Abstract
Compelling evidence from animal models and clinical studies suggest that transplantation of olfactory ensheathing cells (OECs), specialized glia in the olfactory system, combined with specific training may be therapeutically useful in the central nervous system (CNS) injuries and neurodegenerative diseases. The unique function of OECs could mainly attribute to both production of cell adhesion molecules and secretion of growth factors in OECs, which support neuron survival and neurite outgrowth. However, little is known about whether engulfment of neuronal degenerative debris by OECs also equally contributes to neuronal survival and neurite outgrowth. Furthermore, the molecular mechanisms responsible for neuronal degenerative corpses' removal remain elusive. Here, we used an in vitro model of primary culture of spinal cord neurons to investigate the effect of engulfment of degenerative neuron debris by OECs on neuronal survival and neurite outgrowth and the possible molecular mechanisms. Our results showed that OECs can engulf an amount of degenerated neuron debris, and this phagocytosis can make a substantial contribution to neuron growth, as demonstrated by increased number of neurons with longer neurite length and richer neurite branches when compared with the combination of neuron debris and OEC conditioned medium (OECCM). Moreover, p38 mitogen-activated protein kinase (p38MAPK) signaling pathway may mediate the OEC engulfment of debris because the p38MAPK-specific inhibitor, SB203580, can abrogate all the positive effects of OECs, including clearance of degenerated neuron debris and generation of bioactive molecules, indicating that p38MAPK is required for the process of phagocytosis of the neuron debris. In addition, the OEC phagocytic activity had no influence on its generation of bioactive molecules. Therefore, these findings provide new insight into further investigations on the OEC role in the repair of traumatic CNS injury and neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olfactory ensheathing cells (OECs) are a unique type of glial cells in the olfactory system that differ from typical glia by virtue of the fact that they are present in both the peripheral nervous system (PNS) and CNS and share some features and functions of astrocytes as well as Schwann cells [1–4]. In addition, OECs have shown to display distinct molecule expression profiling, which is crucial for creating a regenerative environment within the CNS [1, 4, 5]. Therefore, OECs have been intensively studied as potential candidates for cell-mediated repair of neural injuries. Overall, this may be mainly due to the release of neurotrophic factors from OECs or potentially direct effects on axons by providing some physical substrates and bioactive factors [6–12]. To date, the notion has still been promulgated regarding the use of OECs to facilitate neuroprotective and neuroregenerative repair following spinal cord injury. However, when CNS injuries or neurodegenerative disorders occur, several neural cells including astrocytes and microglial cells respond quickly to the different insults, leading to a gradual deterioration within the CNS and further creation of a damaging degradation environment. All these intricate alterations within the CNS are frequently accompanied with release of several growth inhibitory molecules such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), CXCL8/IL-8, glutamate, myelin-associated glycoprotein (MAG), Nogo, oligodendrocyte-myelin glycoprotein (OMgp), and repulsive guidance molecule a (RGMa), etc. which exhibit inhibitory effects on neuron survival or axonal regrowth. Among them, some directly originate from degenerating or dying neurons and apoptotic neural debris [13–17]. Thus, rapid clearance of these deteriorated cells or debris is essential for establishing a microenvironment beneficial for neuron survival and axonal regeneration after CNS insults. More importantly, efficient removal of these degenerated tissues/cells also avoids the diffusion of damaging degradation products into surrounding tissues [18]. Based on these studies, this is implied that targeted delivery of neurotrophic factors or neural cell adhesion molecules only to lesions cannot efficiently achieve neural regeneration.
Although previous studies have broadly demonstrated the ability of OECs to promote histological and functional neural repair, and that the special efficacy of OECs in axonal regeneration and neuroplasticity in neurodegenerative diseases is usually believed to be due to its properties of generating growth-promoting factors, very little attention was paid to OEC phagocytosis and autoimmune characteristic in the enhancement of neuron survival and axonal outgrowth. Strikingly, a variety of experiments have also concluded that the use of recombinant or virally expressed active factors in OECs following CNS injury could promote nerve regeneration [19–23], whereas the delivery of the same amount of the growth-promoting factors does not reach an equal therapeutic efficacy and even neurological function recovery, suggesting that other biofunctions of OECs may play a pivotal role in nerve regeneration process. Even though it has been found that the combination of several neural outgrowth or survival-promoting molecules could facilitate neuron survival and axonal outgrowth and produce a similar trend to CNS regeneration, both therapeutic efficacies are completely distinct. That is, OEC phagocytosis may exert an important role in the neuron growth. It is well documented that nervous system injuries or diseases can lead to the production of substantial amounts of neural waste material, including neuronal cell corpses as well as axonal, dendritic and synaptic debris[24–26]. These damaged and degraded productions are generally considered to be one of the obstacles for neuronal neurite sprouting and final attainment of regeneration of the injured axons in the CNS [25–28]. Thus, rapid and efficient clearance of these cell degeneration products is essential to prevent cell lysis and the release of pro-inflammatory and antigenic autoimmune components. Also, clearance of these dying cells is crucial for creating a favorable environment for neuronal cell turnover and regeneration. Fortunately, the degenerated neural debris could be engulfed by resident population of microglia [25]. Despite of that, activated microglial cells usually migrate slowly towards lesions and produce an amount of harmful factors which lead to serious secondary disasters.
