Abstract
Fenproporex (Fen) is converted in vivo into amphetamine, which is used to induce mania-like behaviors in animals. In the present study, we intend to present a new animal model of mania. In order to prove through face, construct, and predictive validities, we evaluated behavioral parameters (locomotor activity, stereotypy activity, and fecal boli amount) and brain energy metabolism (enzymes citrate synthase; malate dehydrogenase; succinate dehydrogenase; complexes I, II, II–III, and IV of the mitochondrial respiratory chain; and creatine kinase) in rats submitted to acute and chronic administration of fenproporex, treated with lithium (Li) and valproate (VPA). The administration of Fen increased locomotor activity and decreased the activity of Krebs cycle enzymes, mitochondrial respiratory chain complexes, and creatine kinase, in most brain structures evaluated. In addition, treatment with mood stabilizers prevented and reversed this effect. Our results are consistent with the literature that demonstrates behavioral changes and mitochondrial dysfunction caused by psychostimulants. These findings suggest that chronic administration of Fen may be a potential animal model of mania.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar disorder (BD) is a chronic, and often severe, psychiatric illness, characterized by manic and depressive episodes. Clinical responses of BD patients to different centrally acting drugs have suggested that BD symptoms arise from an imbalance of neurotransmission, consisting of excessive dopaminergic and glutamatergic transmission, and reduced cholinergic muscarinic transmission [1, 2]. In contrast, drugs that stimulate dopamine synthesis (levodopa), bind to dopamine receptors (bromocriptine), or reduce dopamine reuptake (amphetamine), can precipitate mania [3–5].
Among the most effective treatments, mood stabilizers represent the keystone in acute mania, depression, and maintenance treatment of BD. The drugs that are considered first line for BD are lithium (Li) and valproate (VPA). They offer reasonable protection against recurrent mood episodes and have a modest antidepressant property [6]. However, treatment response is a highly heterogeneous trait, thus emphasizing the need for a structured informational framework of phenotypic and genetic predictors [7].
Fenproporex (Fen) is the second most commonly used anorectic substance in the world [8]. Fen is rapidly converted in vivo into amphetamine (AMPH) [9], thus releasing or blocking neuronal reuptake of noradrenaline and dopamine [10, 11]. Previous studies have shown that chronic use of Fen leads to some adverse effects, similar to AMPH, as behavioral alterations, addiction, and abstinence syndrome [12]. Therefore, the Food and Drug Administration has banned most of the AMPH-based anorectics [13]. However, the AMPH-based psychoactive anorexigenic drugs are commonly consumed in many parts of the world, mainly in South America [8].
Previous studies have shown that AMPH induces neurotoxicity through the production of free radicals [14, 15] and mitochondrial apoptotic pathway through cytochrome c release [16, 17], accompanied by a decrease in mitochondrial potential [18]. Mitochondria are important intracellular organelles in adenosine triphosphate (ATP) production [19]. The Krebs cycle occurs within the mitochondrial matrix and is the final common pathway for oxidation of carbohydrates, lipids, and some amino acids, which contributes to the production of large amounts of ATP via mitochondrial oxidative phosphorylation [20]. Another form of ATP production is through creatine kinase, which acts in the brain and other tissues with high and variable rates of ATP metabolism [21–23].
Modeling of human neuropsychiatric disorders in animals is extremely challenging given the subjective nature of many symptoms and lack of biomarkers and objective diagnostic tests. The general argument for using animal models in behavioral research is that such models allow researchers to test specific hypotheses under highly controlled conditions, using methods that are either impossible or unethical to use on humans. Nonetheless, progress in understanding pathophysiology and treatment development would benefit greatly from improved animal models [14, 24, 25].
Validity and reliability are the main criteria for evaluating animal models [26]. The validity of a model refers to the extent to which a model is useful for a given purpose. Thus, depending on the desired purpose of the test that one wishes to validate, different types of validity are relevant. Animal models should meet three sets of criteria: face, construct, and predictive validities. Face validity represents the ability of mimicking the symptoms of a determinate human disorder; this refers to the similarity between the animal model and the specific human behavior of interest. Construct validity refers to the similarity between mechanisms of the animal model and human disorder. Finally, the predictive validity refers to how useful animal models are for predicting the efficacy and safety of drugs for treating psychiatric disorders [27]. An adequate animal model of BD should resemble some features of a manic episode, such as euphoria, irritability, aggressiveness, hyperactivity, insomnia, or increased sexual drive.
Based on the hypothesis that Fen is converted into AMPH, in the present study, we intend to show that Fen is able to induce manic-like symptoms (face validity) and activate similar intracellular mechanisms (construct validity), and that these symptoms are treatable with lithium and valproate (predictive validity), drugs broadly used to treat BD. To accomplish this, it will evaluate behavioral parameters (locomotor activity, stereotypy activity, and fecal boli amount) and brain energy metabolism (enzymes citrate synthase; malate dehydrogenase; succinate dehydrogenase; complexes I, II, II–III, and IV of the mitochondrial respiratory chain; and creatine kinase) in rats submitted to acute and chronic administration of Fen treated with Li and VPA.
Material and Methods
Animals
Adult male Wistar rats (250–300 g) were obtained from the Central Animal House of Universidade do Extremo Sul Catarinense. The animals were caged in groups of five with free access to food and water, maintained on a 12-h light–dark cycle (lights on 7:00 am), at a temperature of 23 ± 1 °C. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care, with the approval of the UNESC Ethics Committee. Moreover, all efforts were made to minimize animal suffering as well as to reduce the number of animals.
