percentage of the total exploration time that the animal spent investigating the novel object was used as a measurement of recognition memory. Between trials, the objects were washed with 10 % ethanol solution. Exploration was defined as sniffing or touching the object with the nose and/or forepaws. Data are expressed as mean ± SD of the percentage of time spent exploring the novel object.
Arsenic Determination in the Blood and Hippocampus
Levels of arsenic species (iAs, MMA, and DMA) in the blood and hippocampus were determined according to our previous report [10]. Briefly, hippocampal samples (50 mg) or blood (0.5 mL) was homogenized by adding 2 mL of deionized water and then mixed with 1 mL of 3 M H2SO4. The samples were digested in a focused microwave field for 10 min in a 10-mL polymethylpentene test tube. The digested samples were assayed using a method based on the hydride generation of volatile arsines, followed by cryogenic separation in liquid nitrogen and the final detection of iAs, MMA, and DMA by atomic absorption spectrophotometry (AAS). Arsenic was detected at 193.3 nm. We report the total arsenic (TAs) contents by summing the concentrations of iAs, MMA, and DMA.
Morphological Observation
The rats were perfused with buffered 4 % paraformaldehyde solution into the left ventricles, and hippocampus tissues were removed and fixed with buffered 2.5 % glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.2–7.4). After 1 h of fixation, a 1-mm-thick slice was made. For electron microscopy, postfixation of the slice in 1 % OsO4 containing 1.25 % potassium ferrocyanide was carried out. Afterwards, the tissues were dehydrated in a graded series of acetone and embedded in spur resin. Finally, the blocks were stained with uranyl acetate and lead citrate and photographed using a JEM-1200EX transmission electron microscope equipped with an ultrascan digital camera.
Determination of Glu and Gln Levels in the Hippocampus and Hippocampal CA1 Region
The extracellular Glu and Gln concentrations in the hippocampus were analyzed by MD coupled with HPLC. Wistar rats were anesthetized with chloral hydrate (300 mg/kg) by intraperitoneal injection and mounted on a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A 0.5-mm hole was drilled in the skull [anterior-posterior (A/P)—3.8 mm, lateral (M/L)—3.0 mm, and dorsal ventral (D/V)—3.0 mm]. A steel guide cannula (20-gauge stainless steel; Small Parts, Miami, FL, USA) was implanted above the hippocampal CA1 region and fixed to the skull with three jewelry screws and dental acrylic cement. After the surgery, the animals were allowed to recover for 24 h in a single cage under the standard conditions, with free access to food and water. The MD experiment was performed within 24–48 h after probe insertion. The implanted MD probes were perfused with modified Ringer's solution (in millimolars: Na+ 145, K+ 4.0, Ca2+ 1.3, Cl− 152) at a flow rate of 2 μL/min driven by a microinjection pump (CMA402, Sweden). Following a 90-min stabilization period, three 20-min dialysate samples (40 μL each) were collected in ice-cold vials and immediately stored at −70 °C until further analysis. Aliquots of 20 μL of microdialysate was mixed with 20 μL of dansyl chloride (6 mM) and 20 μL of Na2CO3-NaHCO3 buffer solution (pH 9.5), and then the mixture was incubated at 65 °C in the dark for 20 min.
The concentrations of the eight amino acid neurotransmitters in the rat hippocampal tissue were also determined after MD. The sample preparation and derivatization procedure were the same as described in our previous paper [10]. Briefly, hippocampal tissue samples (approximately 50 mg) were weighted and homogenized in 2 mL of acetonitrile. The final homogenate was centrifuged at 11,200 g for 10 min at 4 °C. The supernatant was transferred and evaporated to dryness at 40 °C under a gentle stream of nitrogen. The dried residue was reconstituted in 400 μL of a Na2CO3-NaHCO3 buffer solution (pH 9.5). An aliquot of 200 μL of dansyl chloride (10 mM) was added and vortex-mixed. The solution was incubated in the dark at 65 °C for 25 min and cooled to room temperature.
The Glu and Gln analysis was carried out using a Waters 600 separations module with a built-in vacuum degasser and a Thermo ODS column (250 mm × 4.6 mm, i.d.; 5 μm). The mobile phase was composed of a Na2HPO4-NaH2PO4 buffer solution (pH 7.2, A) and acetonitrile (B) with a flow rate of 1.0 mL/min. The column was eluted with a linear gradient of 14–20 % B over 0–7 min and 20–27 % B over 7–14 min and held at 27 % B for 8 min. The detection was performed at 254 nm, and the injection volume was 20 μL. The Glu (or Gln) concentration in the extracellular space = Glu (or Gln) concentration determined in the microdialysate/the MD recovery of Glu (or Gln). The Glu and Gln contents in hippocampal tissues are expressed as micromoles per gram tissue weight.