OECs are an important cell type in the olfactory system. Why the olfactory neurons are continuously replaced throughout the life of mammals is largely associated with OEC characteristics [29–32]. OECs were shown to share some characteristics of inflammatory cells allowing them to prevent microbial infection from the olfactory pathway [10]. More importantly, OECs are also demonstrated to express Toll-like receptors and possess certain bacterial ligands [33–37]. Specifically, OECs can be induced to express OX-42, a microglial cell marker [30]. However, it is unknown whether OECs can function as phagocytes to clear the degenerated neural cell debris for the enhancement of nerve regeneration.
In this study, we developed an in vitro model of an inhibitory spinal cord injury-like environment to investigate whether OECs also serve important roles as phagocytes to clear dying cells debris and are more supportive of neuron survival and neurite outgrowth. Meanwhile, we examined and compared neuron survival and neurite outgrowth in the presence or absence of degenerated neuron debris when cocultured with OECs. Our findings indicated that OECs remarkably strengthen neuron survival and neurite outgrowth by engulfment of neuron debris, displaying the higher proportion of neuron survival and longer neurite length than that cultured with OEC conditioned medium (OECCM). Furthermore, the OEC clearance of degenerating neuron debris and enhancement of neuron growth were mediated through p38MAPK-signaling pathway. In light of our present results, this study on OEC phagocytosis to enhance neuron growth may open new avenues in the search for a therapy to repair injured CNS or neurodegenerative diseases.
Materials and Methods
Main Reagents
Dulbecco's modified Eagle's medium (DMEM)/F12 (DF/12), DMEM, Neurobasal medium, B27 supplement, G5 supplement, Hanks' balanced salt solution (HBSS), fetal bovine serum (FBS), trypsin, collagenase, and normal goat serum were purchased from Gibco (USA); penicillin G, lipopolysaccharide (LPS), streptomycin, forskolin, glutamine, trypsin, poly-l-lysine (PLL), ethylenediaminetetraacetic acid (EDTA), HEPES, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (USA); anti-γ-tubulin and anti-Tuj-1 antibody were purchased from Chemicon (USA); anti-p75 antibody was purchased from Abcam (USA); p38MAPK and phosphorylated p38MAPK were from Cell Signaling (USA); the BCA Kit was from Qiagen (Germany); Chemiluminescence Western Blotting Kit was purchased from Plus (Germany); DAPI Kit, Fluor488-conjugated goat anti-mouse IgG, and Fluor594-conjugated donkey anti-rabbit were purchased from Molecular Probes (USA). Thirty-five-millimeter dishes, cell culture plates, plastic coverslips, and flasks were purchased from Nuncon (Denmark).
Primary Cell Culture
OEC Culture and Purification
All experiments on animals were approved by the Animal Experimentation Ethics Committee of the National Academy of Science and implemented according to the guidelines established by the Ministry of Health of China. The OECs were cultured from olfactory blub and further purified based upon the method of Ramón-Cueto et al. [38] with minor modifications to increase the yield of OECs whilst maintaining a high level of purity. Briefly, the outer nerve fiber and granular layers of olfactory bulbs were dissected from 2-month-old C57/BL6 male mice and washed twice in HBSS without Ca2+/Mg2+. Then, the tissues were cut into small pieces by microscissors and dissociated with 2 mL of 0.25 % typsin and 0.03 % collagenase for 25 min at 37 °C. The digested tissue was triturated and was filtered through an 80-μm metal mesh. The cell suspension was resuspended in DMEM/F12 supplemented with 10 % FBS and seeded on PLL-coated flasks and cultured at 37 °C with 5 % CO2. Media was refreshed half every 3 days. After cells reached over 85 % confluency, the cells were purified by differential cell adhesiveness. In detail, cells were collected and then re-seeded on other uncoated flasks and incubated for additional 36 h after single-cell suspensions generated by digestion. Subsequently, the nonadhesive cell suspension was collected and then replated onto 25-cm2 PLL-coated culture flasks and incubated with 15 % FBS-containing DMEM/F12 supplemented with 2 μM of Forskolin (Sigma).
Neuron Culture of Spinal Cord
Primary neurons were prepared from the spinal cords of 12- to 14-day red fluorescent protein (RFP) transgenic or normal mouse embryos according to the method described by Yang et al. [39]. The spinal cords were dissected and washed with ice-cold HBSS without Ca2+ and Mg2+. After removal of the meninges and blood vessels, the spinal cord tissue was chopped into tiny pieces and trypsinized with 2 mL of 0.125 % (w/v) trypsin at 37 °C for 25 min. Trypsinization was terminated by the addition of 10 mL DMEM supplemented 10 % heat-inactivated FBS, and the tissue was dissociated by mechanical trituration. The cell suspension was filtered and counted, and diluted to an approximated initial plating density of 2 × 105 cells/cm2 with Neurobasal Medium supplemented 2 % B27 and plated into either 35-mm Petri dishes or coverslips coated with PLL. Cultures were maintained in an incubator at 37 °C in a humidified atmosphere of 5 % CO2. The cultures were maintained and prepared for further experiments.
Preparation of Degenerative Neuronal Debris
In order to examine OEC phagocytosis, we developed neuronal degeneration models to produce neural debris. Briefly, when the neurites of the abovementioned neurons from RFP mouse in 35-mm Petri dishes for over 20 days have formed a complicated network, the neurites were longitudinally and latitudinally transected every 0.5 cm by using a blade as described previously [40]. Then the half volume of culture medium were removed and continuously maintained for 12–24 h. Then cell neurites have separated for their bodies and displayed focal swelling and beading, namely, Wallerian degeneration. The degenerated neuronal bodies and axon debris was harvested by scratching with cell blade and further trituration. Thus, degenerated neurites became fragmented debris, consisting of microtubules and neurofilaments, and finally stored at −80 °C until used.