Acute Fen administration
Animals received a single injection of Fen (6.25; 12.5 or 25 mg/kg, intraperitoneal (i.p.)) or Tween 2 % i.p. Locomotor activity was measured 2 h after the injection, and the rats were killed by decapitation right after the open-field task.
Chronic Fen administration
Fen was administered once a day (6.25, 12.5, and 25 mg/kg, i.p.) during 14 days. Control groups received Tween 2 % i.p. at the same volume. The regime of doses was based on previous studies of Rezin and collaborators [28]. Locomotor activity was measured 2 h after the last injection, and the rats were killed by decapitation right after the open-field task.
Reversal Experiment
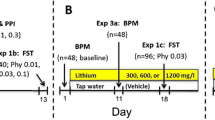
In the reversal experiment, we simulated the treatment of an acute manic episode. Animals received one daily i.p. injection of Fen 12.5 mg/kg or vehicle (Tween 2 %; control group) for 14 days (45 animals per group). On the 8th day of the experiment, the animals in the vehicle and Fen group were divided into three groups (15 animals per group): (1) treatment with Li (47.5 mg/kg i.p.), (2) treatment with VPA (200 mg/kg i.p.), and (3) treatment with vehicle; each treatment was conducted 7 days, twice a day. On the 15th day of experiment, animals received a single injection of Fen or vehicle, and behavior was assessed 2 h after the last injection. Rats were killed by decapitation immediately after the open-field task. The hippocampus, striatum, and prefrontal cortex were dissected, rapidly frozen, and stored at −70 °C until assayed (Fig. 1).
Prevention Experiment
In the prevention experiment, we simulated the maintenance phase of BD treatment. Animals were treated with Li (47.5 mg/kg i.p.), VPA (200 mg/kg i.p.), or vehicle (Tween 2 %), twice a day for 14 days. Between the 8th and 14th days, Li-, VPA-, and Tween 2 %-treated animals additionally received one daily i.p. injection of either Fen 12.5 mg/kg or vehicle (15 animals per group). Locomotor activity was measured 2 h after the last injection. Rats were killed by decapitation, immediately after the open-field task. The hippocampus, striatum, and prefrontal cortex were dissected, rapidly frozen, and stored at −70 °C until assayed (Fig. 2).
Behavior Patterns of Rats in the Open-Field Test
The task was performed in a 40 × 60-cm open field, surrounded by 50-cm-high walls. The floor of the apparatus was constructed with varnished wood and divided into 12 equal rectangles by black lines. The animals were gently placed in the left rear rectangle, in order to explore the arena for 5 min. The open-field test assessed the following behavioral parameters:
-
Crossings—total number of square crossings during the entire test period [29–31].
-
Rearings—total number of erect posture during the entire test period [29, 30].
-
Visits to center—total number of visits to the center of the open field. A center square of 30 × 30 cm was defined as the “center” area of the field.
-
Grooming—total time (in seconds) of grooming behavior during the entire test period. Behaviors include rat paw licking, nose/face grooming, head washing, body grooming/scratching, leg licking, and tail/genital grooming [32–34].
-
Sniffing—total time (in seconds) of sniffing behavior during the entire test period. Rats sniff the environment in moving (walking + rearing) [35–38].
-
Fecal boli—total number of fecal boli produced during the entire test period.
Tissue and Homogenate Preparation
The prefrontal cortex, hippocampus, and striatum were removed and homogenized (1:10, w/v) in SETH buffer, pH 7.4 (250 mM sucrose, 2 mM EDTA, 10 mM Trizma base, 50 IU/mL heparin). The homogenates were centrifuged at 800×g for 10 min, at 4 °C, and the supernatants kept at −70 °C until being used for enzyme activity determination. The maximal period between homogenate preparation and enzyme analysis was always less than 5 days. Protein content was determined using the method described by Lowry et al. [39] using bovine serum albumin as a standard.
Activities of Enzymes of the Krebs Cycle
Citrate Synthase Activity
Citrate synthase (CS) activity was assayed according to the method described by Shepherd and Garland [40]. The reaction mixture, which contained 100 mM Tris, pH 8.0; 100 mM acetyl CoA; 100 mM 5,5′-di-thiobis-(2-nitrobenzoic acid); 0.1 % triton X-100; and 2–4 μg supernatant protein, was initiated with 100 μM oxaloacetate and monitored at 412 nm for 3 min, at 25 °C.
Malate Dehydrogenase Activity
Malate dehydrogenase (MD) was measured as described by Kitto [41]. Aliquots (20 mg protein) were transferred into a medium containing 10 mM rotenone, 0.2 % Triton X-100, 0.15 mM NADH, and 100 mM potassium phosphate buffer, pH 7.4, at 37 °C. The reaction was started by the addition of 0.33 mM oxaloacetate. Absorbance was monitored as described above.
Succinate Dehydrogenase Activity
Succinate dehydrogenase (SD) activity was determined according to the method of Fischer et al. [42], by following the decrease in absorbance, due to the reduction of 2,6-di-chloro-indophenol (2,6-DCIP) at 600 nm, with 700 nm as a reference wavelength (ε = 19.1 mM−1 cm−1), in the presence of phenazine methasulfate (PMS). The reaction mixture, consisting of 40 mM potassium phosphate, pH 7.4; 16 mM succinate; and 8 μM 2,6-DCIP, was preincubated with 40–80 μg homogenate protein at 30 °C, for 20 min. Subsequently, 4 mM sodium azide, 7 μM rotenone, and 40 μM 2,6-DCIP were added, and the reaction was initiated by the addition of 1 mM PMS and monitored for 5 min.