Determination of GS and PAG Activity in the Hippocampus
Determination of GS Activity
The activity of GS was determined by following the formation of l-γ-glutamyl hydroxamate by monitoring the absorbance of the solution at 535 nm as Renis et al. [16] described. The hippocampus was removed on ice, and a 5 % homogenate was made. Then, the homogenate was added to the reaction mixture containing 110 mM Gln, 0.45 mM ATP, 9 mM Na2HAsO4, 110 mM hydroxylamine, 1.8 mM MnCl2, and 0.1 mM dithiothreitol in 50 mM imidazole buffer (pH 7.2), and an enzymatic extract of 100 μg of protein, in a final volume of 1.5 mL. The reaction mixture was incubated for 30 min at 37 °C, stopped using 0.75 mL of 0.6 M Fe(NO3)3 in 40 % trichloroacetic acid, and centrifuged at 10,000 g for 5 min to remove the protein precipitate. The absorbance was determined in the supernatant at 535 nm, and the values were compared with a standard curve prepared with l-γ-glutamyl hydroxamate. GS activity is expressed as micromoles of l-γ-glutamyl hydroxamate per minute per·gram·protein.
Determination of PAG Activity
The PAG activity was measured according to the method of Curi et al. [17]. The hippocampus was removed on ice, and a 5 % homogenate was made. Then, the homogenate was added into an extraction medium containing 150 mM potassium phosphate, 1 mM EDTA, and 50 mM Tris-HCl at pH 8.6. The assay medium consisted of 50 mM phosphate buffer, 0.2 mM EDTA, 50 mM Tris-HCl, 20 mM Gln, and 0.05 % (v/v) Triton X-100, to which 100 μL of homogenate was added. The total volume was 1.0 mL at pH 8.6. Samples of the assay media, in duplicate, were incubated at 37 °C. The reaction was initiated by the addition of freshly prepared Gln, promoting a 10-min linear reaction time course. The reaction was stopped by the addition of 0.2 mL of a 25 % (w/v) perchloric acid solution and then neutralized. The absorbance was determined at 340 nm.
The amount of protein in the preparation was quantified according to the method of Lowry et al. [18].
GLAST, GLT-1, NR1, NR2A, and NR2B mRNA and Protein Expression
Real-Time PCR for GLAST, GLT-1, NR1, NR2A, and NR2B mRNA Expression
Total RNA was extracted using the TRizol reagent and dissolved in RNase-free water. The OD260/OD280 of the total RNA was between 1.6 and 1.8. Quantitative reverse transcription was performed using an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems, Singapore) with a PrimeScript RT Reagent Kit and gDNA Eraser, using GADPH as an endogenous control. The following gene-specific primers were designed by TaKaRa Company: GLT-1, forward primer 5′-GGGTCATCCTGGATGGAGGT-3′ and reverse primer 5′-CGTGTCGTCATAAACGGACT-3′; GLAST, forward primer 5′-GGGTTTTCATTGGAGGGTTGC-3′ and reverse primer 5′-CCACGGGTTTCTCTGGTTCAT-3′; NR1, forward primer 5′-GATCGCCTACAAGCGACACAA-3′ and reverse primer 5′-TTAGGGTCGGGCTCTGCTCTAC-3′; NR2A, forward primer 5′-GCTTGTGGTGATCGTGCTGAA-3′ and reverse primer 5′-AATGCTGAGGTGGTTGTCATCTG-3′; NR2B, forward primer 5′-TGGCTATCCTGCAGCTGTTTG-3′ and reverse primer 5′-TGGCTGCTCATCACCTCATTC-3′; and GADPH, forward primer 5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse primer 5′-ATGGTGGTGAAGACGCCAGTA-3′. For the quantitative PCR reaction, the reaction mixture contained 2 μL of template DNA, 0.8 μL (10 μM) of PCR forward primer, 10 μL of SYBR Green Reagent, 0.4 μL of ROX Reference Dye II, and 6.8 μL of dH2O in a final volume of 20 μL. The initial denaturation was carried out at 95 °C for 30 s, which was followed by amplification in 40 cycles of 95 °C for 3 s and 60 °C for 34 s, using the 7500 Real-Time PCR System (BD, USA). The relative expression analysis was assessed by the 2−ΔΔCt method.
Western Blot for GLAST, GLT-1, NR1, NR2A, and NR2B Protein Expression
Protein extracted from the hippocampal homogenate was mixed with loading buffer (0.25 M Tris-HCl pH 6.8, 10 % SDS, 0.5 % BPB, 50 % glycerol, 5 % 2-ME) and separated by 5 % SDS-PAGE. Following the electrophoretic fractionation, the protein was transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The PVDF membranes were subsequently blocked overnight at 4 °C in Tween 20 Tris-buffered saline (TBS) containing 5 % nonfat powdered milk. Then, the membrane was rinsed briefly in TBS and incubated with a 1:500 dilution of the GLAST and NR1 anti-rabbit primary antibodies, a 1:2,000 dilution of the GLT-1 anti-rabbit primary antibody, a 1:1,000 dilution of either the NR2A and NR2B anti-rabbit primer antibody, or a 1:3,000 dilution of the β-actin anti-rabbit antibody at 4 °C overnight. Specific protein expression was detected by incubating the washed membranes with a 1:5,000 dilution of the HRP-conjugated goat anti-rabbit secondary antibody at room temperature for 2 h. Protein bands were visualized using the ECL plus kit. The intensity of the bands was evaluated semiquantitatively by densitometry using image analysis software (FluorChem v2.0). The changes in the intensities of the GLAST, GLT-1, NR1, NR2A, and NR2B proteins were normalized using the intensity obtained for the internal control bands (β-actin).