Collection of OEC Conditioned Medium
To investigate the relationship between OEC phagocytic activity and secretion of some bioactive molecules to contribute to neuron growth, we collected OEC conditioned medium from the aforementioned cell culture. Briefly, subcultured OECs were grown in DF12 containing G5 supplement on 35-mm dishes, and the cell supernatant was changed and collected once every day. Seven days later, these OEC supernatants were combined, mixed, and centrifuged at 14,000×g for 15 min at 4 °C and then filtered with a sterile 0.45-μm pore to remove cell debris. Finally, these conditioned medium were kept at −80 °C until used.
Phagocytosis Assay
To elucidate the function of OECs in clearance of the neuronal debris following neuronal injury, we attempted to establish an assay for OEC phagocytosis of degenerated debris in vitro. OECs were plated in 35-mm culture dishes prior to pretreatment with LPS at 1 μg/mL for 12 h and then incubated with the collected cell degeneration debris as mentioned above. The ratio of volume of cell degeneration debris poured into OEC culture and total volume is 1:4. After culture for 1, 3, 5, and 7 days, respectively, the photomicrographs of OEC phagocytosis were taken under phase-contrast microscopy. For quantitative analysis, the ability of OECs to engulf the degenerated cell debris was assessed by a phagocytic index (PI). The PI was defined as a percentage of residual debris and phagocytic area relative to control. The detailed analysis was undertaken using ImageJ software. The results were obtained from three independent experiments.
Distinct Treatment of Neurons
To examine if OEC phagocytosis of degenerated cell debris mainly contributes to neuron survival and neurite growth, cells cultured on coverslips were firstly divided as the following: (1) normal neurons with treatment of OEC conditioned medium (OECCM); (2) normal neurons with treatment of OECCM plus degenerative neuronal debris; (3) normal neurons with treatment of degenerative neuronal debris alone; (4) normal neurons cocultured with OECs; (5) normal neurons cocultured with OECs supplemented degenerative neuronal debris; and (6) normal neurons without treatment of exogenous agents. The cells in all groups were then maintained at 37 °C in a humidified 5 % CO2 atmosphere for 5 days, respectively. Notably, OECs should be pretreated with LPS for 12 h prior to coculture with neurons. Concomitantly, cells were processed for the following different assessments.
Immunofluorescent Staining
Neuronal cells on plastic coverslips or OECs of all groups were fixed with 4 % paraformaldehyde (Sigma) for 30 min, treated with 0.01 % Triton X-100 (Sigma) (for OEC staining, not treated with 0.01 % Triton X-100), and 4 % normal goat serum (Sigma) in 0.01 M PBS, incubated with primary mouse monoclonal antibodies against Tuj-1 and p75 (Tuj-1 1:1,000, p75 1:1,500 ), respectively, at 4 °C overnight, washed in PBS thrice and incubated with the corresponding fluorescence-conjugated secondary antibody (Alexa Fluor® 488 goat anti-mouse IgG antibody for Tuj-1, 1:800 dilution; FITC-conjugated donkey anti-rabbit IgG for p75) for 2 h and Hoechst 33,342 (5 μg/mL) at room temperature (RT) for 15 min, coverslipped after rinsed in PBS thrice, and observed under an Olympus BX-51 fluorescence microscope (Olympus, Japan). All images were captured using a FV10-ASW 1.6 photosystem (Olympus, Japan). Independent cultures were used when each experiment was conducted thrice.
Cell Count
To assess the survival of spinal cord neurons with the abovementioned treatments, all Tuj-1-positive cells, regardless of their phenotypes, were counted under fluorescent microscopical observation at as much as ×200 magnification. The observation fields (0.45 mm2) of the culture coverslips were chosen randomly, and the measured cells were Tuj-1-immunoreactive positive. To discriminate between experimental and control groups, this work was completed by a person without knowledge on the experiment according to the same criterion for counting the same amplification, same field area, and same number of cultures. Each coverslip was analyzed by counting cells from 15 randomly chosen fields. In all analysis, the data represent the mean ± SEM of four independent experiments; each performed in four coverslips and is considered as the index of neuron survival.
Quantitative Assays for Neurite Outgrowth
For measurement of neurite outgrowth, Tuj-1-positive neurite lengths were measured as previously described [41, 42]. Briefly, 15 fields in each well were chosen randomly and photographed through the fluorescent microscope. All Tuj-1 immunofluorescent cells were selected to measure neurite length. A total of approximately 400 cells in fields were assessed for each treatment conditions. Neurite length was defined as the distance between the body and the farthest tip of the neurite. Statistical analyses were performed using StatView software (Abacus, Berkeley, CA, USA). In all analysis, the data represent the mean ± SEM of four independent experiments, each performed in duplicate.
Western Blotting
For investigation of the signaling molecules involved in mediating the engulfment of neuronal debris by OECs, in brief, the OEC cocultured neurons in the presence or absence of debris, as well as subsequent specific inhibitor (SB203580), were collected for western blot. The following antibodies were used: p38MAPK (1:500) and phosphor p38MAPK (1:1,000). For analysis of changes of p38MAPK, total cellular extracts were prepared as follows: OECs in three dishes were carefully but briefly rinsed with saline buffer and extracted in ice-cold RIPA as described by Yang et al. [43]. The protein concentrations of clarified lysates were measured using the BCA protein assay, with BSA as a reference. Protein lysates were run on 4–20 % gradient SDS-PAGE, respectively, transferred onto nitrocellulose membrane and blocked with 5 % fat-free milk for 1 h at RT for 30 min, washed with Tris-buffered saline and probed with primary antibodies at 4 °C overnight. β-actin (1:5,000; Santa Cruz) was included as an internal loading control. After washed, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody at RT for 1 h and washed again with TBST. The bound immune complexes were detected using enhanced chemiluminescence and X-ray films. Densitometric analysis of exposed X-ray films was repeated for four times, and intensity of density value (IDV) was calculated.