Activities of Mitochondrial Respiratory Chain Enzymes
Complex I Activity
NADH dehydrogenase (complex I) was evaluated according to Cassina and Radi [43], by the determination of the rate of NADH-dependent ferricyanide reduction at λ = 420 nm.
Complex II Activity
The activities of succinate-2,6-dichloroindophenol (DCIP)-oxidoreductase (complex II) were determined using the method described by Fischer et al. [42]. Complex II activity was measured by following the decrease in absorbance, due to the reduction of 2,6-DCIP at λ = 600 nm.
Complex II–III Activity
The activity of succinate, cytochrome c oxidoreductase (complex III) was determined using the method described by Fischer et al. [42]. Complex II–III activity was measured by cytochrome c reduction, using succinate as substrate at λ = 550 nm.
Complex IV Activity
The activity of cytochrome c oxidase was assayed according to the method described by Rustin et al. [44], by following the decrease in absorbance, due to the oxidation of previously reduced cytochrome c (prepared by the reduction of cytochrome with NaBH4 and HCl) at λ = 550 nm, with 580 nm as the reference wavelength (ε = 19.1 mM−1 cm−1). The activities of the mitochondrial respiratory chain complexes were calculated as nanomoles per minute per milligram of protein.
Activity of Creatine Kinase Enzyme
Creatine kinase (CK) activity was measured in brain homogenates pretreated with 0.625 mM lauryl maltoside. The reaction mixture consisted of 60 mM Tris–HCl, pH 7.5, containing 7 mM phosphocreatine, 9 mM MgSO4, and approximately 0.4–1.2 μg protein, in a final volume of 100 μL. After 15 min of preincubation at 37 °C, the reaction was started by the addition of 3.2 mmol of ADP. The reaction was stopped after 10 min by the addition of 1 μmol of p-hydroxymercuribenzoic acid. The creatine formed was estimated according to the colorimetric method of Hughes [45]. The color was developed by the addition of 100 μL 2 % α-naphtol and 100 μL 0.05 % diacetyl in a final volume of 1 mL and read spectrophotometrically after 20 min at 540 nm. Results were expressed as units/minute × milligram protein.
Statistical Analysis
Data were analyzed by one-way analysis of variance, followed by the Tukey test, when F was significant, and are expressed as mean ± standard error to behavior text and as mean ± standard deviation to biochemical text. All analyses were performed using the Statistical Package for the Social Science (SPSS; version 16.0) software.
Results
Acute or Chronic Administration of Fenproporex on Open-Field Behavior
The acute administration of Fen significantly increased the number of crossings and rearings, at all doses administered. In addition, a single injection of Fen, in high doses (12.5 and 25 mg/kg), increased the number of visits to the center of the open field. No differences were observed for grooming, sniffing (Fig. 3a), and amount of fecal boli (Fig. 4a), after the acute administration of Fen. Once more, the repeated administration of Fen increased the motor behaviors of rats, crossings and rearings. The total visits to the center of the open field, sniffing, and fecal boli were significantly higher after chronic Fen administration, at the doses of 12.5 and 25 mg/kg, compared with the control group (Figs. 3b and 4b). Finally, the repeated administration of Fen significantly increased grooming behavior at the dose of 25 mg/kg (Fig. 3b).
Acute or Chronic Administration of Fenproporex on Enzymes of the Krebs Cycle Activities
In the acute protocol, Fen inhibited CS activity in the prefrontal cortex at the dose of 25 mg/kg, in the hippocampus at all doses administered (6.25, 12.5, and 25 mg/kg), and in the striatum at 12.5 and 25 mg/kg. Acute administration of Fen also inhibited the activity of MD in the prefrontal cortex at higher doses (12.5 and 25 mg/kg) and the hippocampus and striatum in all administered doses of Fen (6.25, 12.5, and 25 mg/kg). SD activity was also measured, and Fen inhibited the enzyme activity only in the hippocampus at all doses (6.25, 12.5, and 25 mg/kg) administered (Fig. 5a). In contrast, after repeated Fen (6.25, 12.5, and 25 mg/kg) administration, only SD was inhibited in the hippocampus (Fig. 5b).
The effects of acute (a) or chronic (b) administration of fenproporex [Fen] 6.25, 12.5, and 25 mg/kg and Tween 2 % (control group) on enzymes of the Krebs cycle activities. Citrate synthase [CS], malate dehydrogenase [MD], and succinate dehydrogenase [SD] activities were measured in the prefrontal cortex, hippocampus, and striatum of rats. Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6), for six independent experiments performed in duplicate. *p < 0.05, different from control
Acute or Chronic Administration of Fenproporex on Mitochondrial Respiratory Chain Complexes
In the acute protocol, administration of Fen at all doses (6.25, 12.5, and 25 mg/kg) resulted in a significant decrease in complexes I, II, II–III, and IV of the mitochondrial respiratory chain in the prefrontal cortex and hippocampus (Fig. 6a). However, administration of Fen in the chronic protocol resulted in a significant inhibition of complexes II and IV of the mitochondrial respiratory chain, only in the hippocampus (Fig. 6b).