Determination of Intracellular Ca2+ in the Hippocampus
The intracellular Ca2+ level in the hippocampus was measured by flow cytometry using the Ca2+-sensitive fluorescence dye Fluo-3/AM. Briefly, hippocampal tissue (50 mg) was dissected for the preparation of a single cell suspension and washed with PBS (pH 7.2–7.4) three to five times. The tissues were minced in PBS supplemented with 0.25 % trypsin for 30 min at 37 °C with vigorous shaking followed by the addition of 3 mL of DMEM supplemented with 10 % bovine serum albumin. The solution was pelleted by centrifugation and resuspended in 2 mL of PBS. The cell concentration was diluted to 1 × 106 cells/mL for flow cytometry. The cells were incubated with 10 μL of Fluo-3/AM. The cell suspension was gently mixed and incubated for 1 h at 37 °C and then rinsed with Hank's solution to remove excess Fluo-3/AM. The intracellular Ca2+ was analyzed immediately for Fluo-3/AM fluorescence intensity by flow cytometry (excitation at 488 nm and emission at 526 nm) (FCM, BD FACSCanto™ II, BD Biosciences, USA). Approximately 10,000 cells were analyzed from each treatment.
Statistical Analysis
The results are expressed as mean ± SD. Comparisons among groups were achieved using one-way ANOVA followed by a least significant difference (LSD) test by SPSS 17.0 (SPSS, Chicago, IL, USA). The level of significance was set at P < 0.05.
Results
Growth and Development and the Hippocampus Weight Coefficient
During the experiment, there were no differences in food intake among the groups. The general appearance and physical condition of the realgar-exposed and control rats, which included movement, alertness, and appearance of the coat, nose, eyes, and limbs, were closely observed, and no obvious differences were noticed. No evident poisoning symptoms were observed in the realgar-exposed rats. No significant differences in body weight were observed among the groups either before or after the 6-week realgar exposure (data not shown). Figure 1 shows that there are no significant differences in hippocampus weight coefficients (hippocampus weight coefficient = hippocampus weight in grams / body weight in grams) between the control and realgar-exposed groups (P > 0.05).
Behavioral Assessment
The Effect of Realgar Exposure on Spatial Learning and Memory Behavior
In the hidden platform acquisition test, the latencies to find the platform after realgar exposure are shown in Table 1. The platform acquisition times in all groups shortened day by day, suggesting the gaining of space allocation memory for the underwater platform. The values of mean total escape latency (Table 1) and mean total swim distance (control, 4.0 ± 1.4 m; low dosage, 5.3 ± 1.9 m; middle dosage, 6.6 ± 3.4 m; high dosage, 8.3 ± 3.7 m) gradually increased on the testing day with increasing realgar dose. Significant differences in the mean total escape latency and the mean total swim distances were found for the middle- and high-dose realgar-exposed groups compared with the control group, which suggested a significant deficit in spatial learning ability. Realgar exposure did not affect the swimming speed of young rats (control, 0.703 ± 0.098 m/s; low dosage, 0.628 ± 0.063 m/s; middle dosage, 0.609 ± 0.065 m/s; high dosage, 0.621 ± 0.062 m/s), suggesting that the spatial learning deficit observed in the hidden platform trial is due to an impairment in spatial memory and not a swimming disability.
In the probe trial, the number of crossings and time spent in target quadrant decreased with increasing realgar dosage. Table 2 shows that a significant decrease in the number of crossings and time spent in the target quadrant was detected in the middle- and high-dose realgar-exposed rats compared with the controls, indicating a spatial memory disability induced by realgar.
The Effect of Realgar on Novel Object Recognition Ability
The ORT test is based upon “spontaneous novelty preference,” i.e., the natural predisposition of rodents to explore novel objects. It allows the assessment of learning and memory processes in rodents [19]. The results of recognition memory task are shown in Fig. 2. The mean exploratory preference for the novel object in the control group was approximately 56 %, which suggests that a greater amount of time was spent exploring the novel object. However, the middle- and high-dose realgar-exposed groups spent significantly less time exploring the novel object compared with the controls (P < 0.05), which suggests that long-term exposure to realgar caused recognition memory impairment in young rats.