Statistical Analysis
All data were presented as mean ± SEM at different time points for each group. The statistical significance was determined using one-way analysis of variance (ANOVA) with SPSS 10.0 software. A value of p < 0.05 was considered statistically significant. For the statistical analyses of the number and length of extended neuritis from cultured neurons, the analyses for the data in each of the three independent experiments were performed in duplicate using StatView software (Abacus Concepts, USA).
Results
OEC Culture, Identification, and Assessment of Clearance of Degenerated Debris
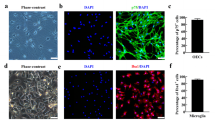
To investigate whether OECs have the capacity to engulf the degenerated neuron debris and further potentiate neuron survival and neurite outgrowth, OECs were firstly cultured and taken under invert phase-contrast microcopy after subcultures, followed by immunostaining identification. After 5 days of culturing, the purified OECs were in a bipolar, multipolar, or an irregular shape with long and thin processes, and interconnected in an intricate network (Fig. 1a). Also, immunocytochemical staining for p75 (a characteristic marker for OECs) demonstrated that over 95 % of cells were OECs (Fig. 1b). In addition, we establish an in vitro assay system to estimate OEC phagocytosis of degenerated neuron debris by observation and calculation of residual debris. The result demonstrated that OECs efficiently cleared degenerated neuron debris, and the amount of residual debris (arrows) and debris area progressively reduced with prolongation of culture time (from 1 to 7 days) (Fig. 1c–g). Quantitative analysis revealed that the residual debris was significantly reduced from 1 to 7 days after pouring neuronal debris into the OEC culture (reduced 3.2 to 49.2 % of control) (Fig. 1h). Moreover, debris area of phagocytosis by OECs was apparently diminished as compared with control (reduced 32.3 to 97.4 % of control) (Fig. 1i).
OEC culture, identification, and phase-contrast microscopic images of OEC phagocytosis of degenerated neuronal debris. a, b Primary cultured OECs and identification of immunocytochemistry for p75, respectively. c Photomicrographs of degenerated neuronal debris in the cultures without OECs. d–g Engulfment of degenerated neuronal debris by OECs at 1, 3, 5, and 7 days. h Quantitative analysis of the relative number of residual debris compared with the control (without OECs). i Quantitative analysis of phagocytic area in OECs cultures. **p < 0.01; ***p < 0.001 compared with control. Arrows indicate cell debris. Scale bars indicate 25 μm
Engulfment of Degenerated Neuron Debris by OECs
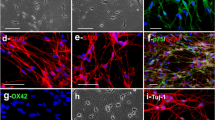
To further validate whether the degenerated neuron debris in vitro were removed owing to OEC phagocytosis, we poured the degenerated neuronal debris from RFP mouse into OEC culture and examined the debris content in OEC cytoplasm. First, the images visualized by an phase-contrast microscopy indicated that the degenerated neuron debris was present inside the cytoplasm of OECs, displaying accumulating red debris in OEC cytoplasm, and that the red cell debris extent in OECs is gradually still accumulating from 1 to 5 days in vitro (Fig. 2(a–c)). This result suggested that OECs can engulf and ingest degenerated neuron corpses. Furthermore, the engulfment of cell debris by OECs was confirmed by immmunofluorescence analysis. Consistent with the images of inverted microscopy, the immunostaining also revealed that the red degenerated neuron debris gradually was ingested into p75 (specific marker for OECs)-positive cells at the abovementioned time points, and the amount of fragmented debris significantly increased with the prolongation of culture time (Fig. 2(d–f)). Importantly, quantitative analysis showed that the number of p75-positive cells inside which cytoplasm contained red cell debris was also increased from 1 to 5 days in culture, displaying a gradual increase in PI (Fig. 2(g)). These results showed that clearance of the degenerated neuron debris (bodies and neurites) in culture is attributed to the primary OEC phagocytosis.