The effects of acute (a) or chronic (b) administration of fenproporex [Fen] 6.25, 12.5, and 25 mg/kg and Tween 2 % (control group) on mitochondrial respiratory chain complex (I, II, II–III, IV) activity in the prefrontal cortex, hippocampus, and striatum of rats. Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6), for six independent experiments performed in duplicate. *p < 0.05, different from control
Acute or Chronic Administration of Fenproporex on Creatine Kinase Activity
Acute Fen administered at all doses (6.25, 12.5, and 25 mg/kg) significantly inhibited CK activity in the rat's hippocampus (Fig. 7a). After repeated Fen (6.25, 12.5, and 25 mg/kg) administration, the CK activity was also inhibited in the hippocampus (Fig. 7b).
The effects of acute (a) or chronic (b) administration of fenproporex [Fen] 6.25, 12.5, and 25 mg/kg and Tween 2 % (control group) on creatine kinase [CK] activity in the prefrontal cortex, hippocampus, and striatum of rats. Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6), for six independent experiments performed in duplicate. *p < 0.05, different from control
Reversal or Prevention Treatment with Lithium and Valproate on Open-Field Behavior
In the present study, we also showed the Fen-induced hyperlocomotion in the reversal experiment. Results showed that there was a significant effect of Fen and mood stabilizers in the behavior, stereotypy, and fecal boli. We showed that the administration of Fen increased the number of crossings, rearings, visits to the center, grooming, sniffing (Fig. 8a, b), and fecal boli (Fig. 9a, b) both in the reversal and prevention experiment, and this effect was reverted and prevented by Li and VPA. Li or VPA alone did not alter behavioral parameters, indicating that mood stabilizers' effects on Fen-treated rats were not associated with sedation.
Open-field test in reversal (a) and prevention (b) experiment (Tween 2 %, fenproporex 12.5 mg/kg [Fen], lithium 47.5 mg/kg [Li], valproate 200 mg/kg [VPA]). Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Mean ± standard error (n = 12). *p < 0.05, different from control
Fecal boli amount in reversal (a) and prevention (b) experiment (Tween 2 %, fenproporex 12.5 mg/kg [Fen], lithium 47.5 mg/kg [Li], valproate 200 mg/kg [VPA]). Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Mean ± standard error (n = 12). *p < 0.05, different from control
Reversal or Prevention Treatment with Lithium and Valproate on Enzymes of the Krebs Cycle, Mitochondrial Respiratory Chain Complexes, and Creatine Kinase Activities
There was inhibited activity of SD (Fig. 10a), complex II (Fig. 11a), complex IV (Fig. 11a), and CK (Fig. 12a) enzymes in the hippocampus. Li and VPA treatments reversed this inhibition. Moreover, CS (Fig. 10a), MD (Fig. 10a), complex I (Fig. 11a), and complex II–III (Fig. 11a) activities were not altered in the brain regions studied. None of the enzymes were altered in the prefrontal cortex and striatum. Our results also showed that Fen, in the prevention experiment, inhibited malate dehydrogenase (Fig. 10b), SD (Fig. 10b), complex II (Fig. 11b), complex IV (Fig. 11b), and CK (Fig. 12b) enzyme activities in the hippocampus, and both Li and VPA treatments were only not able to prevent the complex IV inhibition. CS (Fig. 10b), complex I (Fig. 11b), and complex II–III (Fig. 11b) activities were not altered. None of the enzymes were altered in the prefrontal cortex and striatum.
Citrate synthase, malate dehydrogenase, and succinate dehydrogenase activities in reversal (a) and prevention (b) experiment (Tween 2 %, fenproporex 12.5 mg/kg [Fen], lithium 47.5 mg/kg [Li], valproate 200 mg/kg [VPA]). Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6). *p < 0.05, different from control
Complexes of mitochondrial respiratory chain activities in reversal (a) and prevention (b) experiment (Tween 2 %, fenproporex 12.5 mg/kg [Fen], lithium 47.5 mg/kg [Li], valproate 200 mg/kg [VPA]). Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6). *p < 0.05, different from control
Creatine kinase activity in reversal (a) and prevention (b) experiment (Tween 2 %, fenproporex 12.5 mg/kg [Fen], lithium 47.5 mg/kg [Li], valproate 200 mg/kg [VPA]). Data were analyzed by one-way analysis of variance followed by Tukey test when F was significant. Values are expressed as nanomoles per minute per milligram of protein, mean ± S.D. (n = 6). *p < 0.05, different from control
Discussion
Fen is an AMPH-derived anorectic rapidly converted in vivo into AMPH. Consumption of a low daily dose, 10 mg, of Fen led to the detection of AMPH, within 3 h, in urine with peak urinary levels greater than 4.0 ng/mL [9]. Fen is classified as a dopaminergic agent of indirect action. Thus, it represents complexes' actions on the dopaminergic presynaptic terminal, but its most significant action is the connection to the transporter protein and the induction of reverse transport of dopamine. The resulting action of AMPH in terminals of dopamine is the increase in the concentration of dopamine in the synaptic cleft. Dopamine accumulated in the cleft interacts with dopamine D1 and D2 receptors, initiating a sequence of events that alters the neural activity and behavior [46].
Several studies suggest that AMPH may be used to induce mania-like symptoms in animals [25, 47–50], such as hyperactivity, probably due to extracellular dopamine increase [51]. In this context, studies have reported that BD patients represent dopaminergic and locomotor alterations [52, 53].