Arsenic and Arsenic Metabolite Content in the Blood and Hippocampus
Table 3 illustrates the blood and hippocampal contents of arsenic and its metabolites in rats after 6 weeks of realgar exposure. The metabolites iAs, MMA, and DMA were detected in the blood, and significant increases in these arsenic species were found in the realgar-exposed groups compared with the control group (P < 0.01). Furthermore, a significant increase in blood arsenic levels was detected in the middle- and high-dose realgar-exposed groups compared with the low-dose group. In addition, no significant differences in arsenic levels were found between the middle- and high-dose realgar-exposed groups, which suggests that the levels of arsenic and its metabolites achieved a steady state. No iAs was detected in the hippocampus of any group, while the arsenic metabolites (MMA and DMA) were detected in the hippocampus of the realgar-exposed groups. The contents of MMA, DMA, and total arsenic (TAs, TAs = MMA + DMA) were all significantly higher in the hippocampus of the realgar-exposed groups than those in the control group (P < 0.01). The arsenic metabolites accumulated in the hippocampus of the realgar-exposed groups increased in a dose-dependent manner. The results indicate that the arsenic contained in realgar could be absorbed into the blood, pass through the BBB, and accumulate in the hippocampus.
The Effects of Realgar Exposure on the Ultrastructure of Hippocampal Neurons
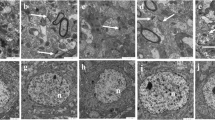
The ultrastructural changes in the neurons of the hippocampus are shown in Fig. 3. In the control group, the nucleus was circular-shaped, had a complete nuclear membrane, and had uniform chromatin. The organelles were abundant and included mitochondria, rough endoplasmic reticulum, and ribosomes, among others (Fig. 3a). In the low-dose realgar-exposed group, the cells presented early damage with the loss of mitochondrial cristae and a slight expansion of the rough endoplasmic reticulum (Fig. 3b). In the middle-dose realgar-exposed group, more advanced damage was observed with the clearing of the mitochondrial matrix and cristae and a slight expansion of rough endoplasmic reticulum (Fig. 3c). The most serious damage to the cell ultrastructure was found in the high-dose realgar-exposed group and included mitochondrial swelling, rupture, and balloon-like damage. The rough endoplasmic reticulum showed mild expansion (Fig. 3d).
Electron microphotographs showing the ultrastructural changes of hippocampal neurons after realgar exposure (n = 2). Images of the slices were collected using a JEM-1200EX transmission electron microscope (×200). The changes in neuronal structures including the nucleus (n), mitochondria (m), rough endoplasmic reticulum (er), and ribosomes (r) are also shown. a Control: 0 g/kg realgar-exposed slices, b 0.3 g/kg realgar-exposed slices, c 0.9 g/kg realgar-exposed slices, and d 2.7 g/kg realgar-exposed slices
Effect of Realgar on Glu and Gln Levels in the Hippocampus and Hippocampal CA1 Region
Table 4 shows the effect of realgar on Glu and Gln levels in the hippocampus and hippocampal CA1 region. Significant increases in Glu concentrations were observed in the hippocampal CA1 region of the realgar-exposed rats compared with the control group (P < 0.05), and a dose-dependent effect was found. The peak area and peak height of Glu (t R = 9.3 min) in the hippocampal CA1 region of the realgar-exposed rats were significantly increased compared with the controls (Fig. 4a). Dose-dependent significant decreases in Glu content were observed in the hippocampus of the realgar-exposed rats compared with the control group (P < 0.05). The peak area and peak height of Glu in the hippocampus of the realgar-exposed rats were significantly decreased compared with the controls (Fig. 4b). No significant differences in Gln levels were found in the hippocampal CA1 region or the hippocampus of the realgar-exposed rats compared with the control group, and no changes in either the peak area or peak height of Gln (t R = 15.3 min) were found in the hippocampus and the hippocampal CA1 region of the realgar-exposed rats. A significant increase in the extracellular/intracellular (E/I) concentration ratio was observed for Glu in the realgar-exposed groups compared with the control group, which indicates an excessive accumulation of Glu in the extracellular space. No significant differences in E/I ratio were observed for Gln.
The Effect of Realgar on GS and PAG Activities
Table 5 shows the effect of realgar on GS and PAG activities. Compared with the control group, the low-, middle-, and high-dose realgar-exposed groups had a dose-dependent increase in GS and PAG activities in the hippocampus.
The Effect of Realgar on the mRNA and Protein Expression of GLT-1, GLAST, NR1, NR2A, and NR2B
The Effect of Realgar on the mRNA Expression of GLT-1 and GLAST
The effects of realgar on the relative expression of GLT-1 and GLAST mRNA are illustrated in Figs. 5a and 6a. Compared with the control group, the middle- and high-dose realgar-exposed groups showed approximately 8 % (P < 0.05) and 10 % (P < 0.05) inhibition of GLT-1 mRNA expression, respectively (Fig. 5a). No significant differences in GLAST mRNA expression were observed in the realgar-exposed groups compared with the control group (P > 0.05, Fig. 6a).