Photomicrographs of the content of engulfed and ingested debris inside OECs. a–c Phase-contrast micrographs of engulfment of degenerated neuronal debris from RFP mouse inside OEC cytoplasm at 1, 3, and 5 days, respectively. d–f To further substantiate the specific phagocytosis by OECs, cells were identified with p75 immunofluorescent staining at the indicated time points. Notably, the content of debris ingested inside OEC cytoplasm was increased with the prolongation of culture time. g Quantitative analysis of the content of debris ingested inside OECs. Arrows indicate cell debris. Scale bars indicate 200 μm
Effect of Engulfment of the Degenerated Cell Debris by OECs on Neuron Survival and Neurite Outgrowth
Next, we examined whether clearance of cell debris by OECs have a role in enhancing neuron survival and neurite outgrowth. The spinal cord neurons were cultured at low density for up to 5 days after the aforementioned treatments, and immunofluorescent staining was then performed using Tuj-1. As Fig. 3(a), exposure of cells to degenerated neuron debris alone resulted in poor neurite outgrowth and reductions in process arborization. Meanwhile, there appeared apparently an amount of scattered debris of degenerated neurons in culture, suggesting that degenerated cell debris suppressed neuron growth and promoted cell degeneration. In contrast, under normal culture condition, no apparent cell debris was seen around neurons, and these neurons extended considerable long and thin neurites. Notably, there were considerable abundant branching and arborizations in neurites (arrows) (Fig. 3(b)). In order to confirm if, apart from various bioactive molecules generated by OECs, the other biofunctions of OECs, such as phagocytosis, is actively responsible for promoting both neuronal survival and neurite, primary neuron cultures were treated with OECCM in the presence of degenerated cell debris. The results showed that OECCM can moderately attenuate inhibition of neurite outgrowth and arborization and cell survival caused by degenerated cell debris, displaying less degenerated neuron debris, a slight increase in neuron survival, and neurite growth (Fig. 3(c)). As to neurons treated with OECCM alone, a significant increase in the neurite growth, branching and cell survival was observed, displaying vigorous extension of neurites, rich process arborization, and higher number of cell survival (Fig. 3(d)). In the presence or absence of degenerated debris with OECs, we found that neuron growth was remarkably potentiated, mainly displaying a significant increase in the number of neuron survival, longer neurites, and more abundant branching. Intriguingly, there is no remarkable difference in cell morphologies between two groups (Fig. 3(e, f)). These results suggest that OEC phagocytosis also plays crucial role in enhancing neuron survival and neurite outgrowth. Furthermore, the enhancement of neuronal survival and neurite by OEC engulfment of degenerated debris was quantitatively analyzed by means of cell count and measurement of neurite length (Fig. 3(g, h)). Statistical analysis showed that degenerated neuronal debris significantly inhibited neuronal survival and neurite extension and arborizations, compared with their respective control (p < 0.01or p < 0.001), whereas the OEC phagocytic activity significantly suppressed this inhibitory effect of debris on neuronal growth. All these data indicated that the phagocytotic function of OECs must be an important enhancer of neuron growth.
Clearance of the degenerated debris by OECs allows neuron survival and neurite outgrowth. a–f Representative images of primary culture of spinal cord neuron at low density following different treatments as indicated. g, h Quantitative assessment of neuron survival and neurite lengthen at the indicated treatments, respectively. **p < 0.01; ***p < 0.001 compared with their respective control. Arrows point to cell debris. Scale bars indicate 200 μm
The Mechanism Underlying Enhancement of Neuron Growth by Phagocytotic Activity of OECs
Numerous studies have demonstrated that LPS can activate phagocytic activity of microglial cells by p38MAPK signaling pathway. Therefore, we next ascertained that the potential of LPS was responsible for the engulfment of degenerated debris by OECs. As shown in Fig. 4(a, c), OECs significantly ingested and engulfed cell debris in the presence or absence of LPS, and the accumulation of red cell debris in OEC cytoplasm were approximately equal (as seen in the inset in the lower right). Furthermore, both displayed approximately the same amount of residual debris in OEC culture environment, suggesting that LPS does not affect OEC phagocytosis. Next, we attempted to explore the signaling pathways that regulate engulfment of cell debris of microglial cells. Previous studies have demonstrated that MAPK signaling pathway is involved in microglial activation, which contributes to cell phagocytosis and the generation of cytokine secretion. Therefore, a specific p38MAPK molecule inhibitor, SB203580, was added to OECs cultures in the presence of degenerated cell debris to elucidate the issue. We found that the p38MAPK inhibitor significantly suppressed the OEC phagocytosis, leading to a striking decrease of degenerated debris ingested inside OECs cytoplasm (as shown in the inset in the lower right of Fig. 4(b, d)). Likewise, the amount of residual debris in OECs cultures significantly increased as compared with their respective controls. Also, statistically analysis revealed that there was a significant difference between two groups (with or without SB203880) (Fig. 4(e)). These results excluded the possibility that LPS directly activated p38MAPK during OECs phagocytic activity. Next, we designed a series of experiments to assess the effect of engulfment of degenerated neuronal debris by OECs on neuron survival and neurite outgrowth, and elucidated possible molecular mechanism. Figure 4(f, g) revealed that OECs significantly enhanced neuron survival and neurite outgrowth in the presence or absence of cell debris, and the specific effects were partially but significantly blocked by p38MAPK inhibitor, SB203580. A marked reduction in cell count and neurite length reached about 47.7 and 52.6 % of their control, respectively. Notably, the presence of degenerated neuronal debris also significantly inhibited neuron survival and neurite growth as compared with their control. Taken together, these data demonstrated the activation of p38MAPK might be required for OEC phagocytosis of neuronal debris, leading to neuron survival and neurite outgrowth.