Firstly, face validity was evaluated through behavior tasks. We observed that acute and chronic administration of Fen increased locomotor and exploratory behavior. We suggest that the changes Fen induced, both behavioral and neurochemical, are via the dopaminergic system. It is well described that psychostimulants have serious side effects, particular behavioral changes, and potential for abuse [54]. We also found that acute and chronic administration of Fen in high doses increases visits to the center of the open field, representing risk-taking behavior. In addition, chronic, but not acute, administration of Fen in high doses induces grooming and sniffing behavior. In agreement with our data, Einat et al. [55] showed that the administration of AMPH increased risk behavior in rats.
We also demonstrated that chronic administration of Fen, in the highest dose (25 mg/kg), increases the amount of feces produced by rats. This observation is an indication that this drug alters the brain–gut axis through central corticotropin-releasing factor receptor signals, an important mediator of the autonomic nervous system, particularly the parasympathetic, to increase colonic motility [56, 57]. It is known that hypersecretion of hypothalamic and extra-hypothalamic corticotrophin-releasing factor (CRF) systems is a major factor in the pathogenesis of affective and anxiety disorders [58]. Pringle and colleagues [59] have demonstrated that CRF2 receptors are increased in the dorsal raphe nucleus 20 h following chronic AMPH administrations and remain elevated up to 6 weeks after withdrawal.
In construct validity parameters, we observed that the activities in enzymes of the Krebs cycle, CS, MD, SD, mitochondrial respiratory chain, and complex I, II, II–III, IV, and CK were inhibited in some cerebral structures of rats submitted to the administration of Fen. Previous studies from our and other laboratories have demonstrated that chronic administration of AMPH also inhibited enzymes of energetic metabolism in rats [60–65].
It is known that AMPH induces the generation of free radicals, via the oxidative catabolism of dopamine, and the subsequent dysfunction of mitochondrial respiration [65–68]. Several studies have demonstrated that AMPH induces oxidative damage in protein, lipids, and DNA in the brain of rats [14, 69]. Another important point to consider is that changes in energy metabolism did not occur similarly in brain structures analyzed. This can be explained by the fact that the brain is the most complex biological structure known, formed by several regions that respond differently, and partly, with different types of neurons. In addition, inside a homogeneous population of cells, there is heterogeneity in terms of physiological and metabolic characteristics [70–72].
Actually, acute or chronic drug manipulations have the advantage of probing the function of a specific receptor or neurotransmitter system that is implicated in the etiology of the disorder, or produces the desired deficit [26]. Here we also observed that the acute treatment causes more changes in energy metabolism than the chronic treatment. Excessive stimulation of dopamine receptors during exposure to psychostimulants induces various molecular adaptive changes in the mesolimbic dopamine pathway [73, 74]. However, the increase in grooming, sniffing, and fecal boli was only observed under chronic treatment, suggesting that changes in energy metabolism are not related to these behavioral changes.
Recent advances regarding underlying neural mechanisms, etiology, genetics, and new pharmacological approaches have facilitated the development of an animal model of mania. In this scenario, we evaluated the last criteria for evaluating an animal model, the predictive validity. Our results for behavior activity of the reversal and prevention experiment showed an increase in the number of crossings, rearings, groomings, sniffing, and visits to the center in rats submitted to chronic administration of Fen, and the treatment with Li and VPA reversed and prevented this alteration. We have also demonstrated increased amount of feces in both experiments, with both Li and VPA treatments reverting and preventing this alteration.
There is a traditional concept among clinicians called class effect, that is to say, all classes of drugs present a specific action. This simplistic concept is supported by recent researches that show a high and global effectiveness for older agents such as lithium and valproate in many facets of bipolar disorder. These old agents are considered to be the “gold standard” [75]. In an animal model of mania using d-AMPH, Li and VPA were able to reverse and prevent amphetamine-induced mitochondrial dysfunction, suggesting that a possible mechanism of action of these mood stabilizers may be decreasing the amount of dopamine available, and normalize mitochondrial function [65]. Besides, another study showed a protective effect of lithium and valproate against amphetamine-induced oxidative stress in vivo, supporting the hypothesis that oxidative stress may be associated with the pathophysiology of BD [76].
It is established that the impairment of the respiratory chain in mitochondria leads to an increase in free radical generation, especially superoxide, and that these reactive oxygen species inhibit the mitochondrial respiratory chain, resulting in the generation of more reactive species, forming a cyclic phenomenon [77, 78]. In this scenario, the oxidative damage induced by AMPH might be responsible for the alterations in the energy metabolism caused by Fen administration in the animal model of mania studied. Damage on the mitochondrial electron transport chain has been suggested to be an important factor in the pathogenesis of a range of psychiatric disorders [79]. The CS, MD, and SD enzymes belong to the Krebs cycle, which is responsible for the generation of approximately 67 % of all reducing equivalents per molecule of glucose. Hence, factors that influence the Krebs cycle flux will be of critical importance for oxidative phosphorylation [80]. In addition, the complexes I, II, II–III, and IV of the mitochondrial respiratory chain belong to the major ATP-regeneration pathway, which supplies more than 95 % of the total energy requirement in the cells [81]. The CK enzyme is present in tissues with high energy demand, and also contributes to maintain ATP levels [21–23].