The Effect of Realgar on the Protein Expression of GLT-1 and GLAST
The effects of realgar on GLT-1 and GLAST protein expression are given in Figs. 5b and 6b. The relative expression of protein between GLT-1 and β-actin was gradually inhibited with an increasing dosage of realgar. Compared with the control group, the low-, middle-, and high-dose realgar-exposed groups showed approximately 24 % (P > 0.05), 33 % (P > 0.05), and 45 % (P < 0.05) inhibition of GLT-1 protein expression, respectively (Fig. 5b). No significant differences in GLAST protein expression were found in the realgar-exposed group compared with the control group (P > 0.05, Fig. 6b).
The Effect of Realgar on the mRNA Expression of NR1, NR2A, and NR2B
The effects of realgar on the relative expression of NR1, NR2A, and NR2B mRNA are illustrated in Figs. 7a, 8a, and 9a. Compared with the control group, there were no significant differences in NR1 mRNA expression observed in the realgar-exposed groups (Fig. 7a). However, the expression of NR2A was enhanced with increasing realgar exposure. Compared with the control group, the low-, middle-, and high-dose realgar-exposed groups showed approximately 8 % (P > 0.05), 16 % (P > 0.05), and 53 % (P < 0.05) enhancement of NR2A mRNA expression, respectively (Fig. 8a). No significant differences in NR2B mRNA expression were observed in the realgar-exposed groups compared with the control group (P > 0.05, Fig. 9a).
The Effect of Realgar on the Protein Expression of NR1, NR2A, and NR2B
The effects of realgar on NR1, NR2A, and NR2B protein expression are given in Figs. 7b, 8b, and 9b. Compared with the control group, the high-dose realgar-exposed group showed approximately 26 % inhibition of NR1 protein expression (P < 0.05, Fig. 7b). The relative expression of protein between NR2A and β-actin increased gradually with an increasing dose of realgar. Compared with the control group, the low-, middle-, and high-dosage realgar-exposed groups showed approximately 2.8 (P < 0.05), 3.5 (P < 0.01), and 4.5 (P < 0.01)-fold increases in NR2A protein expression, respectively (Fig. 8b). No significant differences in NR2B protein expression were found in the realgar-exposed groups compared with the control group (Fig. 9b).
The Effect of Realgar on Intracellular Ca2+ Level in the Hippocampus
Figure 10 shows the effect of realgar on intracellular Ca2+ concentration in the hippocampus. Compared with the control group, the middle- and high-dose realgar-exposed groups showed an approximately 1.5 (P < 0.05) and 1.8 (P < 0.05)-fold increase in Ca2+ levels in the hippocampus.
Discussion
Realgar is widely used clinically in China. There are 32 realgar-containing TCMs included in the China Pharmacopeia (2010 edition), and 14 are used in children. It is well known that the brain is particularly vulnerable during early childhood, and often the damage induced by toxic agents is permanent. The main function of the BBB is to limit the transfer of toxic agents from the blood into the brain. However, as the barrier is immature at the early developmental stages, the transfer of toxic agents from the blood into the brain might not be efficiently limited. Hence, exposure to realgar during early life might be associated with an increased risk of neurotoxicity. In our experiment, weanling rats were selected as the study animals to determine the neurotoxicity of realgar on growth and development. Appropriate dose selection is critical to establishing toxicity. Three dose groups (low, middle, and high) of a test substance plus a control are generally used in toxicological studies to characterize the relationship between doses and responses. In general, the high dose for a toxicological study is recommended to be in the range of 1/5 ~ 1/20 of the LD50 of test substance. The LD50 of realgar in mice is reported to be 20.5 g/kg [2], which is equivalent to 14.2 g/kg in rats according to body surface area conversion factor commonly used in pharmacological tests. In order to achieve the toxicological model (or toxic effects) in short-term exposure, we chose approximately 1/5 of the LD50 of realgar in rat (2.7 g/kg, equivalent to 90 times of the clinical dose) as the highest exposure dose for consecutive 6 weeks of dosing. Clear signs of neurotoxicity and absence of morbidity and mortality were observed in the high-dose realgar-exposed rats. In our study, 10 and 30 times of therapeutic dose were selected as low and middle doses, because the interval between doses is generally 3–10 times geometrically in toxicological studies and the low dose in a toxicological study should ideally demonstrated the no observed adverse effect level (NOAEL). In our study, no neurotoxic effects were observed at the low-dose realgar-exposed group. Therefore, rats were treated with 0.3, 0.9, and 2.7 g/kg realgar for 6 weeks to achieve toxicity in this model. The general appearance and physical condition of the rats, which included movement, alertness, and appearance of the coat, nose, eyes, and limbs, were closely observed during the experiment, and no obvious differences were noticed. No significant differences in hippocampal weight coefficients were found between the control and realgar-exposed groups (P > 0.05).