p38MAPK activation is necessary for ingestion and phagocytosis of OECs. a–d OEC phagocytic activity was not influenced by LPS, but rather by the p38MAPK inhibitor, SB203580. The insets of the lower right corner show engulfment of debris inside OEC cytoplasm. e Quantitative analysis of residual debris compared with the control. f, g After the indicated different treatments, p38MAPK inhibitor affected neuron survival and neurite outgrowth, respectively, displaying a significant decrease in the number of surviving neuron and richer neurite length. h Western blot analysis of p38MAPK and phosphor-p38MAPK in OECs supplemented LPS or not in the presence or absence of debris plus. i Quantitative analysis of the p38MAPK phosphorylation level. Data are represented as mean ± S.E. of three independent experiments. *p < 0.05; **p < 0.01; and **p < 0.001 compared with control
Although the abovementioned data have demonstrated that p38MAPK signaling cascade was involved in the complicated events as SB203580 can significantly block the OEC phagocytosis, it is unclear if the phosphorylation level of p38MAPK in OECs changed after administration of degenerated neuronal debris. Therefore, we further measured the phosphorylation level of p38MAPK in OECs at different time points (1, 4, 8, 16, and 48 h) by western blot assays. The phosphorylation level of p38MAPK (pp38) was elevated 8 h after exposure of cell debris. Intriguingly, the time point of a significant increase in the level of pp38 was at 1 h when LPS was added into OEC cultures. Strikingly, no remarkable changes of the level of pp38 were found in OECs without debris in the presence or absence of LPS (Fig. 4(h)). Furthermore, quantitative analysis (a ratio of phosphor-kinase per kinase) revealed that p38MAPK phosphorylation was significantly enhanced at 8 h in debris-treated OECs and 1 h in debris plus LPS-treated OECs, respectively. For LPS-treated and normal OECs, no significant increase (p > 0.05) in the p38MAPK phosphorylation were found at any of five time points (Fig. 4(i)).
Discussion
OEC transplantation is intensively regarded as a promising therapeutic strategy for CNS injuries and neurodegenerative diseases. One of the major causes regarding its clinical research and trials attributes to OEC's unique intrinsic characteristics, such as cell substrate molecules (L1, Fibronectin, Tenascin and Janic-C etc.) expressed on the surface of OECs and growth-promoting factors (NGF, BDNF, GDNF, NT-3, etc.) secreted by OECs [8, 44–47]. Proverbially, these molecules are likely to actively facilitate neuron survival and axonal growth and also aid in the regeneration of severed axonal after CNS injury, either separately or in concert with NCAM. Therefore, an increasing attention has been paid recently to these molecules of genetically modified OECs in cell-mediated repair of CNS injuries and disorders. However, little is known about OEC enhancement of neuron survival and neurite growth by its engulfment of degenerated cell debris. Also, a clear and precise molecular/cellular mechanism underlying the effect hitherto remains to be addressed.
In the present study, we developed an in vitro model to estimate phagocytosis of neuronal debris by OECs and investigated if this phagocytosis is required for neuronal survival and neurite growth. Our results revealed that OECs efficiently engulfed the degenerated debris which is a potential inhibitory factor of neuron survival and axon growth, and that only a variety of bioactive molecules and growth factors secreted by OECs have limited potential to promote neuron growth when placed in an environment supplemented with degenerated cell debris. These findings provide clear evidence that OECs enhance neuron growth including cell survival and neurite extension and arborizations not only by its secretion of a variety of bioactive molecules, but by its phagocytosis of degenerated cell debris. Thereby the present study highlighted the importance of the OEC engulfment of debris in the neuronal survival and neurite growth concerning OEC transplantation in therapy of CNS injuries or neurodegenerative disorders.
It is well known that when the CNS injuries or neurodegenerative disorders occur, a significant proportion of neurons undergo a complicated degeneration process, leading to a large amount of harmful degeneration productions into surrounding tissues including debris of neuron corpses and inhibitory molecules [48, 49]. These are one of the obstacles for neuron survival and growth and regeneration of the injured neurons in the CNS [50]. Therefore, it is important to rapidly clear these harmful productions for creating a favorable environment within the CNS. In our present study, to confirm if OECs have the capacity to ingest and engulf degenerated nerve tissue debris, the degenerated neuron debris were added into OEC cultures to test the OEC ability. The results indicated that OECs can efficiently clear the degenerated neuron debris, and the substantial amount of scattered debris was reduced with prolongation of culture time, displaying a gradual decrease in residual debris to control and a significant increase in phagocytotic area. But the result also raised the possibility that the removal of debris may attribute to its detachment from the bottom or other cell phagocytotic activity. Accordingly, the OEC phagocytosis was further identified by phase-contrast microscopy and immunofluorescent staining. Strikingly, OECs efficiently recognized and ingested the amount of debris in vitro, displaying a progressive increase in the extent of cellular debris inside the cytoplasm. Thereby, the results excluded the possibility as mentioned above. Although several studies suggested that OECs shared some similar characteristics of inflammatory cells and could function as phagocytes to clear the corpses of degenerated neurons [33, 35–37], the unique properties is required for the neuron survival and neurite growth. Intriguingly, OECs markedly promoted the survival of primary neuron and neurite extension in the presence of the degenerated cell debris, while the secreted growth-promoting factors showed negative results from OECCM experiment in neuron growth. The result suggests that this promotive effect of OECs on neuronal growth is intimately relevant to clearance of the cell debris by OECs. Reportedly, the degenerated neuronal debris generally released much harmful molecules which are inhibitory for neuron survival and growth [25, 35, 51]. Thus, it is important to remove degraded debris and provide a favorable microenvironment for neuron survival and damaged neuron turnover. Based on these literatures, we speculated that OEC's rapid step in the clearance of debris efficiently decreased the amount of these inhibitory products and even further prevented their diffusion into surroundings tissues, resulting in a formation of favorable environment enabling neural regeneration following a CNS injury. On the other hand, the secreted molecules by OECs also actively contributed to CNS regeneration. This may be a main reason why OECs can efficiently promote neural regeneration after CNS injury.