The present results also showed that the administration of Fen in the prevention experiment inhibited the activity of MD, SD, complex II, complex IV, and CK activities in the hippocampus. However, the treatment with Li and VPA prevented this inhibition, except in complex IV. In addition, our results also showed that chronic administration of Fen in the reversal experiment inhibited the activity of SD, complex II, complex IV, and CK enzymes in the hippocampus. But, the treatment with Li and VPA reversed this inhibition in all enzymes. Konradi et al. [82] reported a decreased expression of nuclear gene coding for enzymatic complexes responsible for oxidative phosphorylation, and a reduced expression of nuclear genes related to proteasome degradation in the hippocampus of nine subjects affected by BD. MacDonald et al. [83] presented very interesting results, showing that mRNA levels of CK are decreased in BD patients, especially in the hippocampus. The hippocampus is one region in the brain that has received significant attention in mood disorder research, and the highly plastic, stress-sensitive hippocampal complex may play a central role in mood disorders [84].
Corrêa et al. [60] showed that the animal model of mania induced by AMPH inhibited CS activity in the rat hippocampus. However, VPA administration reversed the CS activity inhibition, and the Li prevented the enzyme inhibition. Streck et al. [62] demonstrated that AMPH inhibited CK activity in rat brains and that VPA and Li were not able to reverse or prevent the enzyme inhibition. Zugno et al. [85] showed that AMPH increased Na+,K+-ATPase activity in rat brains and that VPA or Li reversed this effect. Moreover, VPA and Li did not alter Na+,K+-ATPase activity. Valvassori et al. [64] showed that AMPH inhibited the mitochondrial respiratory chain activity in rat brains. However, VPA, but not Li, reversed AMPH-induced dysfunction. Besides, VPA and Li exert protective effects against this AMPH-induced mitochondrial dysfunction.
In conclusion, the present study showed that acute Fen administration induced manic-like hyperactivity and inhibition of energy metabolism in the rat brain. However, longer periods of exposure to Fen induce hyperactivity, stereotyped behaviors, grooming and sniffing, and increased fecal boli, but do not alter energy metabolism in the rat brain. Moreover, mood stabilizers, such as Li and VPA, reversed and prevented these alterations. Thus, we suggest that the administration of Fen induces behavioral and neurochemical changes similar to those observed with the administration of psychostimulants, such as amphetamine. We showed that Fen achieved the three points for validity as an animal model: face, construct, and predictive validities; thus, this regimen of Fen administration may be used as an animal model of mania for psychiatric diseases like bipolar disorder.
There are clear limitations to this model. Firstly, it remains a pharmacologic challenge model and, as such, cannot fully model human bipolar illness. Secondly, the actual cause of bipolar illness remains unknown. This model is patterned after a hypothesis and not direct in vivo brain measurements in ill humans. Thirdly, only some aspects of the manic syndrome—hyperlocomotion, stereotyped behaviors, grooming and sniffing—were examined. Other aspects, such as sleep, irritability, and increased sexual activity, may be observable in rodents. For example, sleep can be observed indirectly in 24 h in automated activity monitors; aggression may be observed as frequency of fighting when animals are placed together; libido may be quantified as mountings when males are mixed with females. Finally, energy metabolism was observed only in the hippocampus. Nonetheless, the observation that Li and VPA reversed and prevented behavioral changes and mitochondrial dysfunction suggests that Fen administration may be used as an animal model of mania for psychiatric diseases like bipolar disorder.
References
Bunney WEJ, Garland-Bunney BL (1987) Mechanisms of action of lithium in affective illness: basic and clinical implications. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven, New York, pp 553–565
Post RM, Jimerson DC, Bunney WE Jr, Goodwin FK (1980) Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology (Berl) 67:297–305
Fisher G, Pelonero AL, Ferguson C (1991) Mania precipitated by prednisone and bromocriptine. Gen Hosp Psychiatry 13:345–346
Peet M, Peters S (1995) Drug-induced mania. Drug Saf 12:146–153
Sultzer DL, Cummings JL (1989) Drug-induced mania—causative agents, clinical characteristics and management. A retrospective analysis of the literature. Med Toxicol Adverse Drug Exp 4:127–143
Davis LL, Bartolucci A, Petty F (2005) Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord 85:259–266
Squassina A, Manchia M, Del Zompo M (2010) Pharmacogenomics of mood stabilizers in the treatment of bipolar disorder. Hum Genomics Proteomics 3:597–661
Cohen PA (2009) Imported fenproporex-based diet pills from Brazil: a report of two cases. J Gen Intern Med 24:430–433
Cody JT, Valtier S, Stillman S (1999) Amphetamine and fenproporex levels following multidose administration of fenproporex. J Anal Toxicol 23:187–194
Coutts RT, Nazarali AJ, Baker GB, Pasutto FM (1986) Metabolism and disposition of N-(2-cyanoethyl)-amphetamine (fenproporex) and amphetamine: study in the rat brain. Can J Physiol Pharmacol 64:724–728
Mattei R, Carlini EA (1996) A comparative study of the anorectic and behavioral effects of fenproporex on male and female rats. Braz J Med Biol Res 29:1025–1030
Pélissier-alicot AL, Piercecchi-marti MD, Bartoli C, Kuhlmann E, Coiffait PE, Sanvoisin A, Giocanti D, Léonetti G (2006) Abusive prescription of psychostimulants: a study of two cases. J Forensic Sci 51:407–410