The arsenic contained in realgar is an exogenous compound, and only the amount that passes through the BBB can accumulate in the hippocampus and produce toxic effects. The hippocampus is a limbic system structure that is particularly important in forming new memories and connecting emotions and senses. Therefore, in this study, we measured the accumulated arsenic levels in the hippocampus to determine the exposure and metabolism of realgar. The metabolites iAs, MMA, and DMA were detected in the blood of rats after 6 weeks of exposure to realgar. In the hippocampus, only the methylated derivatives (MMA and DMA) of realgar were detected in exposed rats with DMA as the major metabolite (approximately 90 %). A dose-related correlation was found between the arsenic contents and the realgar exposure dose, which indicates that the arsenic contained in realgar and its metabolites can pass through the BBB and accumulate in the hippocampus. iAs was not detected in the hippocampus of any group, which may be the result of brain methylation [20].
Many epidemiological studies conducted in different countries have shown that arsenic exposure through drinking water may impair the intelligence of children [21]. Rodríguez et al. [22, 23] found neurobehavioral changes and alterations in learning tasks in rats following sodium arsenite exposure. Luo et al. [24] demonstrated spatial memory damage in rats exposed to 68 mg/L arsenic for 3 months. The data from our MWM test suggest that rats in all groups developed space allocation memory for the underwater platform. Significant increases in the mean total escape latency and decreases in the number of crossings were found in the middle- and high-dose realgar-exposed groups compared with the control group. A significantly shorter time spent exploring the novel object was also observed in the middle- and high-dose realgar-exposed groups compared with the controls in the ORT test. The results suggest that long-term exposure to realgar causes deficits in spatial learning and memory and novel object recognition in young rats.
In our study, ultrastructural changes in the hippocampus were detected. The amount of heterochromatin was increased, and swelling, rupture, and balloon-like damage in the mitochondria as well as expansion of the rough endoplasmic reticulum occurred.
Glu is involved in brain plasticity and many higher brain functions, such as learning and memory. However, its excessive accumulation could result in neuronal death. The hippocampal CA1 region is critically involved in learning and memory. Therefore, in our study, the MD-HPLC method was applied to determine the extracellular concentration of Glu in the hippocampal CA1 region. Increased levels of extracellular Glu concentrations in the hippocampal CA1 region were observed in the realgar-exposed rats compared with the controls, and a dose-effect relationship was also found. This indicates that realgar can cause the excessive accumulation of Glu in the extracellular space and induce excitotoxicity, which results in declines in learning and memory and novel object recognition.
To prove that realgar can induce excitotoxicity by affecting Glu metabolism and transportation, the contents of Glu and Gln in the hippocampus were also measured. Significant decreases in Glu were found in the hippocampus of the realgar-exposed rats compared with the controls, while no significant alterations in Gln levels were found (P > 0.05). This result may be associated with the alterations of two major enzymes, GS and PAG, in the “Glu-Gln” cycle and the Glu transporters GLT-1 and GLAST. Our results indicate a marked elevation of GS and PAG activities in the realgar-exposed rats compared with the controls. The results were similar to data reported on the effects of subchronic arsenic exposure on the Glu-Gln cycle in the mouse brain [25]. In our study, the inhibition of GLT-1 mRNA and protein expression was observed in the realgar-exposed group compared with the controls, and similar results were also found in astrocytes exposed to arsenite [26]. However, no significant differences in GLAST mRNA and protein expression were observed in the realgar-exposed groups compared with the control group (P > 0.05). This effect may be because GLT-1 is the most physiologically important transporter for Glu, which contributes over 90 % of the total Glu uptake in astrocytes [27] and a quantitative study demonstrates that GLT-1 density is up to 8,500 molecules per square micrometers in the hippocampus glia cell membrane, which is four times higher than GLAST density in the hippocampus [28]. The inhibition of GLT-1 expression observed in our study strongly suggests that GLT-1 is the key Glu transporter subtype that protects neurons from exposure to excitotoxic levels of Glu in the hippocampus of the realgar-exposed rats. It is supposed that the unchanged level of Gln found in the hippocampus of the realgar-exposed rats might result from a compensatory increase in GS activity. The increase in PAG activity results in an increased synthesis of Glu and, consequently, an increased release of Glu into the synaptic cleft. Due to the inhibition of GLT-1 expression, Glu uptake was decreased, which resulted in the excessive accumulation of Glu in the synaptic cleft, producing excitotoxicity. In addition, Glu is the precursor of glutathione; therefore, the decrease in Glu in the hippocampus of the realgar-exposed rats may also be due to the oxidative damage induced by arsenic. This hypothesis requires further study.