Although the abovementioned data showed that clearance of the degenerated cellular debris is thought to be essential for maintaining neural cell growth, the mechanisms underlying enhancement of OECs remain poorly defined. It is well known that the activated microglia can acquire phagocytic activity in the CNS, and clearance of the debris must undergo debris recognition, internalization, and degradation [27, 52, 53]. These separable events were mediated by distinct signaling pathways; thereinto, p38MAPK signaling cascade plays a crucial role in modulating microglial engulfing axonal and dendritic debris [17, 54, 55]. Generally, the molecules on debris were recognized by microglia, leading to microglia activation. The upregulation of clearance of the degenerated debris by microglia was mediated through p38MAPK pathway. Coincidently, our present experiment also revealed that activation of p38MAPK was required for OECs to become phagocytic, as SB203580 can efficiently inhibit engulfment of degenerated neuronal debris by primary OECs, as shown in Fig. 4(a–e). However, stimulation of the OECs with LPS does not result in the apparent improvement of OEC phagocytic activity even though it can promote early phosphorylation of p38 in OECs, suggesting that LPS is not sufficient to induce activation of p38MAPK. This result was confirmed by western blot analysis (Fig. 4(h)). Strikingly, primary neuronal survival and neurite outgrowth were also suppressed when p38MAPK signaling pathways was specifically blocked. These observations indicate that activation of OECs does not change its secretion activity of growth-promoting molecules. On the basis of our present data, we surmised that OEC phagocytosis, like OEC secretion and expression of growth-promoting molecules, exerts crucial effect in enhancing neuron survival and neurite outgrowth.
Nevertheless, it is still unknown what molecules activated p38MAPK signaling pathways responsible for the OEC engulfment. To our knowledge, the complicated process of the OEC engulfing the cellular debris might be mainly due to the OEC characteristic and degenerated neuronal debris. First, OEC was demonstrated to express certain receptors and specific markers of microglia and can be induced to express components of innate immunocytes. Second, neural cell apoptosis or degeneration can produce a large amounts of inflammatory mediators/cytokines. Some of released factors from cellular debris can function as ligands of the receptors on the surface of OECs, efficiently pulling engulfed vesicular materials towards the cell soma. Based on the abovementioned data, we speculated that OECs were activated by direct contact with debris when degenerated neuron debris was delivered into OEC culture. The debris is recognized and internalized by OECs and eventually phagocytosed.
Taken together, our in vitro study in combination with previous studies indicates that in addition to the expressed NACM and the secreted growth factors by OECs, OEC phagocytosis of the degenerated debris played pivotal role in the enhancement of neuron survival and neurite outgrowth. More importantly, the possible underlying mechanism responsible for engulfment of cellular debris by OECs was mediated through p38MAPK signaling pathways, and certain signaling molecules released from neuronal debris might be involved in OEC activation. Therefore, further seeking for these related molecules for the activation of this signaling cascade could potentially promote neuron survival and axonal extension in therapy for CNS injury and neurodegenerative disorders.
References
Ramón-Cueto A, Avila J (1998) Olfactory ensheathing glia: properties and function. Brain Res Bull 46(3):175–187
Lakatos A, Franklin RJ, Barnett SC (2000) Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia 32(3):214–225
Gudiño-Cabrera G, Nieto-Sampedro M (2000) Schwann-like macroglia in adult rat brain. Glia 30(1):49–63
Vincent AJ, Taylor JM, Choi-Lundberg DL, West AK, Chuah MI (2005) Genetic expression profile of olfactory ensheathing cells is distinct from that of Schwann cells and astrocytes. Glia 51(2):132–147
Pellitteri R, Spatuzza M, Russo A, Stanzani S (2007) Olfactory ensheathing cells exert a trophic effect on the hypothalamic neurons in vitro. Neurosci Lett 417(1):24–29
Kafitz KW, Greer CA (1999) Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia 25(2):99–110
López-Vales R, Forés J, Verdú E, Navarro X (2006) Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis 21(1):57–68
Woodhall E, West AK, Chuah MI (2001) Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res 88(1–2):203–213
Li Y, Field PM, Raisman G (2005) Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia 52(3):245–251
Su Z, He C (2010) Olfactory ensheathing cells: biology in neural development and regeneration. Prog Neurobiol 92(4):517–532
Williams SK, Franklin RJ, Barnett SC (2004) Response of olfactory ensheathing cells to the degeneration and regeneration of the peripheral olfactory system and the involvement of the neuregulins. J Comp Neurol 470(1):50–62
Chung RS, Woodhouse A, Fung S, Dickson TC, West AK, Vickers JC, Chuah MI (2004) Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. Cell Mol Life Sci 61(10):1238–1245
Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13(3):757–767
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403(6768):434–439
Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z (2002) Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417(6892):941–944
Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T (2006) RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol 173(1):47–58
Tanaka T, Ueno M, Yamashita T (2009) Engulfment of axon debris by microglia requires p38 MAPK activity. J Biol Chem 284(32):21626–21636
Lauber K, Blumenthal SG, Waibel M, Wesselborg S (2004) Clearance of apoptotic cells: getting rid of the corpses. Mol Cell 14(3):277–287
Chiu SC, Hung HS, Lin SZ, Chiang E, Liu DD (2009) Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J Mol Med (Berl) 87(12):1179–1189