Colman E (2005) Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 143:380–385
Frey BN, Martins MR, Petronilho FC, Dal-Pizzol F, Quevedo J, Kapczinski F (2006) Increased oxidative stress after repeated amphetamine exposure: possible relevance as a model of mania. Bipolar Disord 8:275–280
Valvassori SS, Petronilho FC, Réus GZ, Steckert AV, Oliveira VB, Boeck CR, Kapczinski F, Dal-Pizzol F, Quevedo J (2008) Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 32:1064–1068
Jiménez A, Jordà EG, Verdaguer E, Pubill D, Sureda FX, Canudas AM, Escubedo E, Camarasa J, Camins A, Pallàs M (2004) Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicol Appl Pharmacol 196:223–234
Oliveira MT, Rego AC, Macedo TR, Oliveira CR (2003) Drugs of abuse induce apoptotic features in PC12 cells. Ann N Y Acad Sci 1010:667–670
Cunha-Oliveira T, Rego AC, Cardoso SM, Borges F, Swerdlow RH, Macedo T, de Oliveira CR (2006) Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res 1089:44–54
Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB (2001) Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res 26:739–764
Kelly DP, Gordon JI, Alpers R, Strauss AW (1989) The tissue-specific expression and developmental regulation of two nuclear genes encoding rat mitochondrial proteins. Medium chain acyl-CoA dehydrogenase and mitochondrial malate dehydrogenase. J Biol Chem 264:18921–18925
Bessman SP, Carpenter CL (1985) The creatine–creatine phosphate energy shuttle. Annu Rev Biochem 54:831–865
Schnyder T, Winkler H, Gross H, Eppenberger HM, Wallimann T (1991) Crystallization of mitochondrial creatine kinase. Growing of large protein crystals and electron microscopic investigation of microcrystals consisting of octamers. J Biol Chem 266:5318–5322
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281:21–40
El-Mallakh RS, El-Masri MA, Huff MO, Li XP, Decker S, Levy RS (2003) Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord 5:362–365
Frey BN, Valvassori SS, Gomes KM, Martins MR, Dal-Pizzol F, Kapczinski F, Quevedo J (2006) Increased oxidative stress in submitochondrial particles after chronic amphetamine exposure. Brain Res 1097:224–229
Geyer MA, Markou A (2002) The role of preclinical models in the development of psychotropic drugs. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds) Neuropsychopharmacology: the fifth generation of progress. Lippincott Williams & Wilkins, Pennsylvania, pp 445–455
Riegel RE, Valvassori SS, Elias G, Réus GZ, Steckert AV, de Souza B, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J (2009) Animal model of mania induced by ouabain: evidence of oxidative stress in submitochondrial particles of the rat brain. Neurochem Int 55:491–495
Rezin GT, Scaini G, Ferreira GK, Cardoso MR, Gonçalves CL, Constantino LS, Deroza PF, Ghedim FV, Valvassori SS, Resende WR, Quevedo J, Zugno AI, Streck EL (2012) Inhibition of acetylcholinesterase activity in brain and behavioral analysis in adult rats after chronic administration of fenproporex. Metab Brain Dis 27:453–458
Ericson E, Samuelsson J, Ahlenius S (1991) Photocell measurements of rat motor activity. A contribution to sensitivity and variation in behavioral observations. J Pharmacol Methods 25:111–122
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Wultz B, Sagvolden T, Moser EI, Moser MB (1990) The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylphenidate on exploratory behavior. Behav Neural Biol 53:88–102
Kalueff AV, Tuohimaa P (2004) Grooming analysis algorithm for neurobehavioural stress research. Brain Res 13:151–158
Kalueff AV, Tuohimaa P (2005) The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods 143:169–177
Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P (2007) Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc 2:2538–2544
Casarrubea M, Sorbera F, Crescimanno G (2008) Multivariate analysis of the modifications induced by an environmental acoustic cue on rat exploratory behavior. Physiol Behav 93:687–696
Casarrubea M, Sorbera F, Crescimanno G (2009) Structure of rat behavior in holeboard: I. Multivariate analysis of response to anxiety. Physiol Behav 96:174–179
Casarrubea M, Sorbera F, Crescimanno G (2009) Structure of rat behavior in holeboard: II. Multivariate analysis of modifications induced by diazepam. Physiol Behav 96:683–692
Meyerson BJ, Höglund AU (1981) Exploratory and socio-sexual behaviour in the male laboratory rat: a methodological approach for the investigation of drug action. Acta Pharmacol Toxicol (Copenh) 48:168–180
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Shepherd D, Garland PB (1969) The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114:597–610
Kitto GB (1969) Intra- and extramitochondrial malate dehydrogenases from chicken and tuna heart. Methods Enzymol 13:106–116
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–26
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Hughes BP (1962) A method for estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathologic sera. Clin Chim Acta 7:597–604
Hyman SE (1996) Addiction to cocaine and amphetamine. Neuron, Cambridge, pp 901–904
Machado-Vieira R, Kapczinski F, Soares JC (2004) Perspective for the development of animals models of bipolar disorders. Prog Neuropsychopharmacol Biol Psychiatry 28:209–224
Post RM, Weiss SR (1996) A speculative model of affective illness cyclicity based on patterns of drug tolerance observed in amygdale kindled seizures. Mol Neurobiol 13:33–60
Shaldivin A, Kaptsan A, Belmaker RH, Einat H, Grisaru N (2001) Transcranial magnetic stimulation in an amphetamine hyperactivity model of mania. Bipolar Disord 3:30–34
Gould TJ, Keith RA, Bhat RV (2001) Differential sensitivity to lithium's reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res 118:95–105
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M (2003) Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23:285–300