In our study, the protein expression of NR1 was significantly decreased in the high-dose realgar-exposed group compared with the controls. However, the mRNA expression of NR1 was not significantly changed in the realgar-exposed groups compared with the controls (P > 0.05), which suggests that the differences in NR1 may reflect posttranslational processing. The observation of the posttranscriptional regulation of the NR1 subunit has been reported in several publications [29–32]. The decrease in NR1 expression observed in our study suggested that the cognitive impairments induced by realgar may be associated with its effect on NMDA receptors. Luo et al. [24, 33] have also reported the decreased expression of NMDA receptors in the hippocampus of arsenic-exposed rats. In our study, increases in NR2A mRNA and protein were observed in the realgar-exposed rats compared with those in the controls. Although the mRNA expression of NR2A was not statistical significantly changed in the low- and middle-dose realgar-exposed groups (P > 0.05), a trend towards an increase was observed that may become significant with increasing exposure dose or prolonging exposure time. No significant differences in NR2B mRNA and protein expression were found, which suggests that the NR2A subunit is more sensitive to the toxicity induced by realgar. Additionally, the increased expression of NR2A is associated with the excess accumulation of Glu in the synaptic cleft. Pizzi et al. [34] have confirmed that excitatory amino acids can destroy CNS neurons by the excessive activation of NMDA receptors. In addition, studies show that NMDA receptors are involved in the mechanism of neuronal injury caused by excitotoxicity. The overstimulation of NMDA receptors induces delayed neuronal damage, which is the main consequence of Glu-induced excitotoxic injury. The delayed injury is mainly associated with Ca2+ overload. The disorder of intracellular Ca2+ homeostasis damages mitochondrial function and eventually results in cell death [35, 36]. In our study, the intracellular Ca2+ levels were significantly increased in the middle- and high-dose realgar-exposed groups compared with the controls. Therefore, we infer that realgar induces the inhibition of Glu transport, leading to increased extraneuronal Glu with the consequent overstimulation of the NR2A voltage-dependent calcium channel and excitotoxic processes, which involve intracellular Ca2+ overload and the disturbance of mitochondrial function and eventual cell apoptosis and necrosis.
Conclusion
The present study established a toxic model using weanling rats exposed to realgar for 6 weeks. In conclusion, the study demonstrated that the arsenic contained in realgar and its metabolites can pass through the BBB and accumulate in the hippocampus, and a dose-dependent increase of MMA and DMA was also observed. The arsenic contained in realgar disturbed Glu metabolism and transportation in the hippocampus and resulted in the excess accumulation of Glu in the synaptic cleft and excitotoxicity. On one hand, the excessive Glu accumulation in the synaptic cleft led to the decreased expression of the NR1 subunit. On the other hand, it caused enhanced expression of the NR2A subunit, leading to Ca2+ overload. Together, these alterations resulted in the ultrastructural changes in hippocampal neurons and the impairment of learning and recognition memory. The study provides the experimental basis to explore the mechanism of neurotoxicity induced by realgar in the hippocampus, provides important information for the prevention of realgar-induced learning and memory damage, and provides a theoretical basis for the reasonable application of realgar in clinical situations.
Abbreviations
- TCMs:
-
Traditional Chinese medicines
- BBB:
-
Blood-brain barrier
- MWM:
-
Morris water maze
- ORT:
-
Object recognition task
- Glu:
-
Glutamate
- CNS:
-
Central nervous system
- GLT-1:
-
Glutamate transporter 1
- GLAST:
-
Glutamate/aspartate transporter
- GS:
-
Glutamine synthetase
- Gln:
-
Glutamine
- PAG:
-
Phosphate-activated glutaminase
- NMDA:
-
N-Methyl-d-aspartate
- iAs:
-
Inorganic arsenic
- MMA:
-
Methyl arsine
- DMA:
-
Dimethyl arsine
- ECL:
-
Enhanced chemiluminescence
- CMC-Na:
-
Sodium carboxymethylcellulose
- MD:
-
Microdialysis
- NE:
-
Northeast
- NW:
-
Northwest
- SE:
-
Southeast
- SW:
-
Southwest
- AAS:
-
Atomic absorption spectrophotometry
- TAs:
-
Total arsenic
- HPLC:
-
High-performance liquid chromatography
- PVDF:
-
Polyvinylidene difluoride
- TBS:
-
Tris-buffered saline
- PBS:
-
Phosphate-buffered saline
- NOAEL:
-
No observed adverse effect level
References
Chinese Pharmacopeia Committee (2010) Pharmacopoeia of China. People's Press, Beijing, p 316
Liang A, Li C, Wang J et al (2011) Toxicity study of realgar. Zhongguo Zhong Yao Za Zhi 36:1889–1894
Zhang L, Gao S, Zhou C et al (2006) Safety issues of traditional Chinese medical preparations containing arsenic substances: review starting from Niuhuang Jiedu Pian (Wan). Zhongguo Zhong Yao Za Zhi 31:2010–2013
Vahidnia A, van der Voet GB, de Wolff FA (2007) Arsenic neurotoxicity—a review. Hum Exp Toxicol 26:823–832