Ma YH, Zhang Y, Cao L, Su JC, Wang ZW, Xu AB, Zhang SC (2010) Effect of neurotrophin-3 genetically modified olfactory ensheathing cells transplantation on spinal cord injury. Cell Transplant 19(2):167–177
Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C (2004) Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain 127(Pt 3):535–549
Yang H, Jin WL, Wang CT, You SW, Ju G (2003) Expression and biological activity of human neurotrophin-3 in olfactory ensheathing cells mediated by retroviral vector. Shi Yan Sheng Wu Xue Bao 36(1):5–12
Liu Y, Gong Z, Liu L, Sun H (2010) Combined effect of olfactory ensheathing cell (OEC) transplantation and glial cell line-derived neurotrophic factor (GDNF) intravitreal injection on optic nerve injury in rats. Mol Vis 16:2903–2910
Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW (2005) NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain 128(Pt 4):839–853
Huizinga R, van der Star BJ, Kipp M, Jong R, Gerritsen W, Clarner T, Puentes F, Dijkstra CD, van der Valk P, Amor S (2012) Phagocytosis of neuronal debris by microglia is associated with neuronal damage in multiple sclerosis. Glia 60(3):422–431
Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A (2011) Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem 286(3):2308–2319
Aldskogius H (2001) Microglia in neuroregeneration. Microsc Res Tech 54(1):40–46
Nacher J, Ramírez C, Palop JJ, Molowny A, Luis de la Iglesia JA, López-García C (1999) Radial glia and cell debris removal during lesion-regeneration of the lizard medial cortex. Histol Histopathol 14(1):89–101
Kocsis JD, Lankford KL, Sasaki M, Radtke C (2009) Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci Lett 456(3):137–142
Lankford KL, Sasaki M, Radtke C, Kocsis JD (2008) Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia 56(15):1664–1678
Franssen EH, de Bree FM, Verhaagen J (2007) Olfactory ensheathing glia: their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res Rev 56(1):236–258
Ramón-Cueto A, Valverde F (1995) Olfactory bulb ensheathing glia: a unique cell type with axonal growth-promoting properties. Glia 14(3):163–173
Harris JA, West AK, Chuah MI (2009) Olfactory ensheathing cells: nitric oxide production and innate immunity. Glia 57(16):1848–1857
Raisman G, Li Y (2007) Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci 8(4):312–319
Leung JY, Chapman JA, Harris JA, Hale D, Chung RS, West AK, Chuah MI (2008) Olfactory ensheathing cells are attracted to, and can endocytose, bacteria. Cell Mol Life Sci 65(17):2732–2739
Vincent AJ, West AK, Chuah MI (2005) Morphological and functional plasticity of olfactory ensheathing cells. J Neurocytol 34(1–2):65–80
Vincent AJ, Choi-Lundberg DL, Harris JA, West AK, Chuah MI (2007) Bacteria and PAMPs activate nuclear factor kappaB and Gro production in a subset of olfactory ensheathing cells and astrocytes but not in Schwann cells. Glia 55(9):905–916
Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J (2000) Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25(2):425–435
Yang H, Cheng X, Yao Q, Li J, Ju G (2008) The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res 33(11):2269–2280
Yang H, Cheng XP, Li JW, Yao Q, Ju G (2009) De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol 29(4):455–473
Inatani M, Honjo M, Otori Y, Oohira A, Kido N, Tano Y, Honda Y, Tanihara H (2001) Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Invest Ophthalmol Vis Sci 42(8):1930–1938
Mitchell PJ, Hanson JC, Quets-Nguyen AT, Bergeron M, Smith RC (2007) A quantitative method for analysis of in vitro neurite outgrowth. J Neurosci Methods 164(2):350–362
Yang H, Ling W, Vitale A, Olivera C, Min Y, You S (2011) ErbB2 activation contributes to de-differentiation of astrocytes into radial glial cells following induction of scratch-insulted astrocyte conditioned medium. Neurochem Int 59(7):1010–1018
Miragall F, Kadmon G, Schachner M (1989) Expression of L1 and N-CAM cell adhesion molecules during development of the mouse olfactory system. Dev Biol 135(2):272–286
Navarro X, Valero A, Gudiño G, Forés J, Rodríguez FJ, Verdú E, Pascual R, Cuadras J, Nieto-Sampedro M (1999) Ensheathing glia transplants promote dorsal root regeneration and spinal reflex restitution after multiple lumbar rhizotomy. Ann Neurol 45(2):207–215
Pastrana E, Moreno-Flores MT, Gurzov EN, Avila J, Wandosell F, Diaz-Nido J (2006) Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: a new role for matrix metalloproteinase 2. J Neurosci 26(20):5347–5359
Pastrana E, Moreno-Flores MT, Avila J, Wandosell F, Minichiello L, Diaz-Nido J (2007) BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem Int 50(3):491–498
Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, Noth J, Schwab ME, Brook GA (2005) Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain 128(Pt 2):356–364
David S, Fry EJ, López-Vales R (2008) Novel roles for Nogo receptor in inflammation and disease. Trends Neurosci 31(5):221–226
George R, Griffin JW (1994) Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol 129(2):225–236
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132(Pt 2):288–295
Li YB, Kaur C, Ling EA (1998) Neuronal degeneration and microglial reaction in the fetal and postnatal rat brain after transient maternal hypoxia. Neurosci Res 32(2):137–148
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18(5):1633–1641
Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K (2004) Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 45(1):89–95
Acknowledgments
This work was supported by the Natural Science Foundation of China (grant nos. 81371411, 81071486, 81171137, and 81372056).
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, BR., Xie, ST., Wu, MM. et al. Phagocytic Removal of Neuronal Debris by Olfactory Ensheathing Cells Enhances Neuronal Survival and Neurite Outgrowth via p38MAPK Activity. Mol Neurobiol 49, 1501–1512 (2014). https://doi.org/10.1007/s12035-013-8588-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8588-2