Pantazopoulos H, Stone D, Walsh J, Benes FM (2004) Differences in the cellular distribution of D1 receptor mRNA in the hippocampus of bipolars and schizophrenics. Synapse 54:147–155
Vogel M, Pfeifer S, Schaub RT, Grabe HJ, Barnow S, Freyberger HJ, Cascorbi I (2004) Decreased levels of dopamine D3 receptor mRNA in schizophrenic and bipolar patients. Neuropsychobiology 50:305–310
Zhu J, Reith ME (2008) Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets 7:393–409
Einat H, Yuan P, Szabo ST, Dogra S, Manji HK (2007) Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology 55:123–131
Saito K, Kasai T, Nagura Y, Ito H, Kanazaw M, Fukudo S (2005) Corticotropin-releasing hormone receptor 1 antagonist blocks brain–gut activation induced by colonic distention in rats. Gastroenterology 129:1533–1543
Taché Y, Martínez V, Million M, Rivier J (1999) Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol 13:18A–25A
Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT (1997) Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic like behavior. Regul Pept 71:15–21
Pringle RB, Mouw NJ, Lukkes JL, Forster GL (2008) Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci Res 62:62–65
Bachmann RF, Wang Y, Yuan P, Zhou R, Li X, Alesci S, Du J, Manji HK (2009) Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int J Neuropsychopharmacol 12:805–822
Corrêa C, Amboni G, Assis LC, Martins MR, Kapczinski F, Streck EL, Quevedo J (2007) Effects of lithium and valproate on hippocampus citrate synthase activity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 31:887–891
Moretti M, Valvassori SS, Steckert AV, Rochi N, Benedet J, Scaini G, Kapczinski F, Streck EL, Zugno AI, Quevedo J (2011) Tamoxifen effects on respiratory chain complexes and creatine kinase activities in an animal model of mania. Pharmacol Biochem Behav 98:304–310
Streck EL, Amboni G, Scaini G, Di-Pietro PB, Rezin GT, Valvassori SS, Luz G, Kapczinski F, Quevedo J (2008) Brain creatine kinase activity in an animal model of mania. Life Sci 82:424–429
Valenzuela A, Pla A, Villanueva E (1987) Effects of chronic administration of dextroamphetamine on enzymes of energy metabolism in regions of the rat brain. Neuropharmacology 26:627–631
Valvassori SS, Rezin GT, Ferreira CL, Moretti M, Gonçalves CL, Cardoso MR, Streck EL, Kapczinski F, Quevedo J (2010) Effects of mood stabilizers on mitochondrial respiratory chain activity in brain of rats treated with d-amphetamine. J Psychiatr Res 14:903–909
Burrows KB, Gudelsky G, Yamamoto BK (2000) Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharmacol 398:11–18
Cadet JL, Jayanthi S, Deng X (2005) Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Neurotox Res 8:199–206
Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL (2002) Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology 42:837–845
Andreazza AC, Kauer-Sant'Anna M, Frey BN, Stertz L, Zanotto C, Ribeiro L, Giasson K, Valvassori SS, Réus GZ, Salvador M, Quevedo J, Gonçalves CA, Kapczinski F (2008) Effects of mood stabilizers on DNA damage in an animal model of mania. J Psychiatry Neurosci 33:516–524
Lai YL, Rodarte JR, Hyatt RE (1977) Effect of body position on lung emptying in recumbent anesthetized dogs. J Appl Physiol 43:983–987
Sims DE (1991) Recent advances in pericyte biology—implications for health and disease. Can J Cardiol 7:431–443
Sonnewald U, Hertz L, Schousboe A (1998) Mitochondrial heterogeneity in the brain at the cellular level. J Cereb Blood Flow Metab 18:231–237
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
White FJ, Kalivas PW (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend 51:141–153
Fountoulakis KN, Kasper S, Andreassen O, Blier P, Okasha A, Severus E, Versiani M, Tandon R, Möller HJ, Vieta E (2012) Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry. Eur Arch Psychiatry Clin Neurosci 262(Suppl 1):1–48
Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31:326–332
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7:1140–1149
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Cell Physiol 292:670–686
Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL (2008) Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 34:1021–1029
Bowtell JL, Marwood S, Bruce M, Constantin-Teodosiu D, Greenhaff PL (2007) Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med 37:1071–1088
Rex A, Schickert R, Fink H (2004) Antidepressant-like effect of nicotinamide adenine dinucleotide in the forced swim test in rats. Pharmacol Biochem Behav 77:303–307
Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S (2004) Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry 61:300–308
MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C (2006) Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord 8:255–264
Campbell S, Macqueen G (2004) The role of hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29:417–426
Zugno AI, Valvassori SS, Scherer EB, Mattos C, Matté C, Ferreira CL, Rezin GT, Wyse AT, Quevedo J, Streck EL (2009) Na+, K+-ATPase activity in an animal model of mania. J Neural Transm 116:431–436
Acknowledgments
This work was supported by grants from Programa de Pós-graduação em Ciências da Saúde–Universidade do Extremo Sul Catarinense (UNESC), Núcleo de Excelência em Neurociências Aplicadas de Santa Catarina NENASC project, Conselho Nacional de Desenvolvimento Científico e Tecnológico (PRONEX-FAPESC/CNPq), and Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezin, G.T., Furlanetto, C.B., Scaini, G. et al. Fenproporex Increases Locomotor Activity and Alters Energy Metabolism, and Mood Stabilizers Reverse These Changes: a Proposal for a New Animal Model of Mania. Mol Neurobiol 49, 877–892 (2014). https://doi.org/10.1007/s12035-013-8566-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8566-8