Ehrenstein OS, Poddar S, Yuan Y et al (2007) Children’s intellectual function in relation to arsenic exposure. Epidemiology 18:44–51
Roy A, Kordas K, Lopez P et al (2011) Association between arsenic exposure and behavior among first-graders from Torreón, Mexico. Environ Res 111:670–676
Zhang Y, Qiang S, Sun J et al (2013) Liquid chromatography-hydride generation-atomic fluorescence spectrometry determination of arsenic species in dog plasma and its application to a pharmacokinetic study after oral administration of Realgar and Niu Huang Jie Du Pian. J Chromatogr B 917–918:93–99
Tang Y, Wang N, Mi S et al (2009) Excretion of arsenic in realgar and its metabolites in rats. Pharmacol Clin Chin Mater Med 25:39–42
Li C, Liang A, Wang J et al (2011) Arsenic accumulation following realgar administration in rats. China J Chin Mater Med 36:1895–1899
Huo T, Chang B, Zhang Y et al (2012) Alteration of amino acid neurotransmitters in brain tissues of immature rats treated with realgar. J Pharm Biomed Anal 57:120–124
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. Neurosci Methods 11:47–60
Akkerman S, Prickaerts J, Steinbusch HW et al (2012) Object recognition testing: statistical considerations. Behav Brain Res 232:317–322
Burbaeva GS, Boksha IS, Tereshkina EB et al (2005) Glutamate metabolizing enzymes in prefrontal cortex of Alzheimer’s disease patients. Neurochem Res 30:1443–1451
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Heyser CJ, Ferris JS (2013) Object exploration in the developing rat: methodological considerations. Dev Psychobiol 55:373–381
Renis M, Cardile V, Russo A et al (1998) Glutamine synthetase activity and HSP70 levels in cultured rat astrocytes: effects of 1-octadecyl-2-methyl-rac-glycero-3-phospho-choline. Brain Res 783:143–150
Curi TC, De Melo MP, De Azevedo RB et al (1997) Glutamine utilization by rat neurophils: presence of phosphate-dependent glutaminase. Am J Physiol 273:1124–1129
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
van Goethem NP, Rutten K, van der Staay FJ et al (2012) Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav Brain Res 232:323–334
Rodríguez VM, Del Razo LM, Limon-Pacheco JH et al (2005) Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol Sci 84:157–166
Majumdar KK, Guha Mazumder DN (2012) Effect of drinking arsenic-contaminated water in children. Indian J Public Health 56:223–226
Rodríguez VM, Carrizales L, Jiménez-Capdeville ME et al (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55:301–308
Rodríguez VM, Carrizales L, Mendoza MS et al (2002) Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol 24:743–750
Luo J, Qiu Z, Shu W et al (2009) Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett 184:121–125
Qu C, Niu Y, Zhong Y et al (2007) Effects of subchronic arsenic exposure on glutamate-glutamine cycle in mice brain. J Environ Health 24:751–754
Zhao F, Liao Y, Jin Y et al (2012) Effect of arsenite on glutamate metabolism in primary cultured astrocytes. Toxicol in Vitro 26:24–31
Shachnai L, Shimamoto K, Kanner BI (2005) Sulfhydryl modification of cysteine mutants of a neuronal glutamate transporter reveals an inverse relationship between sodium dependent conformational changes and the glutamate-gated anion conductance. Neuropharmacology 49:862–871
Lehre KP, Danbolt NC (1998) The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18:8751–8757
Gazzaley AH, Weiland NG, McEwen BS et al (1996) Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci 16:6830–6838
Follesa P, Ticku MK (1995) Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Mol Brain Res 29:99–106
Follesa P, Ticku MK (1996) NMDA receptor upregulation: molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J Neurosci 16:2172–2178
Trevisan L, Fitzgerald LW, Brose N et al (1994) Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem 62:1635–1638
Luo J, Qiu Z, Zhang L et al (2012) Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol Lett 11:39–44
Pizzi M, Consolandi O, Memo M et al (1996) Activation of multiple metabotropic glutamate receptor subtypes prevents NMDA-induced excitotoxicity in rat hippocampal slices. Eur J Neurosci 8:1516–1521
Muscoli C, Visalli V, Colica C et al (2005) The effect of inflammatory stimuli on NMDA-related activation of glutamine synthase in human cultured astroglial cells. Neurosci Lett 373:184–188
Caballero-Benitez A, Alavez S, Uribe RM et al (2004) Regulation of glutamate-synthesizing enzymes by NMDA and potassium in cerebellar granule cells. Eur J Neurosci 19:2030–2038
Acknowledgments
The work is financially supported by the National Natural Science Foundation of China (81073144), the China Postdoctoral Science Foundation on the 51th grant program (2012M510848), and the Higher Education Department of Liaoning Province (L2010708).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, Tg., Li, Wk., Zhang, Yh. et al. Excitotoxicity Induced by Realgar in the Rat Hippocampus: the Involvement of Learning Memory Injury, Dysfunction of Glutamate Metabolism and NMDA Receptors. Mol Neurobiol 51, 980–994 (2015). https://doi.org/10.1007/s12035-014-8753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8753